Abstract
Ten subjects balanced their own body or a mechanically equivalent unstable inverted pendulum by hand, through a compliant spring linkage. Their balancing process was always characterized by repeated small reciprocating hand movements. These bias adjustments were an observable sign of intermittent alterations in neural output. On average, the adjustments occurred at intervals of ∼400 ms. To generate appropriate stabilizing bias adjustments, sensory information about body or load movement is needed. Subjects used visual, vestibular or proprioceptive sensation alone and in combination to perform the tasks. We first ask, is the time between adjustments (bias duration) sensory specific? Vision is associated with slow responses. Other senses involved with balance are known to be faster. Our second question is; does bias duration depend on sensory abundance? An appropriate bias adjustment cannot occur until unplanned motion is unambiguously perceived (a sensory threshold). The addition of more sensory data should therefore expedite action, decreasing the mean bias adjustment duration. Statistical analysis showed that (1) the mean bias adjustment duration was remarkably independent of the sensory modality and (2) the addition of one or two sensory modalities made a small, but significant, decrease in the mean bias adjustment duration. Thus, a threshold effect can alter only a very minor part of the bias duration. The bias adjustment duration in manual balancing must reflect something more than visual sensation and perceptual thresholds; our suggestion is that it is a common central motor planning process. We predict that similar processes may be identified in the control of standing.
Balancing an unstable object: bias adjustments
In previous work we described the results obtained when subjects balanced a human proportioned inverted pendulum manually using a compliant linkage (Lakie et al. 2003). We studied the outcome of the balancing process (sway of the inverted pendulum) and the control process (hand movements). As well as providing insights into the way in which an unstable load was manually balanced, we believed that with an appropriate choice of load and linkage our experiment was a useful mechanical analogue of human standing where balancing of the unstable body is carried out by the calf muscles, tendons and feet.
In the hand balancing experiment, there were three components. These were: (A) the inverted pendulum; (B) the hand which provided the force required for balancing; and (C) the spring, which connected hand to inverted pendulum. The spring had a low stiffness which was inadequate for passive static stability. Accordingly, an active dynamic strategy of hand movements was essential to preserve balance.
The dynamic strategy that we reported has two key characteristics. First, for balance to be achieved, hand and inverted pendulum must move in opposite directions at low frequencies (‘paradoxical movement’). Second, the hand movements occur more frequently than the slow pendulum sway and have no consistent instantaneous relationship to pendulum position.
The hand is fixed to one end of the spring. It can adjust the bias of the spring in a way that is mechanically independent of movement of its other end which is moved by the load. Control is a matter of manipulating the bias of the spring in an appropriate manner. We have suggested that the hand movements are ballistic and so we have called the alterations in hand position and spring length a ballistic bias mechanism. However they are generated, the bias adjustments provide an endless series of irregularly repeated nudges which maintain the balance of the inverted pendulum (Lakie et al. 2003; Loram et al. 2005b). As the nudges are effective in preventing the pendulum from collapsing, their size and direction must be controlled by the nervous system. The repeated bias adjustments are a feature that is visually very striking when the experiment is performed. We regard bias adjustments as a clear and direct indication of alteration in neural drive which produces a change in the tension and length of the spring linkage. Bias adjustments, irrespective of their means of regulation, control acceleration of the load. Counting the bias adjustments provides a convenient measure of the rate at which neural adjustments occur.
This approach has much in common with the way in which the rate of manual adjustments is determined in the manual tracking literature. In manually tracking an unpredictable visual target it is well established that there are intermittent adjustments in hand position, submovements, which occur at a low rate, commonly around two per second which is very similar to the rate of the bias adjustments that we observe. They are intermittent in the sense that their occurrence is not predictable from any immediate aspect of the input signal (Craik, 1947; Bekey, 1962; Poulton, 1974; Miall et al. 1993; Reed et al. 2003). In order to generate bias adjustments that are successful in stabilizing the inverted pendulum, the subject must have knowledge of its motion. The intention of the present experiment is to compare the results obtained when different senses are used to obtain this information. Accordingly, we have elaborated upon our previous experiment. Subjects manually balanced their own body or a mechanically equivalent artificial inverted pendulum. By doing this we could control whether subjects had available visual, vestibular or proprioceptive information alone or in combination.
Is bias duration a consequence of visual control?
In our earlier experiment, the bias adjustments were made by the subject who observed directly the movement of the inverted pendulum. Thus vision was the predominating sense available. Visual sensation is associated with long delays attributed to the extensive neural processing required to construct a position or velocity signal from the retinal image. This leads to a considerable delay between unpredictable movement of a visual target and a manual response. Where hand movement is made in response to modification of the velocity of an otherwise predictable visual target, there is still a delay between stimulus and response of more than 200 ms (Brenner et al. 1998). It is possible that the long mean duration of bias adjustments in our previous results might reflect the slowness of visual sensation. Accordingly, the first aim of the present experiment was to determine whether a similarly low frequency of bias adjustment is obtained when balancing is carried out using sensory modalities that are associated with less sensory delay. Particularly relevant are those non-visual senses that contribute to balance in quiet standing. Therefore we have compared the bias duration when balance is performed under purely visual, vestibular or proprioceptive control. Manual movements have been frequently studied when they are executed under visual control; as far as we know the corresponding manual movements made when sensory information flows from vestibular or proprioceptive senses have not previously been investigated. We wished to establish whether or not the duration of bias adjustments is specific to sensory modality.
Is bias duration influenced by sensory abundance?
The mean bias duration in our pendulum balancing experiment was ∼400 ms. It is possible voluntarily to make similar-sized repetitive movements of much shorter duration than this, so why is the average duration so long? One possible answer is that it is set by a perceptual threshold. An appropriate bias adjustment cannot be made until the nervous system can accurately judge the actual movement of the inverted pendulum. If there is a difference between the perceived and actual motion of the inverted pendulum (for example, because the actual motion is too small to generate an accurate perception) the nervous system cannot generate a suitable response. If this type of process dictates the duration of adjustments, then increasing sensory sensitivity should enable an appropriate response to be made more promptly. The latency of the response will decrease as the confidence of the nervous system about the behaviour of the inverted pendulum increases, thus reducing the average duration of the bias adjustments. One common way to increase sensitivity and reduce dead-band is to add more information. Ensemble averaging of sensory information within a modality (for example from muscle spindles (Prochazka, 1996) or cochlear fibres (Rose et al. 1971)) or spatial averaging from multiple sensory modalities (Wolpert et al. 1995), are both techniques used by the nervous system to enhance sensitivity. In contrast to temporal averaging, where a stimulus is integrated with respect to time, they do not intrinsically require longer to perform. The problem faced by the subject balancing the inverted pendulum with solely visual information is that of discriminating the tiny sway signal from irrelevant variation in sensory input (noise). By contrast, when self-balancing such as in quiet standing, additional sensory data are normally available. A body position or velocity signal that cannot be unambiguously discriminated from noise by a standing subject using vision alone may be detected by synergistic activity in proprioceptive and vestibular channels. In the present investigation, we have studied the effect on bias duration of systematically changing the amount of sensory information available to the subjects. Our second aim was to determine whether the rate of adjustment is contingent on the number of sources of sensory data available, or whether it has some other, more intrinsic, limitation.
