Abstract
While standing naturally and when manually or pedally balancing an equivalent inverted pendulum, the load sways slowly (characteristic unidirectional duration ∼1 s) and the controller, calf muscles or hand, makes more frequent adjustments (characteristic unidirectional duration 400 ms). Here we test the hypothesis that these durations reflect load properties rather than some intrinsic property of the human neuromuscular system. Using a specialized set-up mechanically analogous to real standing, subjects manually balanced inverted pendulums with different moments of inertia through a compliant spring representing the Achilles tendon. The spring bias was controlled by a sensitive joystick via a servo motor and accurate visual feedback was provided on an oscilloscope. As moment of inertia decreased, inverted pendulum sway size increased and it became difficult to sustain successful balance. The mean duration of unidirectional balance adjustments did not change. Moreover, the mean duration of unidirectional inverted pendulum sway reduced only slightly, remaining around 1 s. The simplest explanation is that balance was maintained by a process of manual adjustments intrinsically limited to a mean frequency of two to three unidirectional adjustments per second corresponding to intermittent control observed in manual tracking experiments. Consequently the inverted pendulum sway duration, mechanically related to the bias duration, reflects an intrinsic constraint of the neuromuscular control system. Given the similar durations of sway and muscle adjustments observed in real standing, we postulate that the characteristic duration of unidirectional standing sway reflects intrinsic intermittent control rather than the inertial properties of the body.
Duration of sway under postural, voluntary and analogous settings
During quiet stance, humans are not still but are constantly in motion. It is well established that the characteristic duration of sagittal, unidirectional movements of the centre of pressure (CoP) and of the centre of mass (CoM) are approximately 1 s (CoP: mean, s.d., range, 0.97 s, 0.46 s, 0.35–1.85 s; Collins & De Luca, 1993; CoM: mean, s.d., range, 1.25 s, 0.3 s, 0.8–1.9 s; Loram et al. 2005b). (The frequency corresponding to a unidirectional duration of 1.25 s is 0.4 Hz and this represents the mean frequency of the velocity power spectrum (Loram et al. 2005b) which is consistent with a wide range of published measures (Maurer & Peterka, 2005).) Curiously, despite the mechanical differences between sagittal and frontal stability, this characteristic CoP duration is the same for forwards–backwards sway as for the smaller amplitude sideways sway (Collins & De Luca, 1993). Unlike sway size, these durations of CoP and CoM are also unaffected by the presence or absence of vision (CoP: Collins & De Luca, 1995; CoM: Loram et al. 2005b). This duration of 1 s was associated with a transition between ‘persistent’, open loop processes and ‘antipersistent’, closed loop processes (Collins & De Luca, 1993, 1995). These authors hypothesized that open loop processes, i.e. those operating without the benefit of sensory feedback, were responsible for the unidirectional (i.e. ‘persistent’) changes that occur over a time scale of 1 s, and correspondingly, that closed loop processes, i.e. those operating with sensory feedback, were responsible for the regulation of sway that occurs over time scales of more than 1 s.
Standing is usually regarded as a postural activity that is maintained unconsciously and automatically. We have also studied a closely related activity in which subjects are strapped to a vertical support and use their calf muscles and feet to balance an equivalent body, a real inverted pendulum (Loram et al. 2001; Loram & Lakie, 2002b). The inverted pendulum that we used had a moment of inertia and gravitational load stiffness (‘mgh’) equivalent to an adult. Although mechanically similar to standing, this artificial activity lacks the familiarity of real standing and might be regarded as more voluntary than real postural balance. Despite the differences in which subjects were consciously regulating inverted pendulum position, the mean sway duration was also around 1 s (CoM: mean, s.d., range 1.07 s, 0.16 s, 0.7–1.3 s). This duration was unchanged even when the sway size was substantially altered by changes in intention or availability of visual feedback.
Both natural standing and pedal balance of a real inverted pendulum are controlled by the calf muscles, which have been adapted for their postural function. This adaptation includes slow muscle fibres and patterns of innervation via the spinal cord and brain stem that are associated with postural reflexes (Davies et al. 2001). By contrast, manual control utilizes to a greater extent direct cortical innervation and innervation patterns that are associated with fine voluntary control of movement (Brooks, 1986; Davies et al. 2001). We have previously studied manual balance of a real inverted pendulum using a set-up that is mechanically analogous to real standing (Lakie et al. 2003). The inverted pendulum represents the human body, and the subject applies force to the inverted pendulum through a compliant linkage, a spring, which represents the Achilles tendon. Movement of the offset or bias of the spring is mechanically analogous to length changes of the calf muscles. There is now a body of evidence supporting this analogy and providing appropriate values for the spring (Hof, 1998; De Zee & Voigt, 2001; Loram & Lakie, 2002a; Loram et al. 2004, 2005a,b; Casadio et al. 2005). This inverted pendulum was equivalent in characteristics to an adult human and sensory feedback was available visually and via the tension in the spring. The mean unidirectional sway duration for the inverted pendulum was again close to one second (CoM: mean, s.d., range, 1.08 s, 0.28 s, 0.5–2.2 s) for ‘Achilles tendon’ springs varying from 50% to 700% of the gravitational load stiffness of the pendulum.
Bias duration during manual balance and postural sway
Observation of hand movements as well as inverted pendulum sway gives additional insight into the process of balance. The hand is the part that the subject is moving in order to control the pendulum. With a compliant linkage, movement of the inverted pendulum does not mechanically constrain movement of the hand, though movement of the hand directly influences the movement of the pendulum. In fact, by moving the end of the spring (the bias), subjects are directly altering the acceleration of the inverted pendulum (Loram et al. 2005b). Thus by observing hand movements we are observing neural control output more directly than by observing inverted pendulum sway. We found that for springs less than 300% the mean duration of hand movements (∼400 ms) was largely invariant with respect to spring stiffness (Bias: mean, s.d., range 0.40 s, 0.05 s, 0.30–0.65 s). This temporal invariance was observed even though the size of hand movements and sway increased eightfold as relative spring stiffness was decreased from 700% to 50% of mgh.
An obvious question is whether the 400 ms bias duration that we observed is a feature particularly of the visual–manual control loop, which is known to have substantial processing delays. We have specifically tested this question in a separate, detailed series of experiments which are reported elsewhere (Lakie & Loram, 2006). These experiments show that the 400 ms bias duration is not only a feature of the visual–manual loop but is common to manual control of one's own balance (and an inverted pendulum) via all sensory modalities and combinations of modalities including visual, proprioceptive and vestibular.
Using the mechanical analogy between manual balance of a real inverted pendulum and natural standing, the hand position (bias of the spring) represents soleus and gastrocnemius contractile length. Recently, using ultrasound, we have studied the changes in muscle contractile length (bias) under postural conditions during quiet standing and we found that changes in contractile length (bias) show the same 400 ms mean duration as voluntary manual control of a real inverted pendulum (Bias: mean, s.d., range, 0.40 s, 0.05 s, 0.32–0.48 s; Loram et al. 2005b).
What determines the sway duration and bias duration?
The similarity between these observations is intriguing. The central question is what physiological process is responsible for the characteristic durations of body sway and bias adjustment during real standing and the artificial tasks. There are alternative possible answers to this question.
(1) The load might form part of a dynamical system, a continuously acting closed loop feedback system, comprising the load, sensory receptors, controller and actuating muscles. In this case the characteristic durations would be determined by both the gain of the feedback controller and the moment of inertia of the load. For this reason, the frequency of oscillation of the load is usually interpreted as revealing the gain of the feedback controller (Collins & De Luca, 1995; Winter et al. 1998; Carpenter et al. 1999; Masani et al. 2003; Maurer & Peterka, 2005). Consequently if the moment of the inertia of the load is changed, either biologically (e.g. by body growth) or experimentally (e.g. by using artificial loads) the characteristic durations must alter unless there are compensatory changes in gain. The characteristic frequency reflects the load and the dynamic control parameters.
