Abstract
Activation of urethral or genital afferents of the pudendal nerve can elicit or inhibit micturition, and low frequency stimulation of the compound pudendal nerve (PN) is known to produce a continence response. The present study demonstrates that PN stimulation also can elicit a micturition-like response and that the response to PN stimulation is dependent on stimulation frequency. We measured the changes in bladder pressure and external urethral sphincter (EUS) electroneurogram (ENG) evoked by PN stimulation before and up to 16 h after spinal cord transection (SCT) in cats anaesthetized with α-chloralose. Low frequency (10 Hz) stimulation elicited a continence-like response, including inhibition of the bladder and activation of the EUS, but mid-frequency (33 Hz) stimulation produced a micturition-like response, including excitation of the bladder without activation of the EUS. The dependence of the response on stimulus frequency was linked to interpulse interval as the same number of pulses at 10, 33 and 100 Hz produced different responses. Stimulation of the PN at 33 Hz produced bladder contractions before and 8 h after SCT provided the bladder contained a minimum volume of fluid. Only mid-range frequency stimulation with sufficient stimulus train duration produced a reduction in EUS ENG activity before and after SCT. In addition to a continence-like response, PN stimulation can also elicit a micturition-like response, and this response is dependent on stimulation frequency, stimulus train duration, and bladder volume. The ability to control the two principal functions of the bladder by pudendal nerve stimulation is an exciting prospect for neurorehabilitation.
The micturition cycle has two complementary phases, continence, when the bladder stores urine, and micturition, when the external urethral sphincter (EUS) relaxes and the bladder contracts to expel urine. Continence and micturition can be augmented by sensory inputs from pudendal nerve (PN) afferents. Here we demonstrate that electrical stimulation of PN afferents differentially activated either a continence-like reflex or a micturition-like reflex depending on the frequency of stimulation.
Different PN afferents can generate either inhibition or excitation of the bladder. Stimulation of genital (Vodusek et al. 1986) and/or anal (Sundin et al. 1974) sensory pathways in the PN inhibits the bladder by decreasing parasympathetic outflow in the pelvic nerve to the bladder detrusor muscle and by increasing sympathetic outflow in the hypogastric nerve (Sundin et al. 1974; Lindstrom et al. 1983). Bladder inhibition is evoked by low frequency stimulation (2–20 Hz) of PN afferents (Sundin et al. 1974; Vereecken et al. 1984; Vodusek et al. 1986; Jiang & Lindström, 1999a); and inhibition is lost during higher frequency (35 Hz) stimulation (Lindstrom et al. 1983; Walter et al. 1993).
Excitation of the bladder can be produced by stimulation of urethral sensory pathways over a range of stimulus frequencies (Mazieres et al. 1997; Jiang & Lindstrom, 1999b; Shefchyk & Buss, 1998; Boggs et al. 2005). However, higher frequency (20–40 Hz) stimulation is more effective than 10 Hz stimulation at evoking bladder contractions (Boggs et al. 2005), consistent with the firing rates of urethral afferents in response to urethral fluid flow (Todd, 1964), which is also capable of evoking bladder contractions (Garry et al. 1959; Robain et al. 2001).
The compound PN contains both genital and urethral sensory pathways (Martin et al. 1974). Despite the apparent separation between the low- and high-frequency ranges required to activate either pathway, the effect of stimulation of the compound PN (i.e. costimulation of both pathways) has been reported to be exclusively inhibitory (Sundin et al. 1974; McGuire & Herlihy, 1978; Lindström et al. 1983; Ohlsson et al. 1989; Walter et al. 1993). This study demonstrates that the behaviour evoked by PN afferents is dependent on the rate of afferent activity and challenges the perception that the dominant effect of PN afferents is to inhibit the bladder. Excitatory and inhibitory pathways can be activated differentially at the level of the PN according to stimulus frequency. The ability to control selectively both continence and micturition with a single electrode on a peripheral nerve is an exciting prospect for neurorehabilitation.
Methods
Experimental preparation
All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University. Fourteen sexually intact, adult male cats (3.3–5.8 kg) were anaesthetized with ketamine HCl (Ketaset, 35 mg kg−1, i.m., supplemented as required during surgical preparation). Anaesthesia was maintained with α-chloralose (Sigma, 65 mg kg−1, i.v., supplemented at 15 mg kg−1) through a catheter in the cephalic vein. Blood pressure was measured through a catheter placed in the carotid artery, and depth of anaesthesia was monitored via blood pressure, heart rate, blink and withdrawal reflexes. A ventral midline incision was made to expose the bladder, and the ureters were ligated, transected proximal to the ligation, and drained externally. The bladder was cannulated with a 3.5 Fr (1.17 mm) catheter introduced with a hypodermic needle, secured with a purse string suture, and the abdominal incision was closed in layers. A 5 Fr (1.67 mm) catheter was placed in the urethra to occlude the urethra. A heating blanket was used to maintain body temperature between 38 and 39°C, 0.9% saline with 8.4 g l−1 sodium bicarbonate and 5% dextrose added was administered i.v. (10–15 ml kg−1 h−1), and artificial respiration was used to maintain end tidal CO2 between 3 and 4%.
In three animals, the response to PN stimulation was measured after acute transection of the spinal cord. The spinal cord at T12 was exposed via laminectomy, lidocaine was administered on the surface of the spinal cord, and the spinal cord was elevated and transected using a scalpel. The ends of the transected spinal cord were separated, the completeness of transection was confirmed visually, a piece of Gelfoam was inserted between the ends to promote coagulation, and the exposure was closed. Following spinal transection, data were collected for 9 h in one cat, 10 h in another, and 16 h in the third cat.
Recording and stimulation
The pressures produced in the bladder were measured by a solid-state pressure transducer connected to the suprapubic catheter (Deltran DPT-100, Utah Medical Products, Midvale, UT, USA). The pressure signal was amplified, low pass filtered (cutoff frequency = 300 Hz), sampled at 24 kHz and stored on digital tape (CDAT 16 Data Recorder, Cygnus Technology, Inc., Delaware Water Gap, PA, USA), and recorded continuously on a strip chart recorder (TA11, Gould Instrument Systems). In two cats, the intra-abdominal pressure was sampled with a saline filled balloon in the rectum and exhibited a variation of less than 5 cm H2O before, during, and after bladder contractions evoked by PN stimulation.
The PN was exposed by partially transecting and laterally reflecting the gluteus superficialis and gluteofemoralis muscles. The PN was isolated and stimulated with platinum bipolar hook electrodes and a regulated current stimulator (Pulsar 9 bp, FHC Inc., Bowdoinham, ME, USA). Stimulus pulses were cathodic and monophasic with duration of 100 μs and amplitude of 100–900 μA applied as 2 s to 40 s trains with a frequency between 2 Hz and 100 Hz. The minimum current amplitude necessary to elicit bladder contractions was called threshold (PNthr) and ranged from 100 to 500 μA (n = 14). The electrical threshold to evoke a compound nerve action potential in the PN was measured in two cats by stimulating the PN and recording caudally to the stimulation site at the deep perineal branch of the PN, and fell in a similar range (50–300 μA). The data presented here were obtained at 2–4 times the minimum current amplitude necessary to elicit bladder contractions (2–4 × PNthr).