Methods
Subjects
The experiments were performed on 10 healthy subjects; five were male. Their mean age was 23.2 years (s.d., 6.1). Permission was obtained from the local ethics committee and the subjects gave their written informed consent for these simple non-invasive experiments which conformed to the Declaration of Helsinki.
The inverted pendulum
The large inverted pendulum (Fig. 1) consisted of a mass of approximately 60 kg at a height of approximately 1 m on a solid steel pole of 25 mm diameter. It was pivoted on ball races which permitted movement in only one axis. Stops restricted motion to 0.5–8.5 deg from the vertical. The natural tendency of the inverted pendulum was for it to fall backwards until it contacted the 8.5 deg stop. As the angular range is small, the force/angle relationship is almost perfectly linear and it is common to call this the load stiffness (mgh; where m is mass, g is gravitational constant and h is height of centre of mass)). Measured at the point of attachment of the spring, mgh of the pendulum was 1.09 N mm−1. Converting this to angular measure, it was 12.22 Nm deg−1. This approximated to the body characteristic of a man and it had a similar inertia. The same inverted pendulum was used in all the main experiments because it allowed direct comparisons to be made between subjects who controlled an identical load.
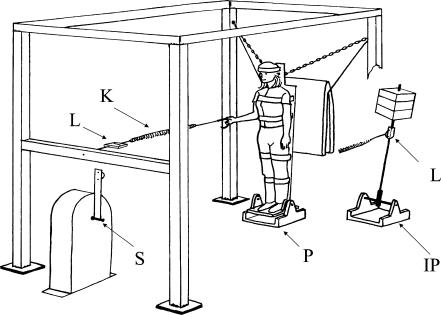
Figure 1. The apparatus.
Only the main components have been labelled. The substantial steel frame acted to anchor all the components. The subject stood on a platform (P) supported on ball bearings at ankle height. The platform was rigidly attached to a plywood backboard to which the subject was tied by Velcro straps at knee, thigh, waist, shoulder and head height. Forward collapse was prevented by the safely chains. The subject is shown in the forward position (approximately upright); the chains are tight. Rearward fall was limited by a mattress which the backboard contacted at approximately 10 deg tilt. In hand balancing, the subject could balance between these limits by pulling on the handle which was attached through the spring (K) and in-line load cell (L) to the rigid frame. In motor balancing, a small hand held joystick was operated to controll the position of the servomotor (S). The output of this device was then coupled through the spring and in-line load cell to a linkage attached to the backboard at approximately waist height (not shown). In inverted pendulum balancing the artificial load (IP) was substituted for the subject. The necessary force to balance it was exerted via the spring by the subject (who now stood in a position close to the servomotor and was supported by the frame) either manually in hand balancing or by the servomotor in motor balancing. In both situations, force was again measured by the in-line load cell (L). Visual registration of body or pendulum angle could be enhanced by a cathode ray tube (CRT) display mounted 1 m in front of the subject.
Pendulum balancing
The inverted pendulum was controlled by a steel extension spring attached to a point on the pole 0.8 m above the axis. Different numbers of identical springs (5.5 N mm−1, Spring Master, UK T32090) were used in all parts of the experiments. For inverted pendulum balancing, six springs were used in series to give a linear stiffness (K) of 0.92 N mm−1. Thus the ratio of K to mgh was 84%; the spring can produce only 84% of the force necessary to stabilize the pendulum. This figure was chosen as it represents a compromise between two recently published values of 64% and 91% for the relative stiffness (intrinsic ankle stiffness/mgh × 100) in human standing (Loram & Lakie, 2002; Casadio et al. 2005). At the point of attachment, a very stiff load cell (K25 Inscale Technology Ltd, UK) measured the force that was exerted on the pendulum through the spring.
Body balancing
Subjects were attached by secure Velcro strapping to a lightweight plywood back support which was rigidly attached to a footplate on which they stood (Fig. 1). The axis of rotation of the whole assembly passed through the ankle joint. Consequently, the subjects were entirely unstable and unable to save themselves and, without intervention, would have crashed to the floor either forwards or backwards. These indignities were prevented by steel chains which did not allow the back support and attached subject to move forward beyond the vertical position. Rearward collapse was allowed, but at approximately 10 deg the backboard contacted thick foam padding attached to the wall and this arrested the fall. There was therefore a zone between approximately vertical and 8 deg of backward inclination where the subject could attempt to maintain balance. The decision to give the body a natural tendency to fall backwards instead of forwards as in ‘real’ standing was made (1) so that the behaviour of the inverted pendulum and the body were the same and (2) for experimental convenience. Because the subject was securely splinted to the backboard and because the footplate was rigidly fixed at right angles with respect to the backboard there was no segmental movement or ankle rotation as the subject toppled. In order to balance themselves, subjects generated a force through a spring. For each subject, mgh was calculated by weighing the subjects and by calculating the position of their centre of mass (CoM) on a horizontal board. The stiffness of the spring was set as closely as possible to 84% of the subject's own mgh, but because this was done by adding or subtracting springs only a limited number of discrete values could be achieved (Table 1).
Table 1.
Subject characteristics and relative spring stiffness
| ID | mgh (N m deg−1) | Desired spring stiffness (N m deg−1) | Actual spring stiffness (N m deg−1) | Actual relative stiffness (K/mgh)% |
|---|---|---|---|---|
| RH | 9.3 | 7.81 | 8.0 | 86.0 |
| GW | 9.2 | 7.73 | 8.0 | 86.9 |
| WG | 11.3 | 9.50 | 9.9 | 88.4 |
| HW | 11.8 | 9.92 | 9.9 | 87.5 |
| NW | 12.0 | 10.10 | 11.0 | 91.6 |
| IL | 11.8 | 9.92 | 9.9 | 83.8 |
| RF | 10.6 | 8.89 | 8.7 | 82.2 |
| RG | 9.2 | 7.72 | 7.6 | 82.7 |
| HT | 10.1 | 8.47 | 8.7 | 86.3 |
| VS | 11.1 | 9.3 | 9.9 | 89.2 |
Mean relative stiffness (all subjects) is 86.5.
The control of the spring length (bias)
Subjects controlled balance in two ways. In hand balancing, the subject directly pulled on the spring by a handle, thus the subject had knowledge of the force being exerted. When hand balancing the inverted pendulum, the spring was connected to the pole through the load cell as described above. The subjects stood upright with the body braced against the steel framework of the apparatus. When hand balancing themselves the load cell was mounted on a rigid frame and the spring was connected to it. In both situations, a flag sprouting from the laboratory floor was used to show the subject the correct height at which the handle was to be held (moment arm of 1.0 m). In order to eliminate knowledge of force in the spring a servo system was employed (motor balancing). In motor balancing, the subject operated a hand-held contactless single axis joystick (HFX Magnetic, CH Products Ltd, USA). The joystick had its restoring spring removed and required little force to operate it (< 0.1 N). The joystick was used to control a powerful geared motor (G19M4, Printed Motors Ltd, UK) configured to act as a position servo. The position servo was attached to the spring. The subject therefore on the spring indirectly using the joystick to control the motor. Thus the subject ‘knew’ only the bias length and could not know the force that was being exerted. The spring was attached to the inverted pendulum by the load cell. The controlling subject stood upright in the same way as when hand balancing the inverted pendulum. When the motor was used to balance the human subject the spring was attached through the load cell to a chain sling which was attached to the backboard at a height of 1.0 m above the pivot.