(2) It is possible that the characteristic durations reflect a non-continuous central control process acting at a certain frequency. For example, if preprogrammed central commands of a predefined time scale are triggered and executed ballistically, this would create a periodicity in the control process corresponding to intermittent control (Collins & De Luca, 1995). Explanations along these lines would imply an intrinsic property of the central nervous system that sets the mean frequency at which balance adjustments can be made. The sway frequency would be a consequence of this bandwidth limited central process. This understanding would differ from reasoning which interprets the frequency of sway as a consequence of postural stiffness (Collins & De Luca, 1993, 1995) or feedback gain (Winter et al. 1998; Carpenter et al. 1999; Maurer & Peterka, 2005). The characteristic frequency is physiologically determined and is independent of load.
Here, using a specialized visual–manual balance task, we test whether varying the moment of inertia and thus the time constant of the inverted pendulum alters the characteristic durations of inverted pendulum sway and bias adjustment. In particular, we decrease the time constant of the inverted pendulum to the point where subjects find the task difficult and we test whether subjects respond to this demand by altering the duration of adjustments.
We make two predictions. (i) If sensory feed-back gain, sensory noise and feedback time delays are unchanged by decreasing the moment of inertia of the load then the frequency of oscillation and frequency of bias adjustment should increase. (ii) If sway frequency and frequency of bias adjustments reflect some intrinsic property of the human neuromuscular system, then sway and bias frequency should be unaltered by the time constant of the load.
We subsequently consider whether the results of this artificial test would generalize to real standing.
Methods
Procedure
Ten healthy subjects seven male and three female, aged 20–52 years (27 ± 11 years, mean ± s.d.) sat quietly in a self-selected position. Subjects controlled a real inverted pendulum using a sensitive joystick which they supported on their leg or in their other hand (Fig. 1). Subjects observed inverted pendulum position on an oscilloscope of full scale range 23 cm placed 120 cm away. For each of five values of moment of inertia, subjects were given up to a maximum of six attempts to perform three successful trials of 40 s duration. A successful trial was one in which the inverted pendulum position remained on screen and thus within a range of 1–9 deg. A trial was terminated once the inverted pendulum position exceeded these limits. Subjects were instructed to keep the inverted pendulum position on a horizontal line near the centre of the oscilloscope and were told that deviation from that central line was the measure of performance.
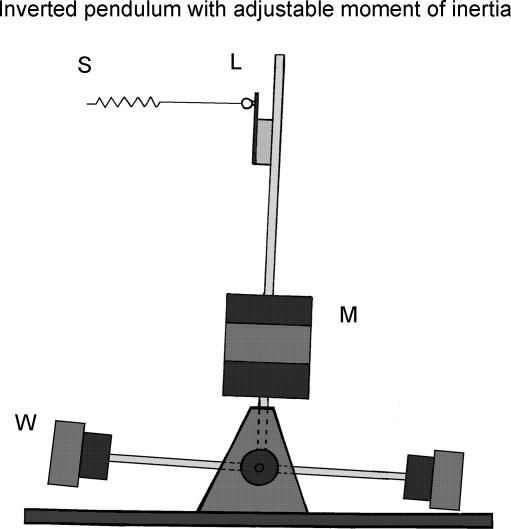
Figure 1. Inverted pendulum with adjustable moment of inertia.
Using a 60 kg mass (M), a real inverted pendulum was pivoted on precision ball races so that it moved in one plane. The moment of inertia was increased by adding symmetrical, perpendicularly mounted poles and weights (W). The inverted pendulum was controlled by a steel cable attached to an extension spring (S) comprising a series combination of identical springs. The springs were attached to the inverted pendulum above the axis giving a stiffness relative to the load which was broadly comparable to the estimated series elastic compliance of the Achilles tendon and foot during quiet standing. At the point of attachment a very stiff load cell (L) measured the force that was exerted on the inverted pendulum through the spring. Inverted pendulum angle was measured by a precision, Hall effect, contactless potentiometer attached to its base.
Previous to the experiment, subjects were given an opportunity to familiarize themselves with the task and gain confidence in their ability to balance the inverted pendulum within the recorded limits. We found that most subjects were able to perform the task within a few minutes of practice and thereafter improved increasingly slowly, if at all within the time scale of the session. An eleventh subject found the task difficult for all levels of inertia and was excluded from the study. By running a familiarization session on a day previous to the recorded trials, subjects began experiments having already solved the initial learning problems. For recorded trials, all subjects started with middle inertia level, the order of subsequent four inertia levels was randomised and the middle inertia level was repeated at the end. Data presented from the middle inertia level represent an average of the initial and final performance. This procedure was adopted to minimize the effects of learning on balance performance.
The subjects gave informed consent, and the study was approved by the local human ethics committee and conformed to the Declaration of Helsinki.
Apparatus and measurements
A real inverted pendulum (Fig. 1) was pivoted on precision ball races so that it moved in one plane. The gravitational load stiffness was determined by experiment to be 2.54 ± 0.03 N m deg−1 (mean ± s.d.). The moment of inertia was increased by adding symmetrical, perpendicularly mounted poles and weights giving five values: 5.6 ± 0.2, 9.1 ± 0.1, 13.9 ± 0.1, 17.6 ± 0.1, 27.3 ± 0.1 kg m2. (Throughout this paper we use the term ‘inertia’ as a short-hand for the ‘moment of inertia of the pendulum’.) This gave ratios of moment of inertia (J) to load stiffness (mgh) of 0.039–0.19 rad s2 which encompasses typical value for a standing adult of kh/g ≈ 0.13 rad s2 where k is a shape factor (∼1.3), h is the height of the centre of mass (∼1 m) and g is the acceleration due to gravity (Morasso & Schieppati, 1999). The inverted pendulum was controlled by a steel cable (1.5 mm diameter) attached to an extension spring comprising a series combination of 17 identical springs (T32 090, Springmasters, UK). The springs were attached to the inverted pendulum pole 0.63 ± 0.005 m above the axis giving a stiffness relative to the load stiffness of 82% ± 2% (mean ± s.d.) (Lakie et al. 2003). This value was broadly comparable with the estimated series elastic compliance of the Achilles tendon and foot during quiet standing (Loram & Lakie, 2002a; Loram et al. 2005a, 2005b). At the point of attachment a very stiff load cell (K25 Inscale Technology Ltd, UK) measured the force that was exerted on the inverted pendulum through the spring. Pendulum angle was measured by a precision, Hall effect, contactless potentiometer attached to its base.
From the equation of motion of the inverted pendulum,
where J is the moment of inertia, mgh is the load stiffness, c is the stiffness of the spring relative to the load stiffness and θ is the angle of the pendulum, the time constant of the inverted pendulum is given by:
giving values of 0.47, 0.59, 0.73, 0.83 and 1.02 s in increasing order of inertia. For an unstable pendulum, this time constant specifies the exponential growth rate of angle through time if no control is applied to the joystick.
The subject operated a hand-held contactless single axis joystick (HFX Magnetic, CH Products Ltd, Leeds, UK) with internal restoring spring removed. The joystick was used to control a powerful geared motor (G19M4, Printed Motors Ltd, UK) using a four-quadrant PID controller configured to act as a position servo. The position servo was attached to the spring. The subject therefore changed the bias of the spring indirectly using the joystick to control the motor and did not know the force that was being exerted. Approximately 3 mm of joystick movement corresponded to a 1 deg change in bias of the spring. Thus the control movements of the joystick were small (∼1–3 mm). All signals including inverted pendulum angle, spring force, and joystick angle were sampled at 100 Hz and recorded to 16 bit resolution on a computer (Measurement Computing PCI-DAS6036, MATLAB).
Data analysis
Calculation of summary measures (for Figs 2 and 3)
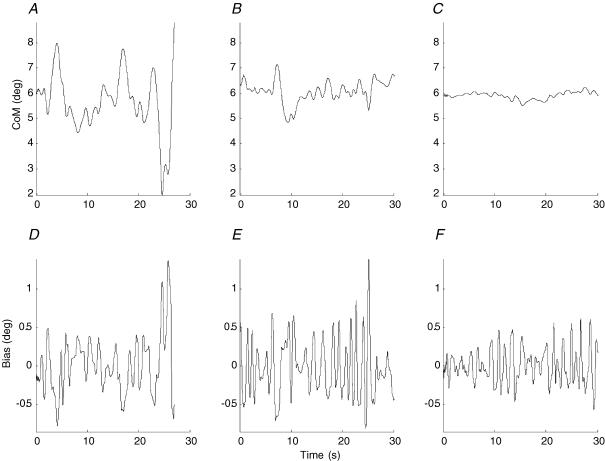
Figure 2. Representative sway and bias for different moments of inertia.
