Abstract
The transmission of light responses to retinal ganglion cells is regulated by inhibitory input from amacrine cells to bipolar cell (BC) synaptic terminals. GABAA and GABAC receptors in BC terminals mediate currents with different kinetics and are likely to have distinct functions in limiting BC output; however, the synaptic properties and localization of the receptors are currently poorly understood. By recording endogenous GABA receptor currents directly from BC terminals in goldfish retinal slices, I show that spontaneous GABA release activates rapid GABAA receptor miniature inhibitory postsynaptic currents (mIPSCs) (predominant decay time constant (τdecay), 1.0 ms) in addition to a tonic GABAC receptor current. The GABAC receptor antagonist (1,2,5,6-tetrahydropyridin-4-yl)methylphosphinic acid (TPMPA) has no effect on the amplitude or kinetics of the rapid GABAA mIPSCs. In addition, inhibition of the GAT-1 GABA transporter, which strongly regulates GABAC receptor currents in BC terminals, fails to reveal a GABAC component in the mIPSCs. These data suggest that GABAA and GABAC receptors are highly unlikely to be synaptically colocalized. Using non-stationary noise analysis of the mIPSCs, I estimate that GABAA receptors in BC terminals have a single-channel conductance (γ) of 17 pS and that an average of just seven receptors mediates a quantal event. From noise analysis of the tonic current, GABAC receptor γ is estimated to be 4 pS. Identified GABAC receptor mIPSCs exhibit a slow decay (τdecay, 54 ms) and are mediated by approximately 42 receptors. The distinct properties and localization of synaptic GABAA and GABAC receptors in BC terminals are likely to facilitate their specific roles in regulating the transmission of light responses in the retina.
Inhibition in the retina is predominantly mediated by two subtypes of ionotropic GABA receptor, GABAA and GABAC receptors, and by glycine receptors. GABAA receptors are present in most retinal cell types whereas GABAC receptors are predominantly localized to bipolar cell (BC) synaptic terminals (Enz et al. 1996; Koulen et al. 1997; Wassle et al. 1998). Here they function to limit BC output (Lukasiewicz & Werblin, 1994; Zhang & Slaughter, 1995; Shen & Slaughter, 2001), resulting in reduced activation of postsynaptic NMDA receptors (Matsui et al. 2001; Sagdullaev et al. 2006) and more transient ganglion cell light responses (Zhang et al. 1997; Dong & Werblin, 1998).
BC terminals receive GABAergic input from amacrine cells, which form both reciprocal and conventional synapses at the terminal (Dowling & Boycott, 1966; Dowling & Werblin, 1969). Activation of amacrine cell synapses evokes a response in BC terminals that comprises both a fast GABAA receptor component and a slow GABAC receptor component (Hartveit, 1999; Vigh & von Gersdorff, 2005; Eggers & Lukasiewicz, 2006). The differing time courses are likely to arise from intrinsic differences in receptor kinetics, as GABAA receptor currents evoked by exogenous GABA are much more transient than GABAC receptor currents (Qian & Dowling, 1995; Lukasiewicz & Shields, 1998; Shields et al. 2000; Du & Yang, 2000; Hull et al. 2006). In addition, GABAC receptors exhibit higher GABA affinity and a lower single-channel conductance (γ) than GABAA receptors (Feigenspan & Bormann, 1994; Qian & Dowling, 1995).
There is currently a lack of physiological evidence for the synaptic colocalization or segregation of GABAA and GABAC receptors in BC terminals. Immunolocalization studies in rat BCs suggest that the receptor subtypes are restricted to separate synaptic sites (Koulen et al. 1998), which would enable independent regulation of the transmission of light responses by GABAA and GABAC receptor pathways. In order to investigate the synaptic properties and functional localization of GABAA and GABAC receptors in BC terminals, I have analysed endogenous GABA receptor currents recorded directly from the synaptic terminals of BCs in goldfish retinal slices.