Thus, in summary, subjects were able to attempt to balance the pendulum or their own body by making dynamic adjustments to the length of a spring that was nominally 84% of the stiffness required for minimum passive stability. The length adjustments were either made by hand (in which case subjects had some indirect knowledge of pendulum or body position because of the amount of force that they were exerting) or by a servomotor which they controlled. Subjects were completely deprived of force information when they controlled the servomotor.
Sources of sensory information in the experiments
Vision
In our previous published report (Lakie et al. 2003), the visual sensory information was provided in the form of an oscilloscope trace that indicated pendulum angle. At the time we noted that allowing the subjects to view the pendulum directly did not make an obvious difference to their bias adjustments. In the present experiments we formally compared direct visualization with more specific position information from a large screen oscilloscope. Body or inverted pendulum angle was displayed to the subject as a continuous horizontal line (timebase, 1 cm ms−1) on a short persistence (50 ms) CRT screen. The screen was at a distance of approximately 1 m from the head and in line with the eyes. A vertical displacement of the line by 2 cm corresponded to 1 deg of rotation of body or pendulum. The information thus provided was more explicit than direct vision of the pendulum or room but the movement was not amplified so the visual gain was not enhanced. We refer to normal direct vision (eyes open; EO) as Vision and CRT enhanced vision as Vision (+). Experiments were carried out where vision was denied (eyes closed; EC).
Proprioception
In hand balancing both the inverted pendulum and the body the only proprioceptive sensation is that which can be registered through the hand and arm because there is no signal corresponding to ankle rotation. For reasons mentioned above, positional information from the hand is ambiguously related to body or pendulum angle. However, as the inverted pendulum angle increases, the force will increase. Accordingly, knowledge of the force that the hand is exerting may provide useful additional information to the subject regarding inverted pendulum or body position and acceleration. This is obviously a much more limited form of proprioceptive information than that normally available from the ankles and feet, accordingly we refer to this as Proprioception (−). In the present experiment, we could remove completely this minor source of proprioceptive information by using the motor to balance.
Vestibule
In standing, a further source of sensory information is the vestibule. In our published inverted pendulum experiments the subject was stationary and vestibular information was not available to them. In the present experiment, we investigated the effect of adding vestibular information by allowing the subjects manually to balance themselves instead of the inverted pendulum (Vestibule).
Summary of experimental conditions
We were able to limit the sources of sensory information. For example, in hand balancing themselves, subjects would have available Vision, Proprioception (−) and Vestibule. Visual information could be removed by closing the eyes (EC). Proprioception (−) could be removed by motor balancing. Vestibule was removed when subjects balanced the inverted pendulum. In Table 2 we have ranked the conditions from A to I in order of increasing sensory impoverishment. The gradations where there is more than a single difference between conditions are debatable (e.g. is H better than I?). However, the ranking is only for convenience in presentation of the results.
Table 2.
Experimental conditions for each balancing task ranked from most available sensory information (A) to least available sensory information (I).
| KEY | Condition | Senses |
|---|---|---|
| A | Body Hand EO | Vision, Vestibule, Proprioception |
| Body Hand EO | (−) | |
| B | Body Motor CRT | Vision (+), Vestibule |
| C | Body Motor EO | Vision, Vestibule |
| D | Pend Hand EO | Vision, Proprioception (−) |
| E | Pend Motor CRT | Vision (+) |
| F | Pend Motor EO | Vision |
| G | Body Hand EC | Vestibule, Proprioception (−) |
| H | Body Motor EC | Vestibule |
| I | Pend Hand EC | Proprioception (−) |
There is also a condition of Pend Motor EC. In this condition there is no sensory information at all that can be used to balance the inverted pendulum. Predictably, no subject was able to balance the pendulum in this situation.
Instructions to subjects
Subjects carried out the tasks in random order. They were asked to balance the inverted pendulum or themselves for a 30 s recording period as close as possible to 3 deg of backward inclination. If the angle exceeded 8 deg, the trial was terminated. The aim was to achieve three 30 s recordings. Subjects were allowed to practice to the point where it seemed unlikely that they would make further rapid improvement. In all cases, recording was not started until the subject had comfortably balanced themselves or the pendulum. If the 30 s target period was not attained, the session was repeated until it was achieved or until it became clear that it would be impossible. The three longest trials were used for analysis and the mean trial length achieved was calculated. The experiments were conducted in a quiet laboratory. Audible cues can provide information about body movement. To minimize this, subjects wore ear defenders.
Signals and analysis
Inverted pendulum angle was measured by a Hall effect precision potentiometer attached to its axle (resolution, 0.01 deg). Body angle was measured by an infra-red reflective rangefinder (HT66MGV80, Wenglor Sensoric, Germany) which was aimed at a target attached to the subject's backboard (resolution, 0.01 deg). Bias (movement of the hand or motor) was computed from the force record from the load cell. The length of the spring was directly proportional to the force at all times. The computed length was then summed with pendulum or body angle to provide a signal of hand position or motor position.
A sway is defined as a unidirectional movement of the body or inverted pendulum between turning points. A bias adjustment is defined as a unidirectional lengthening or shortening of the spring produced by hand or motor. The mean durations and sizes of sway and bias adjustment were calculated using frequency analysis as previously fully described (Loram et al. 2005b). Briefly, the mean frequency of the power spectrum of sway velocity from 0 to 3 Hz was calculated and converted to the mean period (duration of a unidirectional sway). Mean bias adjustment duration was calculated in an identical way using the bias velocity power spectrum. The mean size of bias adjustments was calculated by multiplying their mean duration (as determined above) by the mean speed (modulus of velocity) of the bias adjustment. Sway size was measured as the standard deviation of body (or inverted pendulum) angle for each trial (s.d. A). Statistical analysis of the effect on sway size and bias adjustment durations when one or two sensory conditions were added was by ANOVA or Manova as appropriate.
It is important to note that there is potential confusion concerning the number of movements per second and their frequency. In our experiments a single bias adjustment had a duration of about 0.4 s. Two such alternating movements would make up a complete cycle (forward and back); the number of movements would be 2.5 s−1 but the frequency would be 1.25 Hz. This is similar to the two submovements per second commonly reported in the visuomanual tracking literature. To avoid confusion, we use bias duration rather than bias frequency as a measure of the rate of the adjustments.