For a representative subject, angular sway of the inverted pendulum (A, B and C) and the corresponding fluctuation in bias (joystick) (D, E and F) is shown for the lowest, middle and highest levels of moment of inertia, respectively.
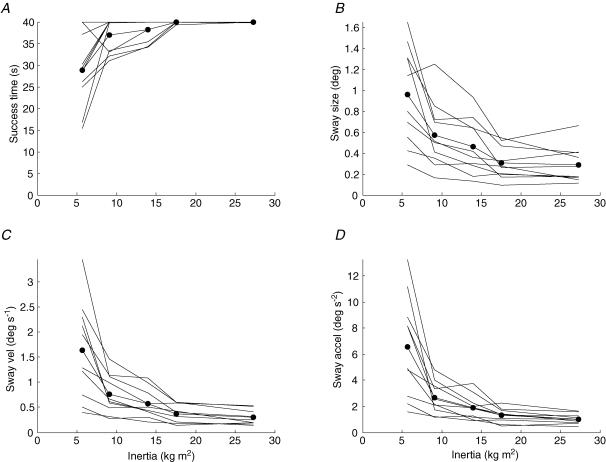
Figure 3. Effect of inertia on successful trial duration and inverted pendulum sway.
A, the mean trial duration for which the inverted pendulum was successfully maintained ‘on screen’. Inverted pendulum sway is shown as the standard deviation of inverted pendulum angle (B), velocity (C), and acceleration (D). Moment of inertia is shown on the abscissa. Dashed lines show the mean for each individual subject. Continuous line with dots shows the mean for all subjects.
Trial duration was calculated as the mean duration for which the inverted pendulum remained on screen. Joystick position was used as the measure of bias angle and actually of hand movements. This measure was cross-checked with bias position calculated from inverted pendulum angle, spring stiffness and spring force. The two measures gave excellent agreement although joystick position gave a superior signal to noise ratio. Inverted pendulum and bias velocity and inverted pendulum acceleration were calculated from angle using a REMEZ, FIR, differentiating filter with a pass band of 10 Hz. Sway measures were calculated from the standard deviation of inverted pendulum angle, velocity and acceleration where for this calculation these signals were low pass filtered with a cut-off at 3 Hz.
Using Welch's averaged, modified periodogram method, we calculated 1024 point power spectra, giving a resolution of 0.1 Hz, for inverted pendulum and bias, angle and velocity.
Calculation of averaged power spectra (for Fig. 4)
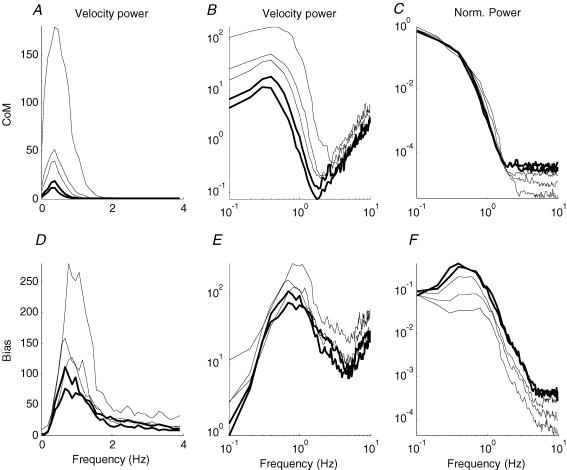
Figure 4. Effect of inertia on power spectra of inverted pendulum and hand.
Power spectra are shown for the inverted pendulum on the top row, and bias (joystick) on the bottom row. A and D, velocity power and bias velocity power on a linear scale. B and E, velocity power and bias velocity power on a log–log scale. C and F, inverted pendulum angle power and bias power, both normalized to the peak power of inverted pendulum velocity. Frequency is in hertz. The lines increase in thickness as the inertia of the inverted pendulum increases. Each line represents a mean for 10 subjects.
The amount of pendulum sway varied from individual to individual. In order to calculate averaged power spectra that were not dominated by the subject with the poorest control, we normalized pendulum and bias power spectra. For each trial we divided all power spectra by the peak power of the inverted pendulum velocity spectrum in the range 0–3 Hz. For each level of inertia we then averaged all trials per subject and then all subjects. The averaged inverted pendulum and bias velocity spectra were restored by multiplying by the inertia mean, peak power of inverted pendulum velocity to produce un-normalized velocity power (Fig. 4A, B, D and E). The power spectra for inverted pendulum angle and joystick bias were un-restored and were presented as normalized (Fig. 4C and F). The bias power fall-off in the range 1–5 Hz was calculated through a linear fit relating log (Bias power) to log (frequency).
Calculation of summary temporal and frequency characteristic (for Fig. 5)
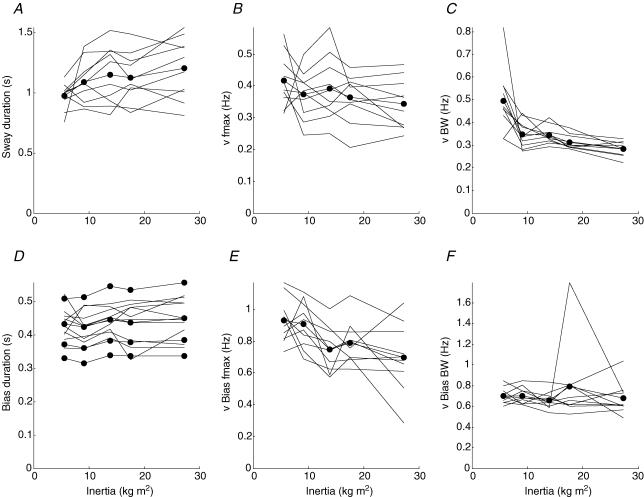
Figure 5. The effect of inertia on temporal control of the pendulum.
A–C, results for angular sway of the pendulum; D–F, bias (joystick). Dashed lines show mean for each individual subject. Continuous lines with dots show mean for all subjects. A and D, mean duration of unidirectional movements for inverted pendulum and bias calculated from the mean power frequency. Pendulum, frequency range 0–3 Hz. Bias, from bottom upwards, lines are derived from frequency ranges 0–5 Hz, 0–4 Hz, 0–3 Hz, 0–2 Hz. B and E, frequency of maximum power for inverted pendulum velocity and bias velocity. C and F, half-power width of the velocity spectral peak.
For each trial, the mean frequency for the postural bandwidth 0–3 Hz was calculated using:
where f is the frequency and Pvv is the velocity power spectrum. This value represents the mean frequency at which the inverted pendulum velocity oscillates. During each velocity cycle there are two unidirectional inverted pendulum sways. Thus the mean sway duration, T, is calculated using the formula
where f is the mean frequency of the velocity spectrum. Similarly, the mean bias movement duration was calculated using the same formula where f is the mean frequency of the bias velocity spectrum. For mean bias duration, additional calculations were presented using the frequency ranges 0–2 Hz, 0–4 Hz and 0–5 Hz. For each trial, and for inverted pendulum and bias, the frequency of peak velocity power was calculated, as was the bandwidth, i.e. the half-power width of the velocity spectral peak.
Statistical analysis
For each quantity of interest, a mean value was calculated for each subject for each level of inertia, giving 10 values for each of five levels of inertia. Unless otherwise stated, Pearson correlation, with N = 50, was used to test for significant differences with changing moment of inertia.
Modelling
We constructed a proportional, integral, derivative (PID) feedback control model to simulate inverted pendulum sway using the methods of Maurer & Peterka (2005). We varied the following parameters – proportional, differential and integral gain, noise amplitude and time delay – and optimized the simulated velocity power spectrum to the velocity power spectrum shown in Fig. 4A. Further details are given in Appendix A.
Estimation of human bandwidth using the Bode integral
Stein (Stein, 2003) has given a mathematical explanation of the difficulty of controlling an inverted pendulum based on the Bode integral. Using the closed loop sensitivity function, and knowing the limiting time constant of the inverted pendulum that can be balanced by subjects, we estimate the bandwidth of the human controller. Further details are given in Appendix B.