A tripolar cuff electrode was placed on the contralateral PN motor branch containing both the caudal rectal nerve and the deep perineal nerve (in 2 cats) or on the contralateral deep perineal nerve (in 1 cat) to record the electroneurogram (ENG) of nerve fibres innervating the EUS. The ENG was preamplified and filtered (100×, 300 Hz to 10 kHz, Stanford SR560, Sunnyvale, CA, USA), further amplified and filtered (1000×, 300 Hz to 3 kHz, Grass P511, Quincy, MA, USA), sampled at 24 kHz, and stored on digital tape. Prior to ENG recording, the deep perineal branch ipsilateral to stimulation was transected to prevent direct activation of the EUS and the resulting afferent response to the EUS contraction that would be recorded by the contralateral cuff electrode. At the conclusion of the experiment, the animal was killed by an overdose of sodium pentobarbital, and the nerves were traced proximally and distally to verify the anatomy.
To determine the threshold volume for distension-evoked contractions, the bladder was first drained and then filled in 1 ml increments every 60 s, and the volume at which a bladder contraction was first observed was considered the volume threshold for distension-evoked contractions. A distension-evoked contraction was defined as having at least two peaks in its pressure waveform to distinguish it from the passive (single peak) response to the 1 ml bolus of saline. To determine the volume threshold for bladder contractions elicited by stimulation of the PN, the PN was stimulated for 20 s at 30 s after each 1 ml increase in bladder volume, and the lowest bladder volume at which PN stimulation elicited a sustained bladder contraction was considered the volume threshold for PN stimulation. During these volume threshold trials, an occluding catheter was placed in the urethra to prevent leakage during the bladder contractions. The bladder was emptied every 1–2 h to determine the accuracy of bladder volume, and the bladder volumes reported were accurate to within ± 2 ml.
Analysis of bladder and urethral sphincter responses
Changes in bladder pressure were quantified to assess the response of the bladder to PN stimulation (Boggs et al. 2005). The pressure during the 2 s interval preceding stimulation was called the baseline pressure (Pbaseline). The standard deviation of Pbaseline was denoted by Std(Pbaseline) and the average of Pbaseline by Avg(Pbaseline). The bladder pressure increased and oscillated slowly (0.2–0.3 Hz) when stimulation elicited sustained bladder contractions, and the pressure returned to baseline when stimulation was terminated. During this period of slow oscillation, the pressure with respect to the Pbaseline was used to quantify the bladder response. A bladder contraction was considered to be evoked during stimulation if the pressure increased over a threshold pressure (Pth):
For bladder contractions evoked by stimulation, the contraction was defined to begin 2 s before the first peak of the contraction and terminate at the end of stimulation if the contraction was sustained. However, if the pressure did not decrease below the average bladder-contraction pressure within 3 s of the termination of stimulus (i.e. the bladder pressure was not gated to the offset of stimulation), then the contraction was considered not to be stimulation evoked. If the contraction was not sustained (i.e. the contraction ended before stimulation was terminated), then the bladder contraction was defined as terminating at the last peak in bladder pressure. If the stimulation failed to evoke a contraction, then the pressure was averaged over the interval of stimulation. Distension-evoked reflex bladder contractions were defined as beginning 2 s before the first peak of the bladder contraction and terminating at the last peak. Similarly, stimulation was considered to inhibit the bladder if the pressure decreased below a threshold pressure (Pth) after onset of stimulation:
The inhibition was considered sustained if the pressure remained below Pth for the duration of stimulation.
The response of the urethral sphincter was assessed by measuring changes in the rectified and integrated (τ = 250 ms) electroneurogram (〈 |ENG| 〉) of the deep perineal nerve contralateral to stimulation. The average rectified ENG during the 0.5 ms preceding each stimulus pulse was used to replace the stimulus artifact (approximately 1–3 ms in duration). The EUS ENG response to stimulation (ENGΔ) was quantified by taking the logarithm base 10 of the ratio of the average 〈 |ENG| 〉 during an interval of stimulation (〈 |ENG| 〉stim) to the baseline 〈 |ENG| 〉 averaged during the 2 s period preceding stimulation (〈 |ENG| 〉baseline).
A one-sample Wilcoxon test was used to determine if 〈 |ENG| 〉stim increased or decreased relative to 〈 |ENG| 〉baseline because ENGΔ was positive if 〈 |ENG| 〉stim increased, negative if 〈 |ENG| 〉stim decreased, and 0 if there was no change. The ENG data were binned in successive intervals during stimulation such that the change in ENG could be calculated for every interval to determine the effect of stimulus duration.
Distribution of experiments
Responses to PN stimulation were investigated in 14 cats prior to spinal cord transection (SCT) and three cats post-SCT. The dependence of the bladder response to PN stimulation on the interaction between stimulus frequency and stimulus train duration was investigated systematically in four spinal intact cats and in one cat after SCT. EUS ENG response to PN stimulation was measured in three cats before and after SCT. The relationship between the volume threshold (Vthr) for bladder contractions evoked by PN stimulation and the Vthr for distension-evoked contractions was investigated in 12 cats with intact spinal cords and in three cats with transected spinal cords. The ability to empty the bladder by PN stimulation was tested in two spinal intact cats.
Bladder emptying trials
The ability to empty the bladder was determined by filling the bladder at a rate of 1 ml min−1 through the urethral catheter until the bladder volume reached 125–200% of the threshold volume for distension-evoked reflex bladder contractions. The urethral catheter was then removed, and the bladder emptied via either PN stimulation or distension-evoked reflex micturition. Bladder emptying typically concluded within 3 min of initiating voiding, and after 5 min voided volumes were considered final and the urethral catheter was reinserted to measure the remaining (residual) bladder volume. Both methods of bladder emptying were tested after unilateral transection of the deep perineal nerve to prevent activation of the urethral sphincter during PN stimulation. Stimuli were 20–60 s trains of 100 μs pulses at 33 Hz and 2–4 × PNthr.
Statistical analysis
Student's t test for paired data or ANOVA followed by the Tukey-Kramer post hoc test was used to perform statistical analyses of bladder pressures. Wilcoxon's test and the Kruskal–Wallis test followed by non-parametric comparisons based on Bonferroni's inequalities (Gibbons, 1993) were used to perform statistical analyses of bladder and EUS response characteristics because the data were not normally distributed. Values are expressed as means ± standard deviation.