Results
Typical subject different sensory conditions
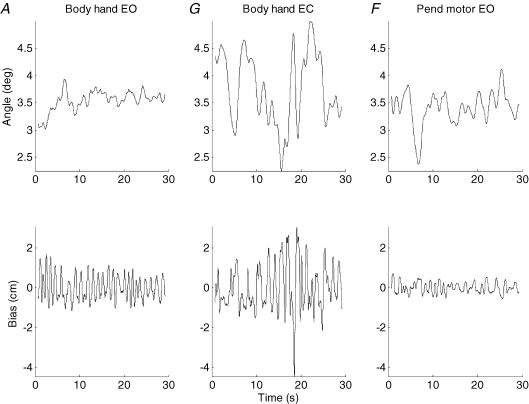
Figure 2 shows the movement of the body (or inverted pendulum) and the associated bias adjustments (hand or motor movements) that occurred during a 30 s recording session in three conditions in a typical subject. The body or inverted pendulum both sway irregularly with reversals of direction occurring at unpredictable intervals. In situations where there is limited sensory information the size of the sway is much bigger than where there are more sources of sensory information. The sway is smallest when all sensory modalities are available (condition A, Body Hand EO, Vision, Vestibule, Proprioception (−)). The task can be performed without visual information using the vestibular and proprioceptive senses but the sway size is very noticeably increased (Condition G, Body Hand EC, Vestibule, Proprioception (−)). Sway size is intermediate when only vision is available (Condition F, Pend Motor EO, Vision). The bias adjustments are also shown. There are clearly more reversals of bias direction than there are of body or inverted pendulum direction.
Figure 2. Typical subject in different sensory conditions.
Movement of the body (or inverted pendulum) is shown with active adjustments (bias) of the hand (or motor). The left pair of traces (condition A) shows all the sensory modalities in play. The body is balanced by hand with the eyes open so the subject has Proprioceptive (−), Visual and Vestibular information. In the middle pair of traces (condition G) only Vestibular and Proprioceptive (−) information are available because the eyes are closed. In the right hand pair of traces (condition F) only Visual information is available because the subject is stationary and the force is provided by the servomotor.
Our subjects found the tasks where sensory information was abundant easy to perform and they could always sustain balance for the target 30 s. With a few repeated attempts, all subjects were able to sustain their balance in condition G (Vestibule, Proprioception (−)) for the full 30 s period. However, with even greater sensory restriction (condition H, Vestibule) balance could not be sustained for 30 s (18 ± 5 s; mean ± s.d.). When subjects balanced the inverted pendulum by hand with no visual information (condition I, Proprioception (−)) the success time with many repeated efforts was only 10 ± 6 s; (mean ± s.d.). Accordingly, the subjects could only sustain balance with difficulty, a lot of sway and for a limited period in these latter two conditions. As we have necessarily recorded the subjects ‘best efforts’, the sway size at the larger end of the scale where the subjects experienced difficulty is likely to be atypically small.
Sway duration is longer than bias duration. Both change little with altered sensation
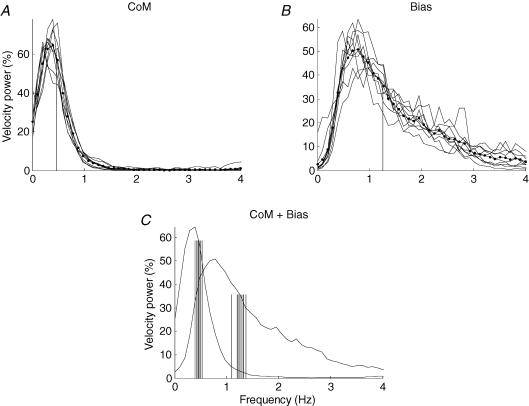
Figure 3 shows the power spectra of the velocity of movement of the body and inverted pendulum. The velocity power spectrum was determined for each trial. The spectra were grouped into the different experimental conditions and subsequently averaged across subjects; their size was normalized. Figure 3A shows the resulting power spectra for the load (body or pendulum) for each of the nine experimental conditions. Figure 3B shows the corresponding power spectra for bias adjustments. These parts of this figure show that the averaged spectra are remarkably similar for each experimental condition. The frequency of the peak and the bandwidth are similar and do not depend in any obvious way on the load (pendulum or body) or the amount of sensory information. However, the spectrum of the load movement is always very different from the spectrum of the bias adjustments. The difference between Fig. 3A and Fig. 3B is summarized in Fig. 3C where the power spectra have themselves been averaged to produce overall averages of the power spectra of the velocity of the bias movements and the velocity of the load movements. It is clear that the load and the controller behave in very different ways. The pendulum and body sway at frequencies that are predominantly below 1 Hz and the controller operates at a frequency that is predominantly above 1 Hz. The mean power frequency has been calculated to quantify these spectra. This is the value of frequency that divides the spectrum into two halves each containing equal power. In Fig. 3C the mean power frequency for each condition is indicated by a dashed line. They are quite similar in each sensory condition. These values of mean power frequency have been converted into half periods (duration of unidirectional movements) in subsequent analysis.
Figure 3. Frequency of sway and bias.
Each curve is a velocity power spectrum of the signal for load movement (CoM) and bias adjustment velocity (Bias) in one sensory condition. There are nine sensory conditions so there are nine curves. For each sensory condition all the trials by every subject have been averaged together to create a single curve. There are three trials and 10 subjects so n = 30 for each curve. A, the normalized load velocity spectra; B, the normalized bias adjustment velocity spectra. The striking finding is that, regardless of the sensory modalities in use, all the load velocity power spectra are very similar and all the bias velocity power spectra are also very similar. Consequently we have not attempted to demarcate the different conditions in A and B. For all sensory conditions, the dashed lines show the mean load frequency and the mean bias frequency. C, the average of all the load spectra in A and all the bias spectra in B are plotted together; the dashed lines indicate the mean frequency of the power spectrum for each sensory condition. For each trial the mean frequency was used to calculate the related duration.
Bias duration is not dependent on sensory modality
Table 3 shows bias duration in the three main conditions where only a single sensory modality was available. F (Vision), H (Vestibule) and I (Proprioception (−)). The bias durations were not significantly different in the different conditions (P = 0.06, n = 78 one way ANOVA).
Table 3.
Bias duration in the three main sensory conditions
| Condition | Bias duration (s) | 95% confidence interval (s) |
|---|---|---|
| F (Vision) | 0.428 | 0.031 |
| H (Vestibule) | 0.412 | 0.027 |
| I (Proprioception–) | 0.479 | 0.063 |
Reduction in bias duration and sway size as sensory channels are added
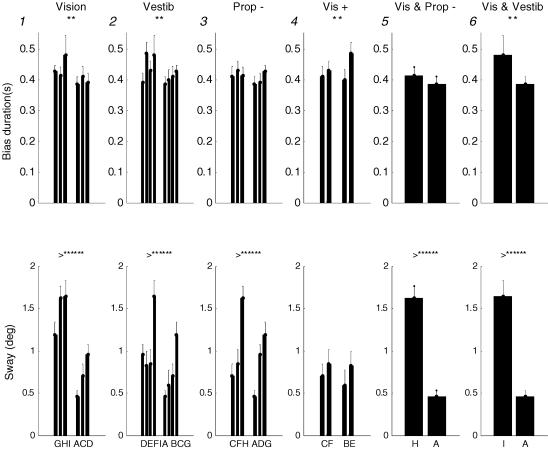
Figure 4 shows the effect on bias duration and sway size of adding one and two sensory modalities. For example in panel 1 the effect of the addition of Vision is shown. The three left hand bars (GHI) are all the EC conditions and the right hand bars (ACD) are the exactly corresponding EO conditions. In panel 2 the comparison is between pendulum balancing (DEFI) and body balancing (ABCG). Thus the additional sense is Vestibule. In panel 3 the comparison is between motor (CFH) and hand balancing (ADG); the difference is Proprioception (−). Panel 4 shows the effect of balancing using normal vision (CF) or enhanced vision (BE) so the additional sense is Vision (+). In panel 5 the effect of the simultaneous addition of two senses Vision and Proprioception (−) is shown. In Panel 6 the effect of the simultaneous addition of two senses Vision and Vestibule is shown. The addition of one or two different sensory modalities always makes a large and very highly significant reduction to mean sway size (bottom row). However, simple enhancement of vision (Vision (+)) does not significantly reduce sway size. Unlike sway size, the effect on bias duration (top row) is much less dramatic. In four cases the effect of the addition of a sense or senses is to reduce bias duration significantly and in two cases no significant difference results. However, even where the differences are unlikely to have arisen by chance, they are very small. The mean bias duration is always close to 400 ms.