Results
Lower inertia pendulums, particularly their acceleration, are more difficult to control
Our representative subject shows inverted pendulum sway of fractions of a degree at the highest inertia level and shows sway increased to several degrees at the lowest level (Fig. 2A–C). For the lower inertia (level 1), this subject lost control before 30 s when the inverted pendulum moved off screen. Curiously, the size of bias movements remains at fractions of a degree changing little as inverted pendulum inertia decreases. The most important observation is that by eye, the frequency of bias movements appears to change little, if at all, as inertia decreases and inverted pendulum sway increases to the point where control is lost. Even the larger bias movements associated with the loss of control at level 1 (t = 22 s onwards) show submovements at the predominant frequency of bias movements.
All subjects found the task more demanding as inertia decreased, and with one exception all subjects were unable to consistently maintain a 40 s duration trial at the lowest levels of inertia (Fig. 3A). From a comfortable 40 s duration at inertia 5, the averaged duration decreased to 29 s at inertia 1 (P = 0.00005). Inverted pendulum sway increased as inertia decreased (Fig. 3B–D). On average, positional sway increased threefold from 0.3 to 1.0 deg (P = 0.00005), velocity increased fivefold from 0.3 to 1.6 deg s−1 (P = 0.00001), and acceleration increased sevenfold from 1.0 to 6.6 deg s−2 (P = 0.00001). Inverted pendulum acceleration and then velocity showed the greatest increases as inertia decreased. The corresponding increases in inverted pendulum jerk and first derivative of jerk reduced to fourfold and threefold, respectively.
Power spectra show similar frequency and bandwidth for all inertias
When we consider the averaged power spectra for inverted pendulum velocity (Fig. 4A) it is clear that there is a peak centred on 0.5 Hz and that the size of this peak increases greatly as inertia decreases. The frequency and bandwidth of the peak appear to change little with inertia although a log–log plot (Fig. 4B) shows visually that both frequency of the peak and the bandwidth increase slightly as inertia decreases. When the power spectra for inverted pendulum position are normalized for the amount of sway (Fig. 4C) it is striking how similar the spectra are for all levels of inertia. The most conspicuous change is the power of inverted pendulum sway in relation to the constant background noise beyond 3 Hz.
The averaged power for bias velocity (Fig. 4D) also shows a clear peak, this time centred around 1 Hz, which shows less variation in size with changing inertia than inverted pendulum velocity did. The most important observation is that centre and width of the peak appears to change little with inertia. Closer examination on a log–log plot (Fig. 4E) shows that the frequency of peak power does increase slightly as inertia decreases although the centre and width of the peak change little if at all. The power spectra for bias position, normalized according to inverted pendulum sway (Fig. 4F), shows that for high inertia there is more bias movement in relation to inverted pendulum sway. There is a clear peak in bias power (Fig. 4F) whereas there is no such peak in inverted pendulum position power (Fig. 4C). Beyond 1 Hz, bias power decreases to the background noise level beyond 5 Hz at a rate of 3.45 decades of power per decade of frequency for all values of inertia. Stated equivalently, this means that bias power falls off in proportion to frequency−3.45.
Pendulum and bias frequency change little with inertia
As inverted pendulum inertia decreases, the mean duration of inverted pendulum sways decreases a little (20%) from 1.2 to 1.0 s (P = 0.02) (Fig. 5A). There appears to be a corresponding increase in the frequency of peak power from 0.34 to 0.42 Hz although statistically this is not significant (P = 0.06) (Fig. 5B). The width of the peak in velocity power increases (70%) from 0.28 to 0.49 Hz as inertia decreases (P = 0.000005) (Fig. 5C). Whereas the duration of inverted pendulum sways decreases, there is no change in the mean duration (440 ms) of bias movements as inertia decreases (P = 0.26) although the low frequency (0–2 Hz) component of bias durations does show a small decrease of 9% from 559 to 511 ms (P = 0.04) (Fig. 5D). The relative invariance of bias duration with inverted pendulum inertia is an important result of this experiment. The frequency of peak power of bias velocity increases slightly from 0.70 to 0.93 Hz as inertia decreases (P = 0.0007) (Fig. 5E) and the width of the peak in bias velocity does not change but remains constant at around 0.7 Hz (P = 0.96) (Fig. 5F).
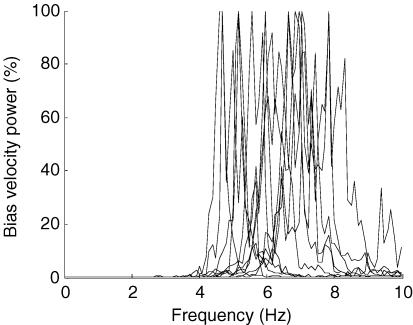
The frequency at which the joystick was operated during balance of the inverted pendulum was not limited by the rate at which the joystick could be physically oscillated by a subject. As shown in Fig. 6, subjects could oscillate the joystick at 6.0 ± 1.0 Hz (mean ± s.d.), which is considerably higher than that used to control the inverted pendulum (∼1 Hz).
Figure 6. The maximum frequency of joystick oscillation.
The bias (joystick) velocity power spectrum is shown for 30 s of sustained oscillation at the maximum frequency that the subject could manage. Each dashed line is the spectrum for one subject, normalized to a peak power of 100%. This figure shows that the ‘manual-joystick’ bandwidth is of the order of 6 Hz
Best fit explanations using a PID model
A PID mechanism with time delays of less than 185 ms could produce a stable system capable of balancing all inertias (not shown). In addition to the stability requirement, it was also necessary that the model reproduce the observed power spectra.
The noisy PID model can simulate the inverted pendulum velocity power spectra very well (R2 > 99%) for time delays of up to 120 ms (Fig. 7). In fact, the degree of fit is hardly distinguishable between delays of 0 ms and 120 ms (Fig. 7I). When the time delay is increased beyond 120 ms, the degree of fit worsens for all levels of inertia and becomes noticeably inadequate beyond 150 ms particularly for the lowest values of inertia and inadequate for all values of inertia beyond 200 ms (Fig. 7I). The ‘best fit’ time delay increases substantially from 10 ms to 93 ms as inertia decreases.
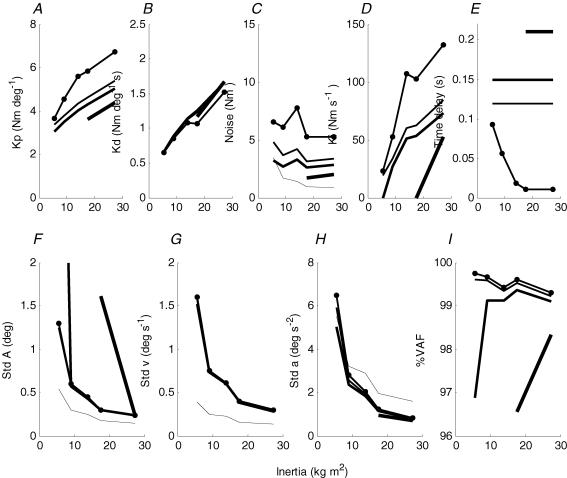
Figure 7. Optimized parameters of PID model simulating inverted pendulum velocity spectra.
A, proportional gain; B, differential gain; C, noise amplitude shown as standard deviation of noise torque; D, integral gain; E, time delay; F–H, inverted pendulum sway shown as standard deviation of inverted pendulum angle, velocity and acceleration, respectively; I, variance accounted for by model. Continuous lines in increasing thickness represent time delays of 0, 120, 150 and 210 ms, respectively, with only two largest values of inertia shown for 210 ms. Continuous line with dots shows time delay optimized as well as gain and noise amplitude parameters. In C, dashed line represents control torque standard deviation for 120 ms delay. In F–H, dashed line represents position, velocity and acceleration noise standard deviation for 120 ms delay.
For a PID controller with no time delay, changes were required in all parameters to reproduce the relatively invariant spectra produced by changes in inertia. As inertia decreases, proportional gain decreased by 46% from 6.7 to 4.7 N m deg−1, derivative gain decreased threefold from 1.5 to 0.5 N m s deg−1, integral gain decreased threefold from 136 to 41 N m s−1 and noise amplitude increased 240% from 5.4 to 12.9 N m (Fig. 7A–E). When we additionally allowed the time delay to adjust freely this gave the model an alternative way of increasing instability and simulating the inverted pendulum velocity power spectrum with almost identical goodness of fit (R2 > 99%) (Fig. 7I). As inertia decreased, time delay increased from 10 ms to a highest value of 93 ms at inertia level 1 (Fig. 7E). Lower maximum time delays could be tolerated at lower levels of inertia.