Methods
The experiments conformed with guidelines laid down by the animal welfare committee of Keele University. Retinal slices were prepared from goldfish (Carassius auratus; 8–14 cm) after 1 h dark-adaptation. Goldfish were killed by decapitation followed immediately by destruction of the brain and spinal cord. The eyeballs were removed and retinae dissected out and treated for 20 min with hyaluronidase to remove vitreous humor. Each retina was quartered, placed ganglion cell layer down on filter paper and kept until needed at 4°C in medium containing (mm): NaCl 127, KCl 2.5, MgCl2 1, CaCl2 1, Hepes 5 and glucose 12, pH adjusted to 7.45 with NaOH. Slices were cut at 250 μm intervals using a Narishige ST-20 slicer, transferred to the recording chamber and perfused (1 ml min−1) with medium containing (mm): NaCl 108, KCl 2.5, MgCl2 1, CaCl2 2.5, NaHCO3 24 and glucose 12, gassed with 95% O2–5% CO2, pH 7.4. Slice preparation and recordings were performed at room temperature (20–23°C), in daylight conditions. Drugs were bath applied in the perfusing medium. Picrotoxin, (1,2,5,6-tetrahydropyridin-4-yl) methylphosphinic acid (TPMPA), 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo-[f]quinoxaline-7-sulfonamide (NBQX) and nifedipine were obtained from Tocris; bicuculline, strychnine and all other chemicals and salts were obtained from Sigma-Aldrich.
Whole-cell recordings were obtained from isolated BC terminals in retinal slices as previously described (Palmer et al. 2003). This technique maximizes the recording resolution of terminal GABA receptor currents and eliminates currents arising from somatodendritic receptors. Patch pipettes (5–8 MΩ) were pulled from borosilicate glass and filled with solution containing (mm): CsCl 115, Hepes 25, TEA-Cl 10, Mg-ATP 3, Na-GTP 0.5 and EGTA 0.5; pH 7.2. CsCl-based intracellular solution was used to increase the driving force through GABA receptors at a holding potential of −60 mV. The majority of recordings (32/39) were made in the presence of the AMPA receptor antagonist NBQX (5 μm) to reduce amacrine cell activity; however, no significant differences in GABA receptor properties were observed between recordings with and without NBQX.
Data acquisition was controlled by Heka Patchmaster software and signals were recorded via a Heka EPC-10 patch-clamp amplifier. Off-line analysis was performed using Wavemetrics IgorPro software. Miniature inhibitory postsynaptic currents (mIPSCs) were identified by rate of rise, aligned for averaging and analysed using IgorPro macros kindly provided by Dr H. Taschenberger. The peak amplitude of average mIPSCs was dependent on the mIPSC detection threshold, which could be lower in low-noise recordings. For comparison between different pharmacological conditions, the threshold was kept constant.
To estimate the frequency of GABAC mIPSCs underlying the tonic current, the plateau current evoked by summated mIPSC waveforms (instantaneous rise followed by exponential decay; amplitude, −10 pA; decay time constant (τdecay), 54 ms) at frequencies of between 1 and 50 Hz was computed using Matlab software. The relationship between mean plateau current and frequency was linear and was approximately described by: mean current = frequency × amplitude × τdecay.
Peak-scaled non-stationary noise analysis of GABAA mIPSCs was performed as previously described for synaptic currents (Traynelis et al. 1993; De Koninck & Mody, 1994). Baseline-subtracted mIPSCs exhibiting a fast rise time and no additional spontaneous activity were averaged, the mean mIPSC was peak-scaled to individual mIPSCs and the variance of the decay around the mean was measured. The average binned variance (σ2) was plotted against mean mIPSC amplitude (I) and fitted with:
to give estimates of single-channel current (i), the average number of channels open at the peak of the current (N) and baseline variance (b). For noise analysis of the GABAC tonic current, the variance of current traces (0.2–0.5 s duration) recorded during the current potentiation by the GAT-1 inhibitor NO-711 was measured, using only traces that were well fitted by a straight line. A plot of variance against mean current amplitude was fitted as above to yield an estimate of i. γ for GABAA and GABAC receptors was obtained from γ = i/V, with V being the driving force for Cl−.
Pooled data are expressed as means ± s.e.m.; statistical significance was assessed using Student's paired t tests, with P < 0.05 considered significant.
Results
Fluctuating inward current was observed in isolated (axon-severed) BC terminals in retinal slices recorded with high intracellular [Cl−] at −60 mV. The current consisted of a tonic component (−18 ± 2 pA, n = 25 terminals) plus mIPSCs (see below; Fig. 1A). The mIPSCs had a mean peak amplitude of −13.7 ± 0.9 pA, a 10–90% rise time of 0.29 ± 0.01 ms and a bi-exponential decay with time constants of 1.01 ± 0.03 and 16.7 ± 1.4 ms, with the fast time constant accounting for 78 ± 1% of the decay (n = 25, 195 ± 12 mIPSCs analysed per terminal; Fig. 1B). In the presence of nifedipine (20 μm) to block voltage-gated Ca2+ channel activation, the mIPSCs reversed polarity at around 0 mV, which is approximately the Cl− equilibrium potential (n = 3; Fig. 1A). Application of the glycine receptor antagonist strychnine (1 μm) had no effect on mIPSC amplitude or kinetics (n = 4; data not shown), consistent with an absence of glycine receptors in goldfish bipolar cells (Kaneko et al. 1991).