Figure 4. Effect of adding sensory modalities.
The effects on mean sway size (measured as s.d.) and mean bias duration of the addition of one or two senses are shown. The upper row is bias duration and the lower row is sway size. In panels 1–4, pairs of conditions that are different by one sensory modality are compared and in panels 5 and 6, pairs of conditions that are different by two sensory modalities are compared. The enhanced condition is the right hand set of bars in each panel. Thus, in panel 1 the three right hand bars are different from the three left hand bars only in the respect that Vision is available to the subjects. Panel 2 shows the effect of adding Vestibular information, panel 3 shows the effect of adding Proprioception (−) information and panel 4 shows the effect of enhancing normal vision with the CRT (Vision (+)). Panel 5 shows the effect of the simultaneous addition of Vision and Proprioception (−), and panel 6 the effect of the simultaneous addition of Vision and Vestibule.
Correlation of sway and bias duration with sway size and correspondence with sensory ranking
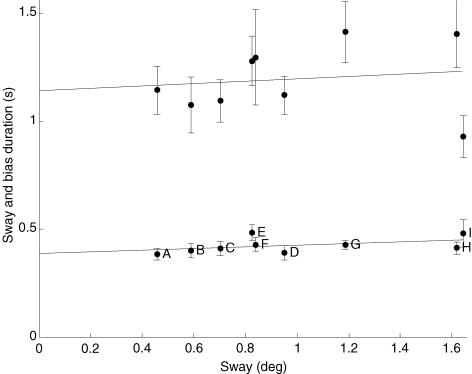
We have no direct way of quantifying the amount of sensory information available. However, from the results shown in Fig. 4 where the removal of a sensory modality or modalities produces a clear and highly significant increase in mean sway size, it seems reasonable to assume that the mean sway size does reflect the degree of sensory deprivation that the subjects are experiencing in that condition. This means that it is possible to use sway size as a substitute measure of sensory deprivation. Accordingly, this provides a means of testing the strength of the correlation between sensory paucity (using sway size as its proxy measure) and bias duration. Furthermore, if the sensory ranking that we proposed in Table 2 is accurate, the sway size should increase progressively from condition A to B to C and so on. These data are summarized in Fig. 5. The mean sway size changes from ∼0.4 to ∼1.6 deg as sensory information is restricted. It is clear that the increase in sway size agrees very satisfactorily with our prior ranking of sensory deprivation (Table 2). The only disparity is that condition D is out of order with conditions E and F. The mean bias durations range from 0.38 to 0.51 s. The mean is 0.44 s for all conditions. The bias duration does appear to increase with sway size; however, this tendency is slight and does not reach statistical significance (r2 = 0.20, P = 0.226). The most striking feature is that the mean bias duration appears to have a minimum value of approximately 0.386 s (intercept value with y-axis). The figure also shows the sway duration of the body or inverted pendulum in the different conditions. The sway duration varies from 0.89 to 1.38 s and also has a slight upward trend with sway size, but it also fails to reach significance (r2 = 0.02, P = 0.712). The sway duration is always approximately 2.5 times longer than the bias duration. Thus sensory deprivation produces a greatly increased sway size but bias duration and sway duration do not vary consistently enough for the increase to be significant. We have not shown the mean bias size which was also calculated for every trial. The size of the bias adjustments scaled in a way that was almost identical to sway size, increasing as sensory information was reduced (r2 = 0.80, P < 0.001).
Figure 5. Sway duration and bias duration plotted against sway size.
Mean values are shown for 10 subjects in three trials. The bars indicate 95% confidence intervals. The gradient of the linear regression line for bias duration is y = 0.038x + 0.386; r2 = 0.20, P = 0.226. Sway duration is also shown. The gradient of the linear regression line for sway duration is y = 0.0554x + 1.14, r2 = 0.02, P = 0.712. The letters correspond to the sensory conditions summarized in Table 2.
Discussion
Manual balancing has clear similarities to standing balance
Although our task involved manual balancing of the subject's own body or a mechanically equivalent inverted pendulum, there were striking similarities to standing balance. The mean sway size (s.d. A) for conditions A–C when most sensory input was available was ∼0.5 deg (Figs 4 and 5); these values are just slightly larger than the values reported for antero-posterior sway observed in quietly standing subjects. Jeka et al. (2004) reported a range of ∼0.2 to ∼0.5 deg for the s.d. of CoM angle in eight normally standing subjects. Fitzpatrick et al. (1994) showed that splinting standing subjects (depriving them of intersegmental movement, as in our experiments) increased mean sway size by ∼50% which may be an explanation for the slightly larger sway size we recorded. The threshold for conscious awareness of sway at the ankle is ∼0.1 deg (angle) and 0.1 deg s−1 (angular velocity) (Fitzpatrick & McCloskey, 1994) so, like standing, manual balancing is controlled at values near threshold. As in standing (Woollacott et al. 1986; Collins & De Luca, 1995; Peterka, 2002; Jeka et al. 2004), the removal of sensory information increased mean sway size; subjects were able to sustain balance in these conditions for a shorter average time and they experienced greater difficulty. The agreement with the prior ranking (based on knowledge of the effect of reduced sensation on sway in standing subjects) confirms the expected relationship (Fig. 5). Diminished accuracy of bias adjustments is a plausible explanation for the large increase in mean sway size that is observed as sensory information is restricted (Figs 4 and 5).