The PID model required a large amount of torque noise input to reproduce the observed sway spectra. In fact the simulated noise torque input was typically three times greater than the actual simulated torque applied to the inverted pendulum (Fig. 7C). We calculated the sensory noise input to the PID controller required to generate the torque noise input. As inertia decreased, the angular noise input typically increased 400% from 0.15 to 0.54 deg and was typically about 60% of the angular sway; the velocity noise increased 280% from 0.14 to 0.39 deg s−1, remaining at 40% of the velocity signal; and the acceleration noise increased 300% from 1.6 to 3.5 deg s−2 and was usually greater (150%) than the acceleration signal.
Estimation of human bandwidth using the Bode integral
Using the Bode integral, a limiting controller sensitivity of 3 ± 1 and a limiting inverted pendulum time constant of 0.5 s, we estimate that the human bandwidth is approximately 1 Hz (range 0.75–1.5 Hz).
Discussion
Manual control of balance is limited to a low frequency (∼1 Hz)
The key facts of this experiment are as follows. By decreasing the moment of inertia, the time constant of the inverted pendulum was decreased by a factor of two from 1 to 0.5 s. Subjects experienced increasing difficulty as the time constant of the unstable inverted pendulum decreased below 0.8 s (Fig. 3). As the task became more difficult the mean frequency of hand movements increased only slightly and never became more than two to three unidirectional hand movements per second corresponding to a mean duration of around 440 ms. The mean duration of unidirectional inverted pendulum movements decreased by a small amount from 1.2 to 1.0 s (Figs 4 and 5). Subjects were not limited by slow postural muscles and subjects had high quality visual information concerning the movement and target position of the pendulum.
In general terms it seems there are intrinsic neuromuscular factors that limit the frequency response of the human controller such that it becomes increasingly difficult to make appropriately modulated bias adjustments beyond the observed frequency. Given that the task demanded a higher frequency response as inertia decreased, subjects lost control rather than increase the frequency of bias adjustment. The bandwidth of control can be quantitatively estimated as approximately 1 Hz (Stein, 2003) (Fig. 8).
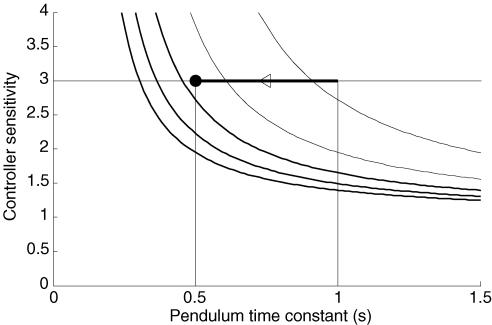
Figure 8. Human bandwidth estimated from the bode integral.
Following Stein (Stein, 2003), it is assumed that the closed loop log sensitivity function is constant up to a limiting bandwidth frequency and is zero at higher frequencies. Here, sensitivity is plotted as a function of inverted pendulum time constant for bandwidths of 0.5, 0.75, 1.0, 1.25 and 1.5 Hz. Bandwidth increases with line thickness. In these experiments, the inverted pendulum time constant was varied from 1 s to 0.5 s, and 0.5 s was the limit at which subject struggled to maintain balance. A sensitivity of 3 ± 1, represents the sensitivity at which control of an unstable system become very difficult.
Why did the task become more difficult as the inertia decreased? In order to maintain a constant pendulum sway, smaller force modulation would be required to balance the lower inertia pendulums and this requires the subject to execute smaller joystick movements. While it is not intrinsically more difficult to generate small movements it may be that the joystick output becomes less accurate because of the disproportionate effect of the subject's motor noise.
Preliminary tests (not shown) confirm that when the joystick sensitivity is progressively reduced up to 20-fold for the same inertia pendulum there is no change in pendulum positional variance and no certain change in velocity variance, but there is a reduced variance in pendulum acceleration and a preferential reduction in bias velocity power at 0.7–2Hz. Thus it is possible that the high sensitivity of our joystick made control of the lower inertia pendulums preferentially more difficult. Three facts point against the actuator biasing the experiment and/or providing the primary limiting constraint. (1) By decreasing the inertia we observed similar increases in pendulum position, velocity and acceleration variance whereas actuator noise appears to affect the higher frequencies preferentially. (2) Control of lower inertia pendulums was associated with larger sway and larger bias movements which would be less not more difficult to actuate. (3) Reduction in joystick sensitivity produced a reduction in control bandwidth, probably because the necessarily larger movements took longer to execute. Thus, decreasing joystick sensitivity may marginally improve performance of the task. However, it does not do this by increasing control bandwidth but by reducing noise.
Probably the main reason for increased difficulty with lower inertia pendulums is that the subject's promptness of control is tested. There may be limitations in the central processing of uncertain information that account for the low bandwidth of control and restrict the frequency at which appropriate bias adjustments can be made to a maximum of two to three corrections per second (Wickens & Hollands, 2002, p. 399). These limitations are considered more fully below.
The frequency of bias adjustments is characteristic of intermittent manual control
It is possible that the peak in the bias velocity spectrum and the bias power spectrum (∼1 Hz, Fig. 4D–F) represents a process that is not particular to balance of an inverted pendulum. Velocity power in the range 0.7–2 Hz is characteristic of intermittent control in a variety of settings including manual tracking of visual targets (Craik, 1947; Vince, 1948; Miall et al. 1993a; Slifkin et al. 2000; Miall & Jackson, 2006) and voluntary arm movements with and without visual feedback (Doeringer & Hogan, 1998). Velocity power in this range represents a lack of smoothness or velocity discontinuity in the hand trajectory that does not relate to the instantaneous motion of the target (Bekey, 1968; Miall et al. 1993a; Miall & Jackson, 2006). Since these manual movements contain frequencies which are not present in the visual stimulus they are a product of the human control process. Intermittency increases in magnitude when the subject is required to follow a target closely and cannot implicitly predict the required trajectory (Miall et al. 1993a). Previously advocated explanations include a sampling mechanism (Bekey, 1962, 1968), blended submovements of predefined duration, a preferential frequency of operation in the spinal cord, non-specific noise in the control process (Doeringer & Hogan, 1998), intermittent reformulation of the motor program with a corresponding refractory period (Craik, 1947; Vince, 1948; Neilson et al. 1988a), a maximum frequency of corrections related to the maximum decision-making speed in the serial reaction-time paradigm (Wickens & Hollands, 2002, p 399) or perceptual dead-bands (Miall et al. 1993a).
The relationship between inverted pendulum sway frequency and bias frequency
The analysis of joystick bias movements in this experiment (Fig. 4) has revealed a peak in the mean bias velocity spectra at approximately 1 Hz. Given the simple relationship of inverted pendulum, compliant linkage and bias actuator, there is an inevitable relationship between the bias θ0 and inverted pendulum θ power spectra (eqn (7) in Loram et al. 2005a) determined by:
where f, I, mgh and c are frequency, moment of inertia, gravitational load stiffness and stiffness of compliant linkage relative to the load stiffness. Bias velocity and inverted pendulum velocity spectra are related by the same equation. If the appropriate bias adjustments of the controller occur at a low and limited range of frequencies (∼1 Hz) then for biomechanical reasons, reflecting the inertia, load stiffness and spring stiffness, the motion of the inverted pendulum (Fig. 4C) will be restricted to a lower frequency range in practice of about 0.5 Hz. Thus a process which limited bias adjustments to a minimum mean duration of ∼400 ms would for biomechanical reasons explain why inverted pendulum sway is limited to a minimum mean unidirectional duration of ∼1 s. Changing the inertia should slightly alter the relationship between joystick bias adjustments and inverted pendulum motion. This accounts for the greater decrease in inverted pendulum sway durations compared with the largely invariant bias duration as inertia decreases (Fig. 5Avs.Fig. 5D).