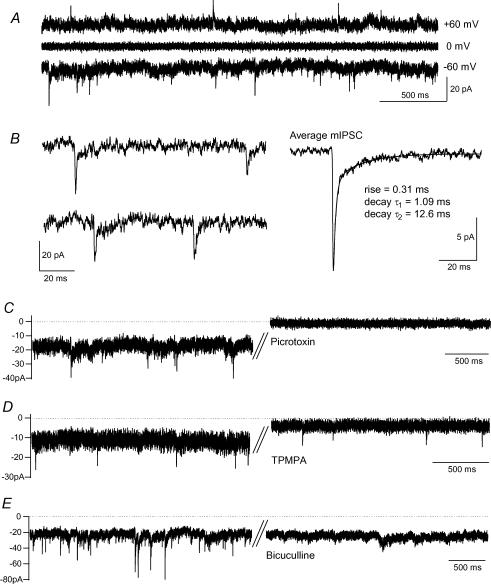
Figure 1. GABAA and GABAC receptors mediate phasic and tonic components of the endogenous GABA current in BC terminals.
A, spontaneous membrane currents recorded with CsCl-based intracellular solution reversed polarity at around 0 mV. Nifedipine (20 μm) was present to inhibit L-type Ca2+ channel activation. B, individual mIPSCs from the terminal in A on an expanded time scale, and the average mIPSC in this terminal (n = 158). C, the mIPSCs and the tonic current were inhibited by the GABAA/GABAC receptor antagonist picrotoxin (50 μm). D, the tonic current alone was inhibited by the GABAC receptor antagonist TPMPA (50 μm). E, the mIPSCs alone were inhibited by the GABAA receptor antagonist bicuculline (50 μm).
The GABAA/GABAC receptor antagonist picrotoxin (50 μm) reduced the tonic current (from −27 ± 7 to −5 ± 3 pA, n = 3, P < 0.05) and abolished the mIPSCs (Fig. 1C). The tonic current was also sensitive to the GABAC receptor antagonist TPMPA (50 μm; current reduced from −23 ± 4 to −7 ± 2 pA, n = 8, P < 0.05) but the mIPSCs were not abolished (Fig. 1D). By contrast, the GABAA receptor antagonist bicuculline (50 μm) inhibited the mIPSCs but left the tonic current intact (control, −19 ± 3 pA; bicuculline, −20 ± 2 pA, n = 3; Fig. 1E). GABAA receptors therefore mediate fast, transient synaptic currents in BC terminals whereas GABAC receptors underlie a slow baseline conductance.
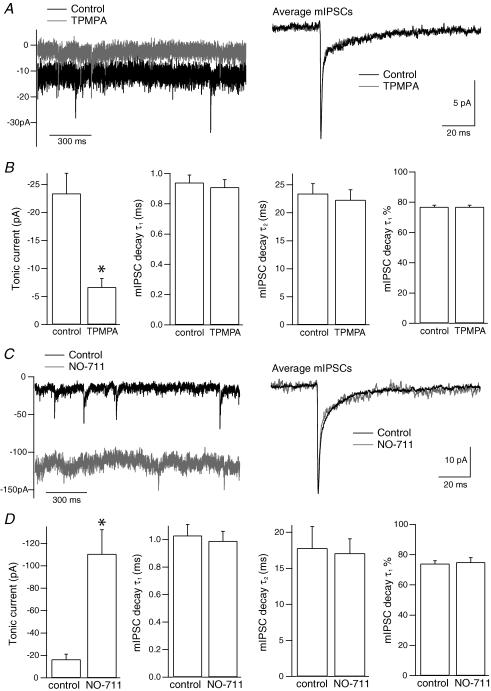
If GABAA and GABAC receptors are present at the same synapses in BC terminals, mIPSCs would be expected to exhibit both receptor components. The kinetics of mIPSCs were therefore compared before and after application of TPMPA (50 μm). TPMPA was found to have no effect on mIPSC decay times, as shown in Fig. 2A and B, or on mIPSC amplitude (control: −13.3 ± 1.3 pA, 195 ± 33 mIPSCs; TPMPA: −13.3 ± 1.4 pA, 183 ± 33 mIPSCs; n = 8 terminals). Inhibition of the GABA transporter GAT-1 has recently been shown to increase the GABAC tonic current in BC terminals (Hull et al. 2006). To determine whether GAT-1 may limit the activation of perisynaptic GABAC receptors at GABAA synapses, mIPSCs were compared in the absence and presence of the GAT-1 inhibitor NO-711 (3 μm). As shown in Fig. 2C and D, mIPSC decay kinetics were unaffected by NO-711 (control: 80 ± 11 mIPSCs; NO-711: 38 ± 8 mIPSCs; n = 8 terminals). Comparison of average mIPSC amplitudes was not meaningful because of the difficulty in detecting small mIPSCs within the increased current noise in the presence of NO-711. The increase in the tonic current was subsequently reversed to baseline with TPMPA (50–100 μm), again with no change in mIPSC kinetics (n = 8; data not shown). Spontaneous exocytosis at GABAA receptor synapses therefore does not appear to activate GABAC receptors, even under conditions of GAT-1 inhibition.