Mean sway was very large when subjects balanced with Vestibule alone probably because the vestibule has a sensitivity which is considerably less than the other senses (Fitzpatrick & McCloskey, 1994; Nagata et al. 2001; Peterka, 2002; Cenciarini & Peterka, 2006). Mean sway was also large and the task was difficult when subjects manually balanced with Proprioception (−) alone. This is different from balancing with the feet because subjects can balance indefinitely with small sway using purely proprioceptive ankle information (Fitzpatrick et al. 1994; Loram et al. 2001). However, in manual balancing the sensory information available (Proprioception (−)) is much more limited (force only, not position) and does not arise from the familiar site (ankle and calf muscles and soles of the feet). Our experiments show that balance could be sustained indefinitely with only moderate sway size using exclusively Visual or Visual (+) sensation (conditions E and F). Vision is sometimes regarded as contributing to postural stabilization only at low frequencies (Lestienne et al. 1977; Nashner & Berthoz, 1978; Diener et al. 1982). Nagata et al. (2001) have stated that it is impossible to maintain stable standing by solely visual means. Using a virtual balancing task, Fukuoka et al. (2001) deduced that the visual feedback system does not produce sufficient phase advance to allow a subject to maintain upright balance. These conclusions are clearly at variance with the present results. The disparity might be explained by the use by Fukuoka et al. of a sway referenced platform to eliminate proprioceptive information from the ankles. It is not generally appreciated that sway referencing also removes the stabilizing effect of the intrinsic ankle stiffness. Recent estimates for intrinsic ankle stiffness suggest a value of 64%–91% mgh (Loram & Lakie, 2002; Casadio et al. 2005; Loram et al. 2005a). Preventing ankle rotation removes sway-induced stretch of the spring, thus eliminating the automatic partial gravitational compensation that the intrinsic ankle stiffness normally contributes. All the balancing torque must then be neurally controlled. This may represent an unrealistically demanding task for the nervous system to accomplish. The sway duration in our experiments was remarkably constant. The mean value was 1.2 s for a unidirectional sway and it did not change significantly over an approximately four-fold range of mean sway sizes (Fig. 5). Very similar values for sway duration have been reported in standing subjects (Peterka, 2002).
Sluggish bias adjustments are not unique to visual control
The mean bias duration was not different when balance was controlled exclusively by Vision, Vestibule or Proprioception (−) (Table 3 and Fig. 5). This is an interesting new finding. It has been suggested that intermittent hand movements in a visual pursuit task may be caused by the inevitable long delays and the error dead-zone associated with processing of visual sensory data (Wolpert et al. 1992; Miall et al. 1993).
When our subjects balanced the inverted pendulum using exclusively visual information, the requirements of the task had obvious similarities to a visual tracking task. (In fact, it is a special example of an error-compensation task where subjects have available only a visual error signal which they must attempt to offset (Costello, 1968; Poulton, 1974). Unusually, the error is not externally applied but is a consequence of the instability of the load and the subject's imperfect control efforts).
Consequently, intermittent hand movements in this situation could also be due to the slowness of visual processing. However, if this were so then intermittency of the same slow time course would be unlikely to occur when manual balancing was carried out under vestibular or proprioceptive control because both these senses are associated with less neural processing and faster responses than vision. Our new finding that the mean bias duration is effectively the same when controlled by visual, vestibular or proprioceptive information argues strongly against the view that intermittency in this compensation task is produced by factors unique to vision. In fact, intermittent arm movements have recently been shown to occur in the absence of visual feedback (Doeringer & Hogan, 1998) and our observations strongly suggest that intermittency may be a fundamental characteristic of accurate motor outputs executed to meet an unpredictably changing demand. The experimental protocol we have used provides a useful new method for studying this issue. The method overcomes the thorny problem of supplying a non-visual target waveform that the subject can pursue. In our experiment, that target is sway minimization of the unstable load which can only be achieved by a precise pattern of hand movements. The information to inform action can be selectively supplied by different sensory sources.
We have chosen to convert the mean frequency of the spectrum into the equivalent mean time taken for a unidirectional hand movement (bias duration). This allows direct comparison with other such durations obtained in manual control tasks (see below). There are other ways of interpreting and quantitatively describing a frequency spectrum. We chose to use the mean frequency of the spectrum because this involves no particular assumption about the mechanism underlying the spectrum as it gives weight to all its components. That is, no particular central frequency of operation is inferred. Had we alternatively used the peak (modal) frequencies in Fig. 3B to calculate the bias duration rather than the mean frequency this would perhaps be a superior measure of a noisy process with a characteristic frequency. This measure gives a lower frequency and correspondingly longer bias durations ranging from 0.45 to 0.7 s. These modal durations are also not significantly different with visual, vestibular or proprioceptive control. It is unclear from the data whether the hand movements are a consequence of a continuously acting control process with a characteristic but noisy frequency of operation or whether the process operates irregularly with discrete outputs with a range of durations. Our method gives us a useful way of counting and measuring the duration of hand movements that are used to preserve balance under different sensory conditions but it does not reveal the underlying mechanism.
Increased sensory abundance only slightly reduces mean bias duration
A large component of the bias adjustment duration is independent of the amount of sensory information available. Thus, pairwise comparisons (Fig. 4) show that whereas the addition of a sense or senses does significantly reduce mean bias duration it does not fall below 380 ms, and Fig. 5 shows that notionally perfect control (zero sway size) might be associated with a mean bias duration of 386 ms. There are therefore two features to explain.
(1) Why does the addition of sensation decrease bias duration? It is well known that some of the time taken to react to a stimulus depends on its intensity (for example, vision (Cattel, 1886) or sound (Chocolle, 1940)). Rather than increasing the size of a stimulus, using additional channels to detect a signal may also provide a signal-to-noise detection advantage. A central problem in balancing is accurate and confident knowledge of motion of the load. The results suggest that a time advantage of approximately 50 ms may be bought by increasing sensory confidence by increasing the number of independent sensory channels. The way in which sensory information is integrated and combined is uncertain. One possibility is a systems approach where visual and vestibular information are integrated and weighted in an essentially linear manner to produce a combined self-motion signal (Peterka, 2002) or a ‘postural state vector’ (Morasso & Schieppati, 1999). Alternatively, McCollum et al. (1996) suggested that the nervous system may distribute monitoring and regulation functions laterally rather than hierarchically. They envisaged a system of weighted choices among several different sensory states. The relatively invariant bias duration in the present experiments is perhaps more compatible with the idea that the nervous system employs a single composite representation of body position rather than switching among several sensory states with different properties. The effect of increasing sensory abundance is very limited. This suggests that the working of the control process does not depend primarily on the rapid detection of motion. The implication is that there is a common feature downstream of the detection and integration stage which sets a minimum limit on the mean bias duration.
(2) Why does the mean bias duration not decrease below 380 ms? Three possibilities are outlined.
(i) The properties of the load. The relatively long time constant of human body and the equivalent inverted pendulum may allow the control process to operate at the sluggish rate that we observed. This raises the question of whether the duration of bias adjustments could be reduced if it became necessary to balance a load with a shorter time constant – for example as in attempting to balance a pencil on the palm of the hand (very difficult) rather than a long stick (easier). We have recently shown that the mean bias duration cannot be reduced when the time constant of the load is reduced to the point where balance becomes very difficult (Loram et al. 2006). The mean bias adjustment duration reflects the biology rather than the load mechanics.