Postulated generalization to real standing
Do the results of this experiment generalize to quiet standing? As described in the introduction, this task differs from standing. However, pedal balance of an inverted pendulum using the calf muscles shows the same duration of unidirectional pendulum sway (Loram et al. 2001; Loram & Lakie, 2002b) as this manual task. Using ultrasound, we have observed the calf muscles during this activity and the power spectra and duration of unidirectional bias adjustments is the same as for manual balance of the inverted pendulum (unpublished data). This pedal task was unfamiliar, like the manual experiment reported here. The similarity in durations supports the prediction that altering the muscles from manual to pedal would not alter the frequency limitation of balance control.
Natural standing is different from pedal balance of the inverted pendulum in that the activity is utterly familiar. The characteristic duration of unidirectional sway is the same as the current experiment. From ultrasonographic analysis of the calf muscles under postural conditions, we know that the spectra and duration of unidirectional bias adjustments is also the same as the current experiment (Fig. 3, Loram et al. 2005b). This finding was unaltered when the eyes were closed leaving the subject dependent on proprioception and vestibular feedback. These observations support the prediction that postural balance in standing follows a similar frequency limited control process as pedal balance of a real inverted pendulum and manual balance of a real pendulum.
Thus we postulate (i) that human balance in real standing as well as manual and pedal balance tasks is controlled via bias adjustments limited to a bandwidth of approximately 1 Hz and (ii) that this bandwidth in combination with biomechanical factors determines the characteristic duration of unidirectional standing sway.
What mechanisms might account for the low and relatively constant frequency of bias adjustments?
The low frequency may be a consequence of a bandwidth limitation. Here we discuss the main possible causes and ask whether they would explain the rather constant spectrum of bias adjustments that is observed when the moment of inertia changes.
(1)Time delays in the neuromuscular system will limit the bandwidth of the system and restrict the range of inertias that it is possible to balance
It is difficult to estimate the true time delay that is relevant to this task. For manual and pedal tracking of continuously moving targets, the mean delay between stimulus and response depends on the predictability of the stimulus and on the order of the system being controlled. If subjects pedally track a temporally unpredictable force target, such as a pseudo random binary sequence, the target is followed imperfectly with a mean delay of around 330 ms whereas a sine wave can be tracked with zero delay (unpublished observations). Zero and first order systems are manually tracked with time delays from 150 to 300 ms whereas for second order systems the delay is 400–500 ms reflecting the more complex error-correction decisions that need to be made (Wickens & Hollands, 2002, p 398). The manual response to single discrete binary tracking stimuli typically reveals a dead reaction time of 250 ms and a movement response time of 250 ms producing a combined delay of 500 ms (Vince, 1948). However, this dead reaction time is not constant and the response to a second discrete stimulus shows an increased dead time of up to 500 ms when the second discrete stimulus follows the first by less than 500 ms (Vince, 1948; Welford, 1959; Poulton, 1974; Pashler et al. 1998); the second movement is delayed until the first is completed. All the above suggests a variable true neuromuscular delay with a mean value of one-third to half a second, which combined with neural prediction produces a commonly observed mean of 70–150 ms in response to continuous stimuli (Neilson et al. 1988b; Miall et al. 1993b; Brenner et al. 1998; Morasso et al. 1999; Foulkes & Miall, 2000; Krekelberg & Lappe, 2001; Nijhawan, 2002; Schlag & Schlag-Rey, 2002). In the present task, the subject knows both the history of pendulum motion and the history of their own control effort thus enabling prediction.
Subjects found it increasingly difficult to balance the inverted pendulum as inertia was reduced and the irreducible effective delay time of 70–150 ms may be the explanation for this. Such a delay will have a more dramatic effect as inertia is reduced and the pendulum accelerates faster for a given control error. The modelling result (Fig. 7) that feedback gains have to be adjusted in some fashion is therefore unsurprising. What is more difficult to explain, is why adjustment to accommodate a time delay should result in pendulum and bias spectra that change so little as the pendulum inertia decreases. (It is also surprising that noise from the sensory organs would change when the inertia changes.) Any successful explanation of this finding must provide a natural biological reason for the frequency spectra to be preserved as the load changes. If the only reason justifying the choice of gains is the constraint that the spectra should change very little as inertia changes, then the adjustment of gains is an arbitrary PID description of a process operating at an observed frequency. If the process is to be explained by a model (PID or other) then we need a biologically appropriate criterion showing how the observed spectra occur naturally when the inertia changes. Using the PID model we have attempted unsuccessfully to find such a criterion. We have optimized the feedback gains to minimize pendulum sway, or maximize relative stability, and we have added low pass filters to simulate a bandwidth limitation. The resulting spectra generally have the wrong profile and alter as pendulum inertia changes. Rather than present our unsuccessful attempts we leave open the search for a criterion as a challenge to those interested in modelling human balance.
More sophisticated controllers include predictors such as Kalman filters (Wolpert et al. 1995; Jeka et al. 2004) and Smith predictors (Morasso et al. 1999) which mitigate the limiting effect of neuromuscular time delays. We don't know whether these would naturally produce the observed pendulum and bias spectra when controlling pendula of different inertia. However, we note that another type of engineering controller, the intermittent open loop controller, is precisely designed to accommodate control of a low bandwidth system and would naturally produce bias power at a defined frequency resulting from the intermittent open loop interval (Ronco et al. 1999; Gawthrop, 2004; Gawthrop & Wang, 2006).
(2)Refractory periods in the initiation of joystick trajectories would limit the frequency at which bias adjustments could be made
Craik observed that humans cannot begin a second response to a discrete stimulus until they have completed the response to the first (Vince, 1948). For Craik this provided an intriguing insight into why subjects intermittently formulate tracking submovements at a mean rate of two per second. Craik hypothesized that humans formulate one submovement at a time and that they are unable to respond to current sensory information for up to 500 ms while actuating the current submovement (Craik, 1947; Vince, 1948; Neilson et al. 1988a; Pashler et al. 1998). This provides a natural explanation for the maximum frequency of hand movement, which is limited by the refractory interval and could be consistent with the time delays which are observed with continuous and discrete stimuli.
This idea of refractory periods is consistent with intermittent open loop control in which the control trajectory is reformulated intermittently on the basis of sensory feedback and the inverted pendulum is controlled open loop through the intermittent interval. In fact, this engineering controller (Ronco et al. 1999; Gawthrop, 2004; Gawthrop & Wang, 2006) was designed for circumstances where slow on-line optimization rules out continuous control. This controller would also explain naturally why pendulum sway increases as the inertia decreases. During the open loop interval pendulum sway resulting from minimal measurement noise increases exponentially through time according to the time constant of the pendulum.
(3)The time required to make hand movements is related to the accuracy of movement that is required
According to the observations encapsulated by Fitts' Law, the duration of unidirectional movements varies from 200 ms for the simplest, shortest distance movements to 800 ms or more for movements of greater difficulty (Fitts, 1954). For this manual balance task, and the other varieties we have reported, the 400 ms mean duration of unidirectional bias movements would be related to the accuracy required to maintain accurate balance. Decreasing the inertia of the pendulum reduces the time available to make adjustments while at the same time increasing the accuracy required to maintain constant sway. Since the required joystick movements are so small, we would expect that 400 ms relates primarily to the processing time required to plan each movement.
(4)A perceptual dead-band would produce periods of time during which the controller is effectively open loop with respect to the error signal until the relevant threshold is crossed
This delay might limit the frequency of intermittent adjustments that are possible (Wolpert et al. 1992; Collins & DeLuca, 1993; Miall et al. 1993a; Collins & De Luca, 1995). Given a certain perceptual threshold, we would expect the delay to shorten as the motion become more prominent in relation to the background uncertainty. This might explain why the frequency of bias movements increases slightly as the inertia decreases.
(5)A low bandwidth actuator such as a slow or velocity restricted muscle would limit the bandwidth of the control process
In addition to the previous discussion, it is not clear that the low pass filtering of an actuator would lead to the observed similarity of spectra with different inertia pendulums.
(6)Predictive controllers usually have a maximum frequency beyond which their internal model is inaccurate
Biological predictors are likely to have an equivalent to the un-modelled dynamics of engineering predictors. For example, muscles are notoriously noisy actuators and there would be little point in the nervous system predicting their output beyond a relatively modest frequency. A hard frequency restriction might limit the nervous system to an intermittent, low frequency trial and error style of control.
How might we discriminate between these mechanisms?