Figure 2. GABAC receptors are not activated by spontaneous release at GABAA synapses.
A, current traces from a terminal before and during application of TPMPA (50 μm), with the superimposed average mIPSCs in the two conditions (control, n = 100; TPMPA, n = 102). B, mean data from eight terminals showing that TPMPA reduced the tonic current but had no significant effect on mIPSC decay kinetics (τ1, fast time constant; τ2, slow time constant; τ1%, percentage contribution of τ1 to the decay). C, current traces from a terminal before and during application of the GAT-1 inhibitor NO-711 (3 μm), with the superimposed average mIPSCs in the two conditions (control, n = 74; NO-711, n = 43). D, mean data from eight terminals showing that NO-711 greatly potentiated the tonic current but had no effect on mIPSC decay kinetics. In B and D, error bars represent s.e.m., *P < 0.05.
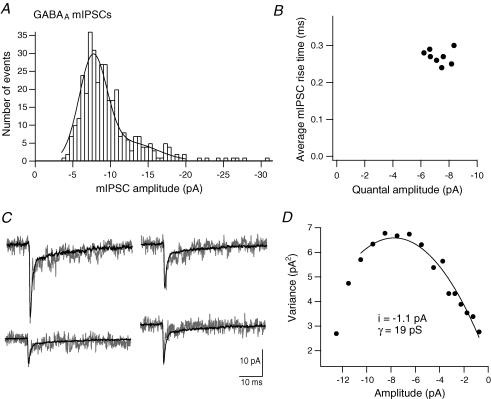
Amplitude histograms of mIPSCs recorded in the presence of TPMPA (50 μm) consisted of a single peak with a tail of larger values and had a mode of −7.9 ± 0.4 pA (n = 8, 386 ± 29 mIPSCs per terminal; Fig. 3A). The histograms were best fitted by the sum of two Gaussians: the mean of the first Gaussian was −7.3 ± 0.3 pA and showed little variability between recordings (coefficient of variation (CV) = 0.10; Fig. 3B); the mean of the second Gaussian was −12.5 ± 1.1 pA (n = 8; Fig. 3A). The histogram peak therefore gives a quantal amplitude of approximately −7 pA for GABAA synapses. The tail of larger amplitude mIPSCs could have a variety of causes, including variability in the number of synaptic GABAA receptors (Nusser et al. 1997) or simultaneous release at several amacrine cell–BC terminal synapses.
Figure 3. Single-channel properties underlying GABAA receptor IPSCs.
A, the mIPSC amplitude histogram for one terminal in the presence of TPMPA (50 μm) fitted with the sum of two Gaussians (first mean, −7.6 pA; second mean, −13.6 pA). B, average mIPSC 10–90% rise time versus quantal amplitude (peak of mIPSC amplitude histogram) for eight terminals. C, example mIPSCs from the terminal in A, with the peak-scaled average mIPSC superimposed for noise analysis. D, plot of mean current variance versus amplitude for this terminal. The curve was fitted to yield an estimate of single-channel current (i).