(ii) The properties of the muscles. Each bias adjustment represents a muscle length change. Muscles (particularly postural muscles) are not particularly fast and this could place an upper limit on the frequency of control. However, it is possible to make small voluntary reciprocating hand movements with durations (∼80 ms) much faster than the bias adjustments. The bandwidth of reflex control of the slow postural muscles is up to at least 8 Hz (Evans et al. 1983; Rack et al. 1983). There was no difference in the present experiments when the pendulum was balanced by hand (requiring some effort from the arm musculature) or by motor (requiring minimal effort from the fingers). The size of the bias adjustments varies with sway size. With sensory restriction the mean size of the bias adjustments increases dramatically but the mean duration of bias adjustments does not change (Fig. 5) so the bias velocity must be increasing in proportion to bias size. There is no suggestion that bias velocity reaches a limit in these experiments. Taken together these observations suggest that the process is not limited by muscle characteristics.
(iii) The neural computation time. The results of our experiment are compatible with an intrinsic manual movement intermittency. Paired visual stimuli experiments (Vince, 1948; Pashler & Johnston, 1998), where the response to the second stimulus is not executed until the response to the first has been run off, suggest that intermittency results from central refractoriness (Craik, 1947) rather than a fixed transmission and sensory processing delay. This central bottleneck may be sensorimotor processing which cannot shape a new motor output until the previous one has been passed to the motor execution stage (Neilson et al. 1988). The effect of the bottleneck is to severely restrict bandwidth by limiting the minimum duration of individual motor outputs. There is evidence that repeated outputs at a low frequency characterize even supposedly static postural efforts. With stable loads and continuous isometric output most power is at low frequencies with a mode at about 1 Hz (Sutton & Sykes, 1967; Stephens & Taylor, 1974; Slifkin & Newell, 1999; Slifkin et al. 2000) this is usually regarded as an underlying control frequency (see also Doeringer & Hogan, 1998). We conclude that the neural controller can only operate at a low and relatively inflexible frequency. A central refractoriness is a plausible, but not exclusive, explanation for this.
Do these bias durations apply to standing balance?
Although our tasks were mechanically analogous to standing, the motive force was obviously different. Balance in quiet standing is accomplished by the calf muscles whereas in the present experiments it was accomplished by movements of the arm or hand with or without servo assistance. Moreover, there may be differences in the way the force is controlled. Standing balance is highly automated and generally unconscious, whereas in our experiments subjects needed at least some concentration on the task. There are well established quantitative differences in the central neural representation of the upper and lower limb and untrained people can do things with the hand that are impossible with the foot. It is possible that standing might have more low level control than manual balance and the control processes may therefore be faster giving this form of postural control a higher bandwidth. For example, there is evidence that vision may ‘drive’ postural responses with a possibly subcortical latency (Day & Brown, 2001; Saijo et al. 2005). It remains to be seen whether quiet standing is more akin to a series of fast responses to perturbations or to a process of slower internally generated adjustments. While our task is certainly not identical to standing, recent work using dynamic ultrasonography (Loram et al. 2005a,b) has revealed very clear similarities between the very small movements of the calf muscles in quietly standing subjects and the larger movements of the hand in manual balancing. The calf muscles exhibit similar bias adjustments to the hand. They are much smaller because the moment arm of the calf muscles in standing is only ∼5 cm and the moment arm in the balancing experiments is 80 cm. However, the similarity in the mean bias duration is very striking (385 ms standing; 400 ms balancing) (Lakie et al. 2003; Loram et al. 2005b) and these durations are very similar to the values reported here. These similarities have lead us to suggest that processes of a similar restricted bandwidth may be involved in controlling pendulum balancing and in standing (Loram et al. 2005b). This is a testable prediction; dynamic ultrasonography can be used to measure the mean bias duration in the calf muscles of standing subjects while sensory conditions are systematically varied. We have noted that bias duration is not different in subjects standing with and without vision (Loram et al. 2005b).
Conclusion
Sensory impoverishment when manually balancing an unstable inverted pendulum produces a large increase in mean sway size. The sluggish manual adjustments that are used to achieve balance have a long mean duration which is independent of sensory modality. In particular, they are not unique to visual control. When sensory modalities are combined there is a significant but very slight decrease in adjustment duration. Together, these findings suggest that the duration is dominated by a downstream process which is common to all these sensory modalities. We suggest that sensory information in manual balancing is used to inform a common central movement planning process which produces motor outputs at a low frequency. Sensory abundance may increase the accuracy but cannot increase the frequency of these adjustments. We predict that corresponding processes may be observed in the calf muscles of standing subjects.
Acknowledgments
We would like to thank the Leverhulme Trust for their support of I.D.L. through this project.
References
- Bekey G. The human operator as a sampled-data system. IRE Transactions on human factors in electronics. 1962:43–51. HFE-3. [Google Scholar]
- Brenner E, Smeets JBJ, de Lussanet MHE. Hitting moving targets – continuous control of the acceleration of the hand on the basis of the target's velocity. Exp Brain Res. 1998;122:467–474. doi: 10.1007/s002210050535. [DOI] [PubMed] [Google Scholar]
- Casadio M, Morasso PG, Sanguineti V. Direct measurement of ankle stiffness during quiet standing: implications for control modelling and clinical application. Gait Posture. 2005;21:410–424. doi: 10.1016/j.gaitpost.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Cattel J. The influence of the intensity of the stimulus on the length of the reaction time. Brain. 1886;9:512–514. [Google Scholar]
- Cenciarini M, Peterka RJ. Stimulus-dependent changes in the vestibular contribution to human postural control. J Neurophysiol. 2006;95:2733–2750. doi: 10.1152/jn.00856.2004. [DOI] [PubMed] [Google Scholar]
- Chocolle R. Variation in auditory reaction times as a function of intensity at different frequencies. Annee Psychol. 1940;41:65–124. [Google Scholar]
- Collins JJ, De Luca CJ. The effects of visual input on open-loop and closed-loop postural control mechanisms. Exp Brain Res. 1995;103:151–163. doi: 10.1007/BF00241972. [DOI] [PubMed] [Google Scholar]
- Costello RG. The surge model of the well-trained operator in simple manual control. IEEE Transactions on man-machine system. MMS-9. 1968;1:2–9. [Google Scholar]