It strikes us that intermittent open loop control is a promising model to be explored alongside continuous models of human control. The intermittent model contains a refractory period, a feature not found in continuous models. This refractory period is not the same thing as a transmission delay, it is a serial process which takes a certain time: the control trajectory cannot be modified and the subsequent process cannot be started until the current process is complete. How can we discriminate between the many continuous and intermittent models that can potentially reproduce the results of this experiment?
Analysing a closed loop system is problematic (Kohn, 2005; Van der Kooij et al. 2005). Can opened loop perturbation experiments resolve this issue? We can see two problems with perturbation techniques.
(1) We think that conventional system identification techniques (e.g. Fitzpatrick et al. (1996) can discriminate differing forms of continuous controller. However, such analysis techniques assume stationary time series and thus implicitly assume a linear time-invariant continuous-time controller. Intermittent control does, by its nature, give non-stationary time series because the effect of a given stimulus is dependent on when the stimulus arrives with respect to the controller's intermittent interval; thus applying such conventional methods could give erroneous or misleading results. A current challenge is to devise an appropriate system identification technique that can test the hypothesis of intermittent open loop control.
(2) Perturbations may stimulate and measure a different process to the one controlling the spontaneous activity. Perturbations degrade the predictability of the task and increase the necessity to respond rapidly. Thus perturbations are likely to stimulate shorter latency mechanisms that are less accurate and which make less use of forward prediction. It is usually assumed that perturbations add noise to an invariant system. But since the human system adjusts to the perturbed activity the results bear an uncertain relationship to spontaneous balance where accuracy is the essence of fine control.
Conclusions
We found that sway frequency and the frequency of manual bias adjustments are altered very little by changes in load inertia. The simplest explanation is that manual balance is maintained by a process of bias adjustments intrinsically limited to a maximum mean frequency of 2–3 unidirectional adjustments per second. A central process operating at this frequency via a compliant coupling provides a natural explanation for the 1 s duration of inverted pendulum sway. The similar durations of sway and bias adjustments in quiet standing and pedal balancing of an inverted pendulum support a new hypothesis: that the characteristic duration of postural sway is primarily explained by the limited bandwidth of control. More complex models must provide a criterion explaining why changing the moment of inertia has so little effect on the frequency spectra of pendulum and bias.
Acknowledgments
We would like to thank the Leverhulme Trust for their support of Dr I.D. Loram throughout this project. We would also like to thank Dr B. Day (Institute of Neurology, London) for originally posing the question which led to this experiment.
Appendix A
A. PID model and procedures
We write the equation of motion of the inverted pendulum subject to PID feedback and torque noise input as follows:
where θ is inverted pendulum angle, J is the moment of inertia, mgh is gravitational load stiffness, KP, KD and KI are proportional, derivative and integral gains, N is the input torque noise and F is the feedback delay function, where F = e−sτd, s is the Laplace variable and τd is the feedback loop delay (Maurer & Peterka, 2005). Using Laplace transformation and rearrangement we obtain the system equation as:
F was calculated using a 15th order pade function (Dorf & Bishop, 1998), N was calculated as low pass filtered white noise using:
where KN is the noise amplitude, and the first order time constant τN was set to 100 following Maurer & Peterka (2005). Using s = 2jπf where f is frequency is hertz and j is the square root of −1, the inverted pendulum velocity power spectrum was calculated using:
Using constrained optimization of a ‘variance accounted for’ error function (Mirbagheri et al. 2000), the model velocity spectrum was fitted to the experimental spectrum. Parameters KP, KD and KI were bounded within the range 10, 1, 0 and 2000 N m rad−1, 1000 N m rad−1 s, and 200 N m s−1. We used initial search values of 350 N m rad−1, 60 N m rad−1 s, and 34 N m s−1.
Before each evaluation of the error function, the system was checked for stability by calculating the system poles. If any positive real poles were found the error function was multiplied by the magnitude of the phase margin and a large number.
Simulink (Maurer & Peterka, 2005) was used to calculate time series of the inverted pendulum angle and calculate the standard deviation of inverted pendulum angle, velocity and acceleration as well as inverted pendulum torque and noise torque. The inverse transfer function of the PID part of the controller was used to calculate angular noise from torque noise. Velocity noise and acceleration noise were calculated by differentiation of angular noise.
Appendix B
B. Estimation of the human bandwidth using the Bode integral
The open loop inverted pendulum has a time constant τ corresponding to a real, positive pole p = 1/τ in the Laplace plane where:
Assuming that the inverted pendulum is stabilized by feedback of loop gain L(jw), where w is angular frequency in radians s−1, the closed loop sensitivity function (Dorf & Bishop, 1998) is defined as:
S is constrained by the following Bode integral (the right hand side of the equation reflects the single unstable system pole with value p):
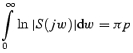 |
Following Stein's simplifying analysis (Stein, 2003) the controller behaviour is approximated by a constant sensitivity function S(jw) = S0up to a bandwidth of w0 rad s−1 such that S(jw) = S0 for ω = ω0 and S(jw) = 1 for ω > ω0.
The Bode Integral then becomes:
or equivalently:
where f0 = w0/2π. Figure 8 shows |S0| plotted against τ for different values of bandwidth f0. Sensitivity values of |S0| > 3 make control very difficult. The limiting bandwidth of the controller is estimated from the intersection of |S0| = 3 and the limiting time constant of the pendulum.
References
- Bekey G. The human operator as a sampled-data system. IRE Transactions on Human Factors in Electronics. 1962;HFE-3:43–51. [Google Scholar]
- Bekey G. Identification of sampling intervals in sampled-data models of human operators. IEEE Transactions on Man-Machine System. 1968;MMS-9:138–142. [Google Scholar]
- Brenner E, Smeets JBJ, de Lussanet MHE. Hitting moving targets – Continuous control of the acceleration of the hand on the basis of the target's velocity. Exp Brain Res. 1998;122:467–474. doi: 10.1007/s002210050535. [DOI] [PubMed] [Google Scholar]
- Brooks VB. The Neural Basis of Motor Control. Oxford: Oxford University Press; 1986. [Google Scholar]
- Carpenter MG, Frank JS, Silcher CP. Surface height effects on postural control: a hypothesis for a stiffness strategy for stance. J Vestib Res. 1999;9:277–286. [PubMed] [Google Scholar]
- Casadio M, Morasso PG, Sanguineti V. Direct measurement of ankle stiffness during quiet standing: implications for control modelling and clinical application. Gait Posture. 2005;21:410–424. doi: 10.1016/j.gaitpost.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Collins JJ, De Luca CJ. The effects of visual input on open-loop and closed-loop postural control mechanisms. Exp Brain Res. 1995;103:151–163. doi: 10.1007/BF00241972. [DOI] [PubMed] [Google Scholar]
- Collins JJ, De Luca CJ. Open-loop and closed-loop control of posture: a random-walk analysis of centre-of-pressure trajectories. Exp Brain Res. 1993;95:308–318. doi: 10.1007/BF00229788. [DOI] [PubMed] [Google Scholar]
- Craik KJW. Theory of the human operator in control systems. I. The operator as an engineering system. Br J Psychol. 1947;38:56–61. doi: 10.1111/j.2044-8295.1947.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Davies A, Blakeley A, Kidd C. Human Physiology. Edinburgh: Churchill Livingstone; 2001. [Google Scholar]