Results
The changes in bladder pressure and EUS activity evoked by PN stimulation were dependent on stimulus train duration, stimulus frequency, and bladder volume, and these dependencies were similar across animals. Mid range frequency (33 Hz) stimulation of the compound PN evoked bladder contractions (n = 14 of 14) and decreases in EUS ENG activity (n = 3 of 3). Conversely, low frequency (10 Hz) stimulation of the compound PN inhibited bladder contractions (n = 8 of 8) and evoked increases in urethral sphincter ENG activity (n = 3 of 3). Increasing stimulation amplitudes beyond twice the threshold to evoke a bladder contraction (2 × PNthr) did not increase the magnitude of the bladder contractions nor did it change the ability of stimulation to evoke a sustained bladder contraction. Bladder pressure and sphincter ENG activity were gated to stimulation and typically returned to prestimulus levels when stimulation ended.
Following SCT, distension-evoked reflex bladder contractions disappeared and PN stimulation failed to elicit bladder contraction. As time post-spinalization increased the responses returned (n = 3 of 3), and by 8 h after SCT 33 Hz stimulation evoked synergic contraction of the bladder and reduction in EUS ENG, while 10 Hz stimulation evoked bladder contraction in fewer than 20% of trials and generated an increase in EUS ENG.
Voiding was evoked by 33 Hz stimulation of the PN confirming that the evoked bladder contraction and EUS relaxation were indeed a micturition reflex. Following ipsilateral transection of the PN branch innervating the urethral sphincter (distal to the stimulating electrode) and removal of the urethral catheter, bladder emptying produced by PN stimulation (56 ± 10% of the initial volume, n = 4 trials across 2 cats) was comparable to bladder emptying produced by distension-evoked reflex bladder contractions (42 ± 8%, n = 4 trials across 2 cats).
Bladder response was dependent on frequency of stimulation and duration of stimulus train
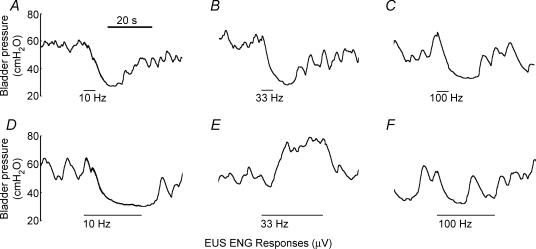
Whether PN stimulation evoked bladder contraction, bladder inhibition, or no response depended on the frequency of stimulation, the duration of the stimulus train, and the interaction between frequency and duration (P < 0.001 for all 3, Kruskal–Wallis tests adjusted for ties, n = 208 trials across four cats with 53 ± 15 samples per cat, and bladder volume was above threshold for distension-evoked bladder contractions in all trials). Figure 1 shows representative bladder pressure responses to stimulation of the PN over a range of frequencies (10, 33 and 100 Hz) and with short (5 s) and long (25 s) duration stimulus trains. A decrease in bladder pressure was observed in response to PN stimulation for short durations at all frequencies (Fig. 1A–C). When stimulation was applied for longer durations, mid-range frequency stimulation (33 Hz) evoked robust bladder contractions (Fig. 1E), while low (10 Hz) and high (100 Hz) frequency stimulation did not evoke bladder contractions (Fig. 1D and F).
Figure 1. Bladder responses evoked by PN stimulation for 5 s (A, B and C) and 25 s (D, E and F) at stimulation frequencies 10 Hz (A and D), 33 Hz (B and E), and 100 Hz (C and F) in the same animal.
Short duration stimulation at all frequencies produced bladder inhibition, but the bladder response to 25 s stimulus trains was dependent on stimulation frequency. Stimuli were trains of 100 μs, 900 μA pulses, and bladder volume was above the threshold for distension-evoked reflex contractions.
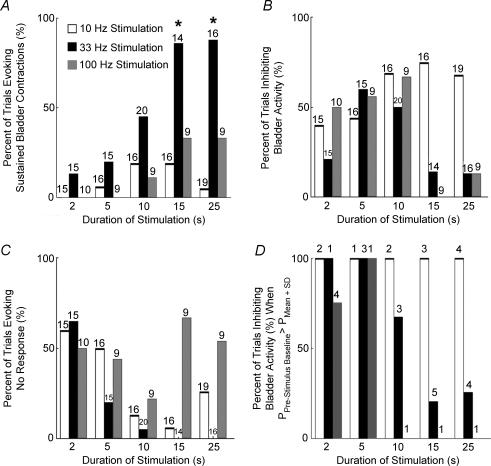
The relative incidence of generation of sustained bladder contractions, inhibition of bladder activity, or neither response was quantified. Figure 2 shows the relative incidence of excitation or inhibition in four animals, and is representative of the relative incidence within each of the animals. Stimulation at 33 Hz for 15 s or longer produced a greater percentage of sustained contractions than all other combinations (P < 0.05, Kruskal–Wallis test adjusted for ties). Conversely, 10 Hz stimulation produced bladder inhibition most successfully across all stimulus train durations (P < 0.05) and inhibited ongoing bladder activity in approximately 60% of all trials across all durations. Stimulation at 100 Hz inhibited bladder activity in approximately 60% of all short duration (≤ 10 s) trials, but for longer durations (≥ 15 s) 100 Hz stimulation neither consistently evoked nor inhibited bladder contractions. Thus, mid-range frequency stimulation applied for at least 15 s was more likely to elicit and sustain bladder contractions than either low or high frequency stimulation applied for any duration.
Figure 2. Bar graphs of the character of the bladder responses evoked by PN stimulation at 10 Hz (white bar), 33 Hz (black bar), and 100 Hz (grey bar) as a function of stimulus train duration.
A, the percentage of trials where stimulation generated sustained bladder contractions depended on the stimulation frequency, stimulus train duration, and the interaction between frequency and duration. B, the percentage of trials in which PN stimulation inhibited the bladder depended on stimulation frequency and the interaction between stimulus frequency and train duration, but not on train duration alone. C, the percentage of trials where PN stimulation evoked neither a sustained bladder contraction nor sustained inhibition of the bladder was dependent on stimulus duration, but on neither stimulus frequency nor the interaction between stimulus frequency and train duration. D, bladder inhibition in those trials where PN stimulation was delivered during elevated bladder pressure. The numbers above the bars are the number of trials across cats. *Stimulus parameters evoking a significantly greater percentage of sustained bladder contractions (P < 0.05, non-parametric comparison based on Bonferroni inequalities (Gibbons, 1993). All stimulation amplitudes were between 2 × and 4 × PNthr and all bladder volumes were above threshold for distension-evoked contractions.