- Craik K. Theory of the human operator in control systems. I. The operator as an engineering system. Br J Psychol. 1947;38:56–61. doi: 10.1111/j.2044-8295.1947.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Day BL, Brown P. Evidence for subcortical involvement in the visual control of human reaching. Brain. 2001;124:1832–1840. doi: 10.1093/brain/124.9.1832. [DOI] [PubMed] [Google Scholar]
- Diener HC, Dichgans J, Bruzek W, Selinka H. Stabilisation of human posture during induced oscillations of the body. Exp Brain Res. 1982;45:126–132. doi: 10.1007/BF00235771. [DOI] [PubMed] [Google Scholar]
- Doeringer JA, Hogan N. Intermittency in preplanned elbow movements persists in the absence of visual feedback. J Neurophysiol. 1998;80:1787–1799. doi: 10.1152/jn.1998.80.4.1787. [DOI] [PubMed] [Google Scholar]
- Evans CM, Fellows SJ, Rack PM, Ross HF, Walters DK. Response of the normal human ankle joint to imposed sinusoidal movements. J Physiol. 1983;344:483–502. doi: 10.1113/jphysiol.1983.sp014953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R, McCloskey DI. Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. J Physiol. 1994;478:173–186. doi: 10.1113/jphysiol.1994.sp020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R, Rogers DK, McCloskey DI. Stable human standing with lower-limb muscle afferents providing the only sensory input. J Physiol. 1994;480:395–403. doi: 10.1113/jphysiol.1994.sp020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka Y, Nagata T, Ishida A, Minamitani H. Characteristics of somatosensory feedback in postural control during standing. IEEE Trans Neural Syst Rehabil Eng. 2001;9:145–153. doi: 10.1109/7333.928574. [DOI] [PubMed] [Google Scholar]
- Jeka J, Kiemel T, Creath R, Horak F, Peterka R. Controlling human upright posture: velocity information is more accurate than position or acceleration. J Neurophysiol. 2004;92:2368–2379. doi: 10.1152/jn.00983.2003. [DOI] [PubMed] [Google Scholar]
- Lakie M, Caplan N, Loram ID. Human balancing of an inverted pendulum with a compliant linkage: neural control by anticipatory intermittent bias. J Physiol. 2003;551:357–370. doi: 10.1113/jphysiol.2002.036939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestienne F, Soechting JF, Berthoz A. Postural readjustment induced by linear motion of visual scenes. Exp Brain Res. 1977;45:363–384. doi: 10.1007/BF00235717. [DOI] [PubMed] [Google Scholar]
- Loram ID, Gawthrop PJ, Lakie M. The frequency of human, manual adjustments in balancing an inverted pendulum is constrained by intrinsic physiological factors. J Physiol. 2006;577:417–432. doi: 10.1113/jphysiol.2006.118786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Kelly S, Lakie M. Human balancing of an inverted pendulum: is sway size controlled by ankle impedance? J Physiol. 2001;532:879–891. doi: 10.1111/j.1469-7793.2001.0879e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Lakie M. Direct measurement of human ankle stiffness during quiet standing: the intrinsic mechanical stiffness is insufficient for stability. J Physiol. 2002;545:1041–1053. doi: 10.1113/jphysiol.2002.025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. Active, non-spring-like muscle movements in human postural sway: how might paradoxical changes in muscle length be produced? J Physiol. 2005a;564:281–293. doi: 10.1113/jphysiol.2004.073437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. Human postural sway results from frequent, ballistic bias impulses by soleus and gastrocnemius. J Physiol. 2005b;564:295–311. doi: 10.1113/jphysiol.2004.076307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum G, Shupert CL, Nashner LM. Organizing sensory information for postural control in altered sensory environments. J Theor Biol. 1996;180:257–270. doi: 10.1006/jtbi.1996.0101. [DOI] [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Stein JF. Intermittency in human manual tracking tasks. J Mot Behav. 1993;25:53–63. doi: 10.1080/00222895.1993.9941639. [DOI] [PubMed] [Google Scholar]
- Morasso PG, Schieppati M. Can muscle stiffness alone stabilize upright standing? J Neurophysiol. 1999;82:1622–1626. doi: 10.1152/jn.1999.82.3.1622. [DOI] [PubMed] [Google Scholar]
- Nagata T, Fukuoka Y, Ishida A, Minamitani H. Analysis of role of vision in human upright posture control. Engineering Med Biol Soc Proc 23rd Annu Int Conf IEEE. 2001;2:1155–1158. [Google Scholar]
- Nashner LM, Berthoz A. Visual contributions to rapid motor responses during postural control. Brain Res. 1978;150:403–407. doi: 10.1016/0006-8993(78)90291-3. [DOI] [PubMed] [Google Scholar]
- Neilson PD, Neilson MD, O'Dwyer NJ. Internal models and intermittency: a theoretical account of human tracking behavior. Biol Cybern. 1988;58:101–112. doi: 10.1007/BF00364156. [DOI] [PubMed] [Google Scholar]
- Pashler H, Johnston JC. Attentional limitations in dual-task performance. In: Pashler H, editor. Attention. UK: Psychology Press; 1998. [Google Scholar]
- Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. 2002;88:1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- Poulton EC. Tracking Skill and Manual Control. New York: Academic Press; 1974. [Google Scholar]
- Prochazka A. Proprioceptive feedback and movement regulation. In Handbook of Physiology. In: Rowell L, Sheperd JT, editors. Exercise: Regulation and Integration of Multiple Systems. New York: American Physiological Society; 1996. pp. 89–127. [Google Scholar]
- Rack PM, Ross HF, Thilmann AF, Walters DK. Reflex responses at the human ankle: the importance of tendon compliance. J Physiol. 1983;344:503–524. doi: 10.1113/jphysiol.1983.sp014954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DW, Liu XG, Miall RC. On-line feedback control of human visually guided slow ramp tracking: effects of spatial separation of visual cues. Neurosci Lett. 2003;338:209–212. doi: 10.1016/s0304-3940(02)01389-7. [DOI] [PubMed] [Google Scholar]
- Rose JE, Hind JE, Anderson DJ, Brugge TF. Some effects of stimulus intensity on the response of auditory nerve fibres in the squirrel monkey. J Neurophysiol. 1971;34:685–699. doi: 10.1152/jn.1971.34.4.685. [DOI] [PubMed] [Google Scholar]
- Saijo N, Murakami I, Nishida S, Gomi H. Large-field visual motion directly induces an involuntary rapid manual following response. J Neurosci 25. 2005;20:4941–4951. doi: 10.1523/JNEUROSCI.4143-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifkin A, Newell KM. Noise, information transmission and force variability. J Exp Psychol Hum Percept Perform. 25:837–851. doi: 10.1037//0096-1523.25.3.837. [DOI] [PubMed] [Google Scholar]
- Slifkin A, Vaillancourt D, Newell KM. Intermittency in the control of continuous force production. J Neurophysiol. 2000;84:1708–1718. doi: 10.1152/jn.2000.84.4.1708. [DOI] [PubMed] [Google Scholar]
- Stephens J, Taylor A. The effects of visual feedback on physiological muscle tremor. Electroencephalogr Clin Neurophysiol. 1974;36:457–476. doi: 10.1016/0013-4694(74)90202-8. [DOI] [PubMed] [Google Scholar]
- Sutton G, Sykes K. The effect of withdrawal of visual presentation of errors upon the frequency spectrum of tremor in a manual task. J Physiol. 1967;190:281–293. doi: 10.1113/jphysiol.1967.sp008208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince MA. The intermittency of control movements and the psychological refractory period. Br J Psychol. 1948;38:149–157. doi: 10.1111/j.2044-8295.1948.tb01150.x. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269:1880–1882. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Winter JL, Stein JF. Evidence for an error deadzone in compensatory tracking. J Mot Behav. 1992;24:299–308. doi: 10.1080/00222895.1992.9941626. [DOI] [PubMed] [Google Scholar]
- Woollacott M, Shumway-Cook A, Nashner LM. Aging and postural control: changes in sensory organisation and muscular coordination. Int J Aging Hum Dev. 1986;23:97–114. doi: 10.2190/VXN3-N3RT-54JB-X16X. [DOI] [PubMed] [Google Scholar]