- De Zee M, Voigt M. Moment dependency of the series elastic stiffness in the human plantar flexors measured in vivo. J Biomech. 2001;34:1399–1406. doi: 10.1016/s0021-9290(01)00133-6. [DOI] [PubMed] [Google Scholar]
- Doeringer JA, Hogan N. Intermittency in preplanned elbow movements persists in the absence of visual feedback. J Neurophysiol. 1998;80:1787–1799. doi: 10.1152/jn.1998.80.4.1787. [DOI] [PubMed] [Google Scholar]
- Dorf RC, Bishop RH. Modern Control Systems. Menlo Park, CA, USA: Addison-Wesley; 1998. [Google Scholar]
- Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol. 1954;47:381–391. [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, Gandevia SC. Loop gain of reflexes controlling human standing measured with the use of postural and vestibular disturbances. J Neurophysiol. 1996;76:3994–4008. doi: 10.1152/jn.1996.76.6.3994. [DOI] [PubMed] [Google Scholar]
- Foulkes AJM, Miall RC. Adaptation to visual feedback delays in a human manual tracking task. Exp Brain Res. 2000;131:101–110. doi: 10.1007/s002219900286. [DOI] [PubMed] [Google Scholar]
- Gawthrop PJ. Intermittent constrained predictive control of mechanical systems. In: Petersen IR, editor. Proceedings of the 3rd IFAC Symposium on Mechatronic Systems. Australia: Manly; 2004. [Google Scholar]
- Gawthrop PJ, Wang L. Intermittent predictive control of an inverted pendulum. Con Eng Prac. 2006;14:1347–1356. [Google Scholar]
- Hof AL. In vivo measurement of the series elasticity release curve of human triceps surae muscle. J Biomech. 1998;31:793–800. doi: 10.1016/s0021-9290(98)00062-1. [DOI] [PubMed] [Google Scholar]
- Jeka J, Kiemel T, Creath R, Horak F, Peterka R. Controlling human upright posture: velocity information is more accurate than position or acceleration. J Neurophysiol. 2004;92:2368–2679. doi: 10.1152/jn.00983.2003. [DOI] [PubMed] [Google Scholar]
- Kohn AF. Cross-correlation between EMG and center of gravity during quiet stance: theory and simulations. Biol Cybern. 2005;93:382–388. doi: 10.1007/s00422-005-0016-x. [DOI] [PubMed] [Google Scholar]
- Krekelberg B, Lappe M. Neuronal latencies and the position of moving objects. Trends Neurosci. 2001;24:335–339. doi: 10.1016/s0166-2236(00)01795-1. [DOI] [PubMed] [Google Scholar]
- Lakie M, Caplan N, Loram ID. Human balancing of an inverted pendulum with a compliant linkage: neural control by anticipatory intermittent bias. J Physiol. 2003;551:357–370. doi: 10.1113/jphysiol.2002.036939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakie M, Loram ID. Manually controlled human balancing using visual, vestibular and proprioceptive senses involves a common, low frequency neural process. J Physiol. 2006;577:403–416. doi: 10.1113/jphysiol.2006.116772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Kelly S, Lakie M. Human balancing of an inverted pendulum: is sway size controlled by ankle impedance? J Physiol. 2001;532:879–891. doi: 10.1111/j.1469-7793.2001.0879e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Lakie M. Direct measurement of human ankle stiffness during quiet standing: the intrinsic mechanical stiffness is insufficient for stability. J Physiol. 2002a;545:1041–1053. doi: 10.1113/jphysiol.2002.025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Lakie M. Human balancing of an inverted pendulum: position control by small, ballistic-like, throw and catch movements. J Physiol. 2002b;540:1111–1124. doi: 10.1113/jphysiol.2001.013077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. Paradoxical muscle movement in human standing. J Physiol. 2004;556:683–689. doi: 10.1113/jphysiol.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. Active, non-spring-like muscle movements in human postural sway: how might paradoxical changes in muscle length be produced? J Physiol. 2005a;564:281–293. doi: 10.1113/jphysiol.2004.073437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. Human postural sway results from frequent, ballistic bias impulses by soleus and gastrocnemius. J Physiol. 2005b;564:295–311. doi: 10.1113/jphysiol.2004.076307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masani K, Popovic MR, Nakazawa K, Kouzaki M, Nozaki D. Importance of body sway velocity information in controlling ankle extensor activities during quiet stance. J Neurophysiol. 2003;90:3774–3782. doi: 10.1152/jn.00730.2002. [DOI] [PubMed] [Google Scholar]
- Maurer C, Peterka RJ. A new interpretation of spontaneous sway measures based on a simple model of human postural control. J Neurophysiol. 2005;93:189–200. doi: 10.1152/jn.00221.2004. [DOI] [PubMed] [Google Scholar]
- Miall RC, Jackson JK. Adaptation to visual feedback delays in manual tracking: evidence against the Smith Predictor model of human visually guided action. Exp Brain Res. 2006;172:77–84. doi: 10.1007/s00221-005-0306-5. [DOI] [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Stein JF. Intermittency in human manual tracking tasks. J Motor Behav. 1993a;25:53–63. doi: 10.1080/00222895.1993.9941639. [DOI] [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Wolpert DM, Stein JF. Is the cerebellum a Smith Predictor. J Motor Behav. 1993b;25:203–216. doi: 10.1080/00222895.1993.9942050. [DOI] [PubMed] [Google Scholar]
- Mirbagheri MM, Barbeau H, Kearney RE. Intrinsic and reflex contributions to human ankle stiffness: variation with activation level and position. Exp Brain Res. 2000;135:423–436. doi: 10.1007/s002210000534. [DOI] [PubMed] [Google Scholar]
- Morasso PG, Baratto L, Capra R, Spada G. Internal models in the control of posture. Neural Networks. 1999;12:1173–1180. doi: 10.1016/s0893-6080(99)00058-1. [DOI] [PubMed] [Google Scholar]
- Morasso PG, Schieppati M. Can muscle stiffness alone stabilize upright standing? J Neurophysiol. 1999;82:1622–1626. doi: 10.1152/jn.1999.82.3.1622. [DOI] [PubMed] [Google Scholar]
- Neilson PD, Neilson MD, O'Dwyer NJ. Internal models and intermittency: a theoretical account of human tracking behavior. Biol Cybern. 1988a;58:101–112. doi: 10.1007/BF00364156. [DOI] [PubMed] [Google Scholar]
- Neilson PD, O'Dwyer NJ, Neilson MD. Stochastic prediction in pursuit tracking: an experimental test of adaptive model theory. Biol Cybern. 1988b;58:113–122. doi: 10.1007/BF00364157. [DOI] [PubMed] [Google Scholar]
- Nijhawan R. Neural delays, visual motion and the flash-lag effect. Trends Cogn Sci. 2002;6:387. doi: 10.1016/s1364-6613(02)01963-0. [DOI] [PubMed] [Google Scholar]
- Pashler H, Johnston JC, Pashler H. Attention. Hove: Psychology Press; 1998. Attentional limitations in dual-task performance; pp. 155–189. [Google Scholar]
- Poulton E. Tracking Skill and Manual Control. New York: Academic Press; 1974. [Google Scholar]
- Ronco E, Arsan T, Gawthrop PJ. Open-loop intermittent feedback control: Practical continuous- time GPC. IEEE Proc-Control Theory Applications. 1999;146:426–434. [Google Scholar]
- Schlag J, Schlag-Rey M. Through the eye, slowly: Delays and localization errors in the visual system. Nat Rev Neurosci. 2002;3:191–215. doi: 10.1038/nrn750. [DOI] [PubMed] [Google Scholar]
- Slifkin AB, Vaillancourt DE, Newell KM. Intermittency in the control of continuous force production. J Neurophysiol. 2000;84:1708–1718. doi: 10.1152/jn.2000.84.4.1708. [DOI] [PubMed] [Google Scholar]
- Stein G. Respect the unstable. IEEE Control Systems Magazine. 2003;23:12–25. [Google Scholar]
- Van der Kooij H, Van Asseldonk E, Van der Helm FCT. Comparison of different methods to identify and quantify balance control. J Neurosci Methods. 2005;145:175–203. doi: 10.1016/j.jneumeth.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Vince MA. The intermittency of control movements and the psychological refractory period. Br J Psychol. 1948;38:149–157. doi: 10.1111/j.2044-8295.1948.tb01150.x. [DOI] [PubMed] [Google Scholar]
- Welford AT. Evidence of a single channel decision mechanism limiting performance in a serial reaction task. Q J Exp Psychol. 1959;11:193–210. [Google Scholar]
- Wickens CD, Hollands JG. Engineering Psychology and Human Performance. Taiwan: Pearson Education Taiwan; 2002. [Google Scholar]
- Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K. Stiffness control of balance in quiet standing. J Neurophysiol. 1998;80:1211–1221. doi: 10.1152/jn.1998.80.3.1211. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269:1880–1882. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Winter JL, Stein JF. Evidence for an error deadzone in compensatory tracking. J Motor Behav. 1992;24:299–308. doi: 10.1080/00222895.1992.9941626. [DOI] [PubMed] [Google Scholar]