Sphincter response was dependent on frequency of stimulation and interval of stimulation
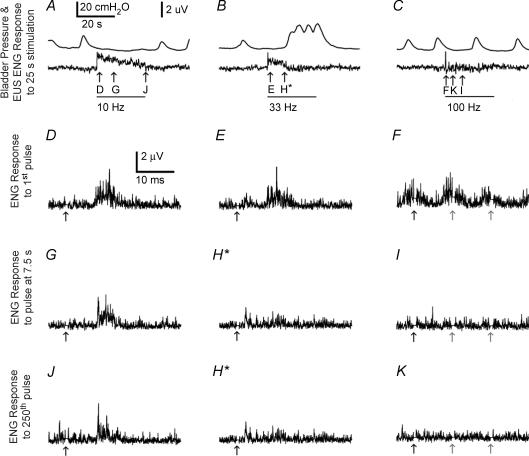
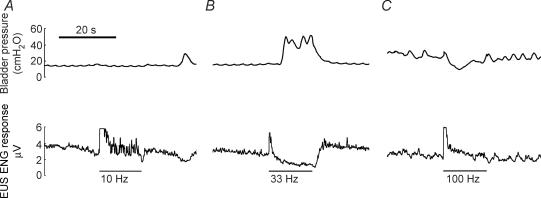
The response of the EUS to PN stimulation depended on the frequency of stimulation, the interval during stimulation, and the interaction between frequency and interval (P < 0.001 for all 3, Kruskal–Wallis tests adjusted for ties, n = 80 across three spinal intact cats with a minimum of 24 intervals per cat). Figure 3 shows typical bladder pressure and EUS ENG responses to stimulation over a range of frequencies (10, 33 and 100 Hz), and Fig. 4 shows summary data across three cats. Stimulation at 10 Hz evoked an increase in EUS ENG activity that waned as stimulation continued but remained above baseline for the duration of stimulation (Figs 3A and 4B). Stimulation at 33 Hz evoked a transient increase in EUS ENG activity, but the ENG activity returned to baseline as bladder pressure increased (Figs 3B and 4). Stimulation at 100 Hz evoked a large transient increase in EUS ENG followed by a return to baseline (Fig. 3C). PN stimulation always produced an initial burst of EUS ENG activity, but the succeeding ENG response depended on stimulation frequency and time following stimulation onset.
Figure 3. Bladder pressure (top trace) and ENG from the contralateral deep perineal nerve evoked by PN stimulation.
ENG from the contralateral deep perineal nerve evoked by PN stimulation is indicated with bars below the pressure and rectified and integrated ENG traces in A–C and arrows below the rectified ENG traces in D–K. A, EUS ENG increased above baseline and bladder pressure remained at baseline during PN stimulation at 10 Hz. B, EUS ENG activity increased initially during PN stimulation at 33 Hz but decreased to baseline as bladder pressure increased. C, EUS ENG activity increased initially during PN stimulation at 100 Hz and returned to baseline shortly after stimulation onset, and PN stimulation at 100 Hz had little influence on bladder pressure. D–F, stimulation evoked compound nerve action potentials (CNAPs) in the contralateral deep perineal nerve approximately 7–15 ms after the first pulse at the onset of stimulation at all frequencies (10, 33 and 100 Hz). Stimulus pulses at 10 Hz continued to evoke CNAPs 7.5 s after stimulation onset (G), but stimulation at 33 Hz (H) or 100 Hz (I) did not. A burst of EUS activity is observable in response to the 250th pulse of 10 Hz stimulation (J) but not in response to the 250th pulses of either 33 Hz (H) or 100 Hz (K) stimulation. Stimuli were 25 s trains of 100 μs, 900 μA pulses, and bladder volume was above the threshold for distension-evoked reflex contractions. Traces D–K show ENG responses to individual stimuli averaged over 3 sweeps. *The 250th pulse of 33 Hz stimulation occurs 7.5 s after stimulation onset; thus, trace H depicts the ENG response to both because they are the same pulse. The grey arrows (F, I and K) represent succeeding stimulus pulses shown in the 30 ms time frame because the interpulse interval of 100 Hz stimulation is 10 ms.
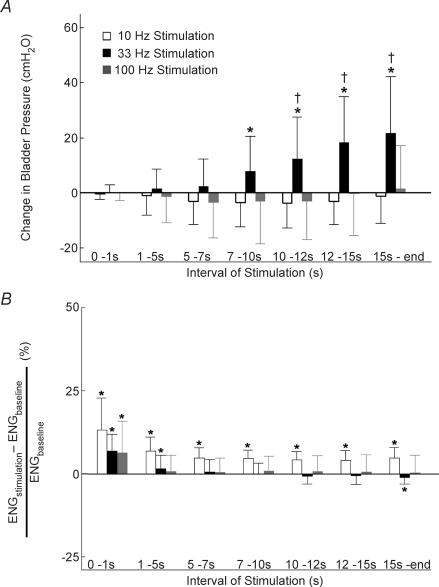
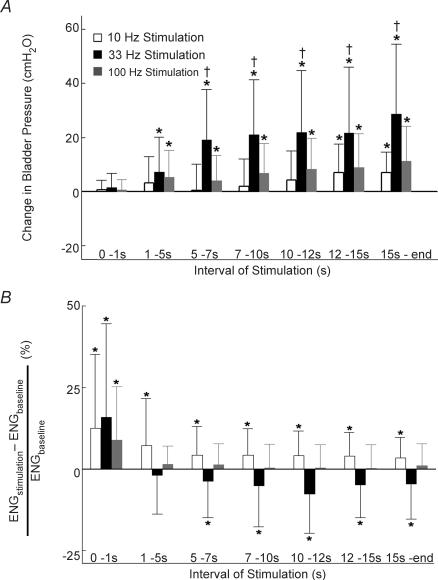
Figure 4. The mean and standard deviation of bladder pressure (A) and rectified and integrated ENG (B) from the contralateral deep perineal nerve evoked by PN stimulation as a function of interval during stimulation at 10 Hz, 33 Hz and 100 Hz.
A, stimulation at 10 Hz decreased bladder pressure during all intervals greater than 1 s (P > 0.05 for each interval, Wilcoxon test, n = 18 for each interval across 3 cats with a minimum of 5 trials per interval per cat), while 33 Hz stimulation evoked progressively larger increases in bladder pressure during each interval > 1 s. Stimulation at 100 Hz evoked no significant change from baseline pressure during any interval. B, stimulation at all frequencies elicited an initial increase in ENG during the first second of stimulation which remained above baseline during all intervals of stimulation at 10 Hz, but progressively decreased during 33 Hz stimulation, and dropped to baseline in response to 100 Hz stimulation. Stimuli were 20–40 s trains of 100 μs pulses at 2–4 × PNthr, and bladder volume was above the threshold for distension-evoked reflex contractions. *Statistically significant increase or decrease from baseline with P < 0.05. †Significant difference from pressures produced by other stimulation frequencies during the same interval of stimulation with P < 0.05.
The changes in ENG and bladder pressure were quantified during different intervals of stimulation over a range of stimulation frequencies (10, 33 and 100 Hz). During the 1st second of stimulation, the response to each frequency was the same: the ENG discharge increased (P < 0.005), but the bladder pressure did not change (P > 0.05) (Fig. 4). During all successive intervals of 10 Hz stimulation, the ENG discharge remained greater than baseline (P < 0.05) and the bladder pressure decreased slightly (P > 0.05) (Fig. 4). In response to 33 Hz stimulation, the ENG discharge decreased after the initial burst and dropped below the prestimulus baseline 15 s after the onset of stimulation (P < 0.05). Conversely, the bladder pressure increased during each successive interval of 33 Hz stimulation, and increased significantly above baseline by 7 s after the onset of stimulation (P < 0.05) (Fig. 4). After the initial burst of activity, 100 Hz stimulation did not elicit an ENG response different from baseline (P > 0.05) nor did it evoke a change in bladder pressure during any interval (P > 0.05) (Fig. 4). Thus, the ENG response depended on both stimulation frequency and interval of stimulation. Stimulation at 10 Hz elicited increased ENG activity in all intervals, but after at least 15 s of 33 Hz stimulation ENG activity returned to baseline.
The dependence of the ENG response on frequency may be due solely to the number of stimulus pulses because higher frequencies apply more pulses within a fixed period, or it may be due also to the interpulse interval. These two possibilities were examined by comparing the ENG responses after equal periods of stimulation (immediately following stimulus onset and 7.5 s after onset) and after equal numbers of pulses (1st pulse and 250th pulse after stimulus onset). The first pulse evoked an ENG discharge with a latency of approximately 7 ms and a duration of approximately 7 ms (Fig. 3D–F). However, after 7.5 s the response evoked by 33 Hz stimulation was greatly diminished, there was no response evoked by 100 Hz stimulation, and only 10 Hz stimulation continued to elicit a discharge (Fig. 3G–I). Similarly, the ENG response to the 250th pulse of 33 Hz stimulation was greatly diminished, there was no response evoked by 100 Hz stimulation, and only 10 Hz stimulation elicited a discharge (Fig. 3J, H and K). Thus, the ENG response to PN stimulation was dependent on the interpulse interval and not just the number of stimulus pulses. Higher stimulus frequencies (33 and 100 Hz) produced less ENG discharge than the lower stimulus frequency (10 Hz) following the same number of pulses.
Bladder and sphincter responses after spinal cord transection
Eight to ten hours after SCT, the character of the bladder response to PN stimulation was still dependent on the frequency of stimulation (P < 0.001), but did not depend on the train duration (P > 0.05). Similar to pre-SCT, 33 Hz stimulation evoked sustained bladder contractions in all trials with stimulus train durations of 5 s and greater. However, the lack of distension-evoked reflex contractions reduced the potential for stimulation to produce inhibition of the bladder and either 10 Hz or 100 Hz stimulation produced only modest (< 3 cmH2O) inhibition of bladder activity in approximately 50% of trials. In fewer than 20% of trials 10 Hz or 100 Hz stimulation produced sustained contraction of the bladder.
Shortly after SCT, ENG activity did not respond consistently to any stimulation frequency, but as time progressed the responses recovered and closely resembled prespinalization activity. Eight hours after acute SCT, the response of the EUS to PN stimulation was still dependent on the stimulus frequency, the interval during stimulation, and the interaction between frequency and interval (P < 0.001 for all 3, Kruskal–Wallis test adjusted for ties, n = 116 across 3 spinal transected cats with a minimum of 31 trials per cat). Stimulation at 10 Hz evoked a sustained increase in sphincter ENG (Figs 5A and 6B), while 33 Hz stimulation evoked a decrease in sphincter ENG and a sustained increase in bladder pressure (Figs 5B and 6). PN stimulation at 33 Hz decreased ENG activity in all intervals greater than 5 s after stimulation onset. The decrease in ENG activity occurred earlier in the stimulus train, and larger decreases were found for all intervals after the initial 5 s of stimulation (P < 0.05) after SCT than before SCT. The earlier reduction in EUS ENG was accompanied by an earlier (1 s) increase in bladder pressure (Fig. 6). Similar to pre-SCT, the ENG increased insignificantly in response to 100 Hz stimulation (P > 0.05), while the bladder pressure increased slightly (P < 0.05). Thus, following SCT the continence-like response produced by 10 Hz stimulation was less robust during later intervals of stimulation, and the micturition response evoked by 33 Hz stimulation appeared sooner after stimulus onset and became more robust during later intervals compared to that before SCT.
Figure 5. Bladder pressure and rectified and integrated ENG from the deep perineal nerve evoked by PN stimulation 8 h after SCT.
PN stimulation is indicated with bars below the pressure and ENG traces. A, PN stimulation at 10 Hz generated an increase in deep perineal nerve ENG, which remained above baseline while bladder pressure remained at baseline. B, PN stimulation at 33 Hz initially produced an increase in ENG activity, but the ENG activity decreased below baseline as bladder pressure increased and remained elevated. C, PN stimulation at 100 Hz also increased ENG activity initially, and the ENG activity remained above baseline as bladder pressure decreased transiently. Stimuli were 15 s trains of 100 μs, 900 μA pulses, and bladder volume was above the threshold for distension-evoked reflex contractions prior to spinal transection.
Figure 6. The mean and standard deviation of bladder pressure (A) and rectified and integrated ENG (B) from the contralateral deep perineal nerve evoked by PN stimulation after acute spinalization as a function of interval during stimulation at 10 Hz, 33 Hz, and 100 Hz.
A, 10 Hz stimulation did not elicit a significant change in bladder pressure during the initial intervals and only evoked a small increase in bladder pressure during the successive intervals. However, 33 Hz stimulation evoked progressively larger increases in bladder pressure during each interval > 1 s. Stimulation at 100 Hz produced small increases in bladder pressure during each interval > 1 s. B, stimulation at all frequencies elicited an initial increase in ENG during the 1st second of stimulation, which remained above baseline during all intervals of stimulation at 10 Hz, but decreased below baseline during 33 Hz stimulation and dropped to baseline in response to 100 Hz stimulation. Stimuli were 20–40 s trains of 100 μs pulses at 2–4 × PNthr, and bladder volume was above the threshold for distension-evoked reflex contractions prior to SCT. *Statistically significant increase or decrease from baseline with P < 0.05. †Significant difference from pressures produced by other stimulation frequencies during the same interval of stimulation with P < 0.05.
Bladder response to pudendal nerve stimulation was dependent on bladder volume
The bladder response to PN stimulation depended on bladder volume in all of the 12 spinal intact cats and three spinal transected cats in which bladder volume dependence was investigated. The bladder was filled in 1 ml increments and between each increase in volume, the PN was stimulated. PN stimulation evoked bladder contractions only when the bladder contained more than a minimum threshold volume (13 ± 5 ml, 1–20 ml, n = 12 spinal intact cats). This volume was always less than the minimum volume required to elicit a distension-evoked bladder contraction (18 ± 7 ml, 4–30 ml, n = 12 spinal intact cats) (P < 0.001, paired t test). PN stimulation elicited contractions at 66 ± 17% of the volume required for the 1 ml saline bolus to evoke a contraction (n = 12 spinal intact cats). The volume dependence was also present after spinal transection. The threshold volume for PN stimulation decreased to 11 ± 7 ml (5–18 ml, n = 3 spinalized cats) or 41 ± 28% of the volume required for the saline bolus to evoke a contraction prior to spinalization, but this decrease was not significant (P > 0.10).
Discussion
Activity in PN afferents differentially elicited continence and micturition reflexes dependent on the frequency of stimulation. The ability to control the principal functions of the bladder (storage and elimination) provides exciting prospects for clinical neurorehabilitation. Low frequency stimulation of the compound PN with any train duration elicited a continence-like response. When low frequency stimulation was applied during a bladder contraction, the bladder pressure decreased and the activity of the external urethral sphincter increased (Fig. 2D). When the bladder was quiescent and the pressure was already at or near baseline, application of low frequency stimulation did not decrease the bladder pressure further, but did increase the activation of the EUS. Conversely, mid-range frequency stimulation evoked a micturition-like response. In cats with intact spinal cords, this response included an initial increase in the activity of the EUS followed by a return to prestimulus baseline as the bladder pressure increased. After SCT, the synergy of the micturition-like response was more pronounced such that the activity of the EUS decreased below prestimulus levels as the bladder pressure increased. In a similar manner, different frequencies of epidural spinal cord stimulation evoke different leg motor responses, and these responses can be generated by interactions between temporal summation and presynaptic inhibition (Jilge et al. 2004).
The bladder contraction and reduction in EUS activity evoked by PN stimulation were confirmed to be part of a micturition reflex because PN stimulation produced voiding (56 ± 10%) that was comparable to voiding produced by distension-evoked bladder contractions (42 ± 8%). PN stimulation voided roughly half of the volume in the bladder, and this percentage is consistent with voiding produced by distension-evoked reflex bladder contractions in other studies (Rudy et al. 1991; Boggs et al. 2006). Further, intermittent PN stimulation empties the bladder more efficiently before and after spinalization than either distension-evoked reflex contractions or sacral nerve root stimulation without any nerve transections in the cat anaesthetized with α-chloralose, but it is still incomplete (64 ± 14% of initial volume) (Boggs et al. 2006). The anaesthetic α-chloralose, chosen because of its minimal interference with autonomic function and spinal reflexes relative to other anaesthetics (Balis & Monroe, 1964; Bonvento et al. 1994), may have contributed to the incomplete voiding in the present and previous studies as it can reduce maximal bladder contraction pressures and induce bladder-sphincter dyssynergia (Rudy et al. 1991). Distension-evoked micturition typically produces complete bladder emptying in the absence of α-chloralose (Rudy et al. 1991; Angel et al. 1994), and PN stimulation also may empty the bladder completely in the absence of α-chloralose. However, the presence of detrusor-sphincter dyssynergia following chronic spinal cord injury (SCI) may impair bladder emptying. If future studies determine that stimulation of the PN can indeed empty the bladder in persons with SCI, it may serve as a valuable method of restoring the two principal functions of the bladder: continence and micturition.
Low frequency PN stimulation elicited a continence-like response presumably by activating genital (Lindström et al. 1983) or anal (Sundin et al. 1974) afferent pathways contained in the PN (Martin et al. 1974). Activation of either the genital or anal pathway with low frequency (5–10 Hz) stimulation can inhibit bladder activity (Sundin et al. 1974; Lindström et al. 1983), and low frequency (2–20 Hz) stimulation is used clinically to inhibit the bladder detrusor (Vodusek et al. 1986). Stimulation of genital (vaginal) PN afferents at higher frequencies (20–100 Hz) is ineffective in reducing bladder activity (Jiang & Lindström, 1999a), and inhibition is ‘lost’ at mid-range frequency (35 Hz) stimulation of the PN (Walter et al. 1993). In the present study, stimulation was most often delivered between increases in bladder pressure generated by distension-evoked reflexes, and segregation of trials with stimulation delivered during elevated bladder pressure suggested that the incidence (Fig. 2) and degree (Figs 4 and 5) of bladder inhibition evoked by low frequency stimulation (10 Hz) may have been underestimated.
In the present study, 33 Hz stimulation of the PN initially inhibited on-going bladder contractions (Figs 1B and 2B), but the inhibition was short-lived and gave way to excitation if the stimulation duration was extended (Figs 1E and 2). The increase in bladder pressure was accompanied by a decrease in EUS ENG activity, suggesting that the ‘loss’ of a continence-like response reported in previous studies was actually the initiation of a micturition-like response. This biphasic effect is typical of the response generated by stimulating afferent nerve fibres in the rostral urethral sensory branch (Shefchyk & Buss, 1998) or in the deep perineal branch (Boggs et al. 2005) of the PN; this two-stage response may be analogous to the increase followed by decrease in urethral sphincter ENG activity produced by high frequency stimulation of pelvic detrusor afferents following spinalization (Bradley & Teague, 1972). The initial increase in EUS ENG activity evoked by PN stimulation may indicate activation of perineal afferents leading to excitation of EUS motoneurons typical of a continence response (Fedirchuk et al. 1994). The subsequent decrease in EUS ENG coincident with the generation of a sustained bladder contraction may indicate activation of micturition circuitry and suppression of a continence response via presynaptic depolarization of perineal afferents that would otherwise trigger sphincter contraction (Buss & Shefchyk, 1999), hyperpolarization of EUS motoneurons (Fedirchuk et al. 1994), or both.
The micturition-like response could be evoked only if stimulation was applied for a sufficient duration and if the bladder contained a sufficient volume, suggesting the reason why previous accounts of activation of micturition circuitry by pudendal afferents are uncommon. Previously, the effect of stimulation of the compound PN has been reported to be inhibitory (Sundin et al. 1974; McGuire, 1978; Lindström et al. 1983; Ohlsson et al. 1989; Walter et al. 1993). However, bladder contractions may not have been seen when the PN was stimulated at higher frequencies (20–30 Hz) because stimulus train durations (≤ 3 s) were too short (Tanagho & Schmidt, 1988) to evoke bladder contractions (Boggs et al. 2005 and Fig. 4) or bladder volumes were too low.
Stimulation of the PN generated bladder contractions, but only if the bladder contained more than a minimum volume, similar to responses evoked by stimulation of the deep perineal branch of the PN (Boggs et al. 2005). Stimulation of the rostral urethral sensory branch elicits bladder contractions at high volumes (Shefchyk & Buss, 1998) but fails to excite bladder preganglionic parasympathetic neurons if the bladder contains less than a threshold volume (Mazieres et al. 1997). The bladder response to flow of urine through the urethra has a similar dependence on bladder volume (Barrington, 1931, 1941); urethral fluid flow evokes bladder contractions at large bladder volumes and inhibits bladder contractions at small volumes (Garry et al. 1959). Urethral fluid flow generates bladder contractions when the bladder contains 65 ± 17% of the volume required for a bolus of saline to evoke a contraction (Robain et al. 2001), and PN stimulation produced bladder contractions when the bladder contained at least 66 ± 17% of the volume required for the saline bolus to evoke a contraction. Thus, it is possible that PN stimulation elicits bladder contractions via the same circuitry activated by urethral fluid flow.
The micturition-like increases in bladder pressure evoked by 33 Hz stimulation are distinct from rebound responses following removal of stimulation that inhibits bladder activity (Bycroft et al. 2004). Rebound responses are only observed occasionally and their latencies are unpredictable (Bycroft et al. 2004). In contrast, the response to 33 Hz stimulation was quite reliable, and 33 Hz stimulation resulted in sustained bladder contractions in approximately 90% of all trials with stimulus durations of 15 s or longer (Fig. 2). Further, 33 Hz stimulation evoked bladder contractions at repeatable latencies, the pressure was sustained during stimulation, and the pressure returned to baseline at stimulation offset.
Previous studies have reported the EUS response to PN stimulation to be dependent on the frequency of stimulation (Bradley & Teague, 1972; Rampal & Mignard, 1975a,b), and the present data demonstrated that the frequency dependence was due to the interpulse interval and not just the number of pulses. The number of pulses alone could not account for frequency dependence because the same number of pulses evoked an increase in ENG activity when applied with an interpulse interval of 100 ms (10 Hz) but did not evoke an increase in activity when applied at interpulse interval of 30 ms (33 Hz).
The EUS response to 10 Hz stimulation often waned during the stimulus train (Fig. 3A), and this reduction of ENG activity may have been due to habituation of the reflex response to stimulation at a constant frequency. Previous studies have shown that continuous stimulation of afferent fibres at a constant frequency can lead to habituation, resulting in decreased efferent output (Granat et al. 1993; Floeter et al. 1998; Cariga et al. 2001).
Approximately 8 h after SCT, PN stimulation generated micturition-like responses closely resembling pre-SCT responses. Though spinal shock was not measured directly, it likely altered the bladder and sphincter activity immediately after SCT (Hassouna et al. 1992; Zvara et al. 1998), and the variability across animals of the response to stimulation following spinalization is consistent with the variable rate of recovery after SCT in previous studies (Boggs et al. 2005). Spinalization also decreased the latencies from the onset of stimulation to the increase in bladder pressure (Figs 4A and 5A) and to the decrease in sphincter ENG activity (Figs 4B and 5B). Elimination of descending inhibitory inputs (Holstege et al. 1986) may have contributed to the decrease in delay. The present study did not investigate the response more than 16 h after spinalization, and it is possible that the response to PN stimulation could change as a result of hyper-reflexia, bladder-sphincter dyssynergia, and bladder hypertrophy resulting from chronic spinal cord injury (de Groat et al. 1981; Krenz & Weaver, 1998). Despite these changes, activation of urethral afferents is able to generate bladder contractions in persons with chronic spinal cord injury (Gustafson et al. 2004).
Acknowledgments
This work was supported by NIH grants R21 NS-43450 (W.M.G.), K25 HD-40298 (K.J.G.), R01 NS-50514 (W.M.G.), and a Whitaker Graduate Student Fellowship (B.J.W.). The authors thank Kerri Leder and Gilda Mills for their outstanding technical assistance.
References
- Angel M, Fyda D, McCrea D, Shefchyk S. Primary afferent depolarization of cat pudendal afferents during micturition and segmental afferent stimulation. J Physiol. 1994;479:451–461. doi: 10.1113/jphysiol.1994.sp020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balis G, Monroe RR. The pharmacology of chloralose. A review. Psychopharmacologia. 1964;6:1–30. doi: 10.1007/BF00710911. [DOI] [PubMed] [Google Scholar]
- Barrington FJF. The component reflexes of micturition in the cat, Parts I and II. Brain. 1931;54:177–188. [Google Scholar]
- Barrington FJF. The component reflexes of micturition in the cat III. Brain. 1941;64:239–243. [Google Scholar]
- Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Spinal micturition reflex mediated by afferents in the deep perineal nerve. J Neurophysiol. 2005;93:2688–2697. doi: 10.1152/jn.00978.2004. [DOI] [PubMed] [Google Scholar]
- Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Bladder emptying by intermittent electrical stimulation of the pudendal nerve. J Neural Eng. 2006;3:43–51. doi: 10.1088/1741-2560/3/1/005. [DOI] [PubMed] [Google Scholar]
- Bonvento G, Charbonne R, Correze J, Borredon J, Seylaz J, Lacombe P. Is α-chloralose plus halothane induction a suitable anesthetic regimen for cerebrovascular research? Brain Res. 1994;665:213–221. doi: 10.1016/0006-8993(94)91340-4. [DOI] [PubMed] [Google Scholar]
- Bradley W, Teague CT. Electrophysiology of pelvic and pudendal nerves in the cat. Exp Neurol. 1972;35:378–393. doi: 10.1016/0014-4886(72)90162-8. [DOI] [PubMed] [Google Scholar]
- Buss R, Shefchyk SJ. Excitability changes in sacral afferents innervating the urethra, perineum and hindlimb skin of the cat during micturition. J Physiol. 1999;514:593–607. doi: 10.1111/j.1469-7793.1999.593ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bycroft J, Craggs M, Sheriff M, Knight S, Shah P. Does magnetic stimulation of sacral nerve roots cause contraction or suppression of the bladder? Neurourol Urodyn. 2004;23:241–245. doi: 10.1002/nau.20009. [DOI] [PubMed] [Google Scholar]
- Cariga P, Catley M, Mathias CJ, Ellaway PH. Characteristics of habituation of the sympathetic skin response to repeated electrical stimuli in man. Clin Neurophysiol. 2001;112:1875–1880. doi: 10.1016/s1388-2457(01)00647-2. [DOI] [PubMed] [Google Scholar]
- de Groat W, Nadelhaft I, Milne R, Booth A, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst. 1981;3:135–160. doi: 10.1016/0165-1838(81)90059-x. [DOI] [PubMed] [Google Scholar]
- Fedirchuk B, Downie J, Shefchyk SJ. Reduction of perineal evoked excitatory postsynaptic potentials in cat lumbar and sacral motoneurons during micturition. J Neurosci. 1994;14:6153–6159. doi: 10.1523/JNEUROSCI.14-10-06153.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floeter MK, Gerloff C, Kouri J, Hallett M. Cutaneous withdrawal reflexes of the upper extremity. Muscle Nerve. 1998;21:591–598. doi: 10.1002/(sici)1097-4598(199805)21:5<591::aid-mus5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Garry RC, Roberts TD, Todd JK. Reflexes involving the external urethral sphincter in the cat. J Physiol. 1959;149:653–665. doi: 10.1113/jphysiol.1959.sp006366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons JD. Nonparametric Statistics: An Introduction. Newbury Park, CA, USA: Sage; 1993. [Google Scholar]
- Granat MH, Heller BW, Nicol DJ, Baxendale RH, Andrews BJ. Improving limb flexion in FES gait using the flexion withdrawal response for the spinal-cord injured person. J Biomed Eng. 1993;15:51–56. doi: 10.1016/0141-5425(93)90093-e. [DOI] [PubMed] [Google Scholar]
- Gustafson K, Creasey G, Grill WM. A urethral afferent mediated excitatory bladder reflex exists in humans. Neurosci Lett. 2004;360:9–12. doi: 10.1016/j.neulet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Hassouna M, Li J, Sawan M, Duval F, Latt R, Elhilali MM. Effect of early bladder stimulation on spinal shock: experimental approach. Urology. 1992;40:563–573. doi: 10.1016/0090-4295(92)90418-v. [DOI] [PubMed] [Google Scholar]
- Holstege G, Griffiths D, de Wall H, Dalm E. Anatomical and physiological observations on supraspinal control of bladder and urethral sphincter muscles in the cat. J Comp Neurol. 1986;250:449–461. doi: 10.1002/cne.902500404. [DOI] [PubMed] [Google Scholar]
- Jiang C, Lindstrom S. Optimal conditions for the long-term modulation of the micturition reflex by intravesical electrical stimulation: an experimental study in the rat. BJU Int. 1999a;83:483–487. doi: 10.1046/j.1464-410x.1999.00947.x. [DOI] [PubMed] [Google Scholar]
- Jiang C, Lindstrom S. Prolonged enhancement of the micturition reflex in the cat by repetitive stimulation of bladder afferents. J Physiol. 1999b;517:599–605. doi: 10.1111/j.1469-7793.1999.0599t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilge B, Minassian K, Rattay F, Dimitrijevic MR. Frequency-dependent selection of alternative spinal pathways with common periodic sensory input. Biol Cybern. 2004;91:359–376. doi: 10.1007/s00422-004-0511-5. [DOI] [PubMed] [Google Scholar]
- Krenz N, Weaver LC. Sprouting of primary afferent fibers after spinal cord transection in the rat. Neuroscience. 1998;85:443–458. doi: 10.1016/s0306-4522(97)00622-2. [DOI] [PubMed] [Google Scholar]
- Lindstrom S, Fall M, Carlsson C, Erlandson BE. The neurophysiological basis of bladder inhibition in response to intravaginal electrical stimulation. J Urol. 1983;129:405–410. doi: 10.1016/s0022-5347(17)52127-8. [DOI] [PubMed] [Google Scholar]
- Martin W, Fletcher T, Bradley WE. Innervation of feline perineal musculature. Anat Rec. 1974;180:15–29. doi: 10.1002/ar.1091800104. [DOI] [PubMed] [Google Scholar]
- Mazieres L, Jiang C, Lindstrom S. Bladder parasympathetic response to electrical stimulation of urethral afferents in the cat. Neurourol Urodyn. 1997;16:471–472. [Google Scholar]
- McGuire E, Herlihy E. Bladder and urethral responses to isolated sacral motor root stimulation. Invest Urol. 1978;16:219–223. [PubMed] [Google Scholar]
- Ohlsson B, Fall M, Frankenberg-Sommar S. Effects of external and direct pudendal nerve maximal electrical stimulation in the treatment of the uninhibited overactive bladder. Br J Urol. 1989;64:374–380. doi: 10.1111/j.1464-410x.1989.tb06046.x. [DOI] [PubMed] [Google Scholar]
- Rampal G, Mignard P. Organization of the nervous control of urethral sphincter. A study in the anaesthetized cat with intact central nervous system. Pflugers Arch. 1975a;353:21–31. doi: 10.1007/BF00584508. [DOI] [PubMed] [Google Scholar]
- Rampal G, Mignard P. Behaviour of the urethral striated sphincter and of the bladder in the chronic spinal cat. Implications at the central nervous system level. Pflugers Arch. 1975b;353:33–42. doi: 10.1007/BF00584509. [DOI] [PubMed] [Google Scholar]
- Robain G, Combrisson H, Mazieres L. Bladder response to urethral flow in the awake ewe. Neurourol Urodyn. 2001;20:641–649. doi: 10.1002/nau.1014. [DOI] [PubMed] [Google Scholar]
- Rudy D, Downie J, McAndrew JD. α-Chloralose alters autonomic reflex function of the lower urinary tract. Am J Physiol. 1991;261:R1560–R1567. doi: 10.1152/ajpregu.1991.261.6.R1560. [DOI] [PubMed] [Google Scholar]
- Shefchyk S, Buss RR. Urethral pudendal afferent-evoked bladder and sphincter reflexes in decerebrate and acute spinal cats. Neurosci Lett. 1998;244:137–140. doi: 10.1016/s0304-3940(98)00155-4. [DOI] [PubMed] [Google Scholar]
- Sundin T, Carlsson C, Kock NG. Detrusor inhibition induced from mechanical stimulation of the anal region and from electrical stimulation of pudendal nerve afferents. An experimental study in cats. Invest Urol. 1974;11:374–378. [PubMed] [Google Scholar]
- Tanagho E, Schmidt RA. Electrical stimulation in the clinical management of the neurogenic bladder. J Urol. 1988;140:1331–1339. doi: 10.1016/s0022-5347(17)42038-6. [DOI] [PubMed] [Google Scholar]
- Todd JK. Afferent impulses in the pudendal nerves of the cat. Q J Exp Physiol Cogn Med Sci. 1964;49:258–267. doi: 10.1113/expphysiol.1964.sp001730. [DOI] [PubMed] [Google Scholar]
- Vereecken R, Das J, Grisar P. Electrical sphincter stimulation in the treatment of detrusor hyperreflexia of paraplegics. Neurourol Urodyn. 1984;3:145–154. [Google Scholar]
- Vodusek DB, Light JK, Libby JM. Detrusor inhibition induced by stimulation of pudendal nerve afferents. Neurourol Urodyn. 1986;5:2381–2389. [Google Scholar]
- Walter J, Wheeler J, Robinson C, Wurster RD. Inhibiting the hyperreflexic bladder with electrical stimulation in a spinal animal model. Neurourol Urodyn. 1993;12:241–252. doi: 10.1002/nau.1930120306. discussion 253. [DOI] [PubMed] [Google Scholar]
- Zvara P, Sahi S, Hassouna MM. An animal model for the neuromodulation of neurogenic bladder dysfunction. Br J Urol. 1998;82:267–271. doi: 10.1046/j.1464-410x.1998.00676.x. [DOI] [PubMed] [Google Scholar]