An estimate of the single-channel conductance (γ) of the GABAA receptors was obtained from peak-scaled non-stationary noise analysis of mIPSCs recorded in the presence of TPMPA (Fig. 3C and D). The mean single-channel current obtained from variance versus amplitude plots was −1.0 ± 0.1 pA, equating to γ of 17 ± 1 pS (n = 7, 113 ± 13 mIPSCs analysed per terminal). From this estimate, a BC terminal quantal GABAA response is mediated by, on average, seven receptors.
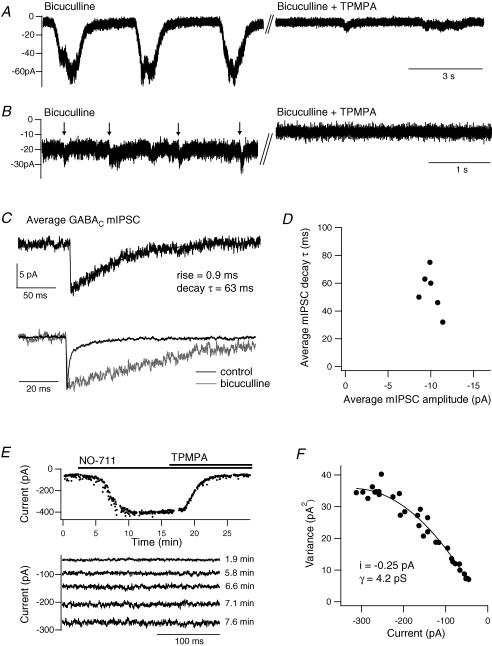
For comparison, I investigated whether quantal GABAC receptor events could be observed in the presence of GABAA receptor antagonism. Application of bicuculline (25–50 μm) often evoked or potentiated slow oscillations in the tonic current (Fig. 4A), which were variable in amplitude and duration between recordings (−20 to −130 pA, 0.5–9 s; n = 9). The oscillations were reduced or blocked by a high concentration of TPMPA (100–200 μm, n = 5; Fig. 4A) or by picrotoxin (50 μm, n = 2). In some terminals, smaller TPMPA-sensitive events that resembled postsynaptic currents were occasionally observed within the tonic current (arrows in Fig. 4B). A subpopulation of these events exhibited a single, fast rising phase and were identified as GABAC mIPSCs (Fig. 4C). Average GABAC mIPSCs had a peak amplitude of −10.0 ± 0.4 pA, which showed little variability between terminals (CV = 0.10; Fig. 4D), a 10–90% rise time of 1.0 ± 0.1 ms and a mono-exponential decay with a time constant of 54 ± 6 ms (n = 6, 13 ± 2 mIPSCs per terminal). This decay time is very similar to the value of 51 ms reported for putative GABAC IPSCs in mouse rod BCs (Frech & Backus, 2004). GABAC mIPSCs therefore exhibit significantly slower decay kinetics than GABAA mIPSCs (Fig. 4C). Assuming that the GABAC tonic current arises from the summation of spontaneous mIPSCs, a simple convolution model of the GABAC mIPSC waveform was used to estimate the frequency of those events. The average TPMPA-sensitive tonic current of −17 ± 2 pA (n = 8) would be evoked by mIPSCs at a frequency of approximately 30 Hz.
Figure 4. Single-channel properties underlying GABAC receptor currents.
A, example traces showing the slow current oscillations in the presence of bicuculline (25 μm) and their inhibition by a high concentration of TPMPA (200 μm). B, in some terminals, small synaptic currents (marked by arrows) were observed within the tonic current. They were inhibited by TPMPA (50 μm). C, the average GABAC mIPSC in the presence of bicuculline (50 μm) in one terminal (n = 16). Only events with a single, fast rising phase were included. Below, the GABAC mIPSC is peak scaled and superimposed with the GABAA mIPSC from the same terminal prior to application of bicuculline (n = 180). D, average mIPSC decay time constant versus peak amplitude for six terminals. E, mean current amplitude versus time for a terminal in the presence of bicuculline (25 μm), showing the potentiation of the tonic current by NO-711 (3 μm) and subsequent inhibition by TPMPA (100 μm). Below are example current traces from selected time points during the potentiation. F, current variance versus amplitude for the terminal in E, fitted to yield an estimate of single-channel current (i).
GABAC receptor γ was estimated from noise analysis of the tonic current during potentiation by the GAT-1 inhibitor NO-711 (3 μm), in the presence of bicuculline (25–50 μm; Fig. 4E). Plots of current variance versus amplitude gave a mean single-channel current of −0.24 ± 0.05 pA, equating to a γ value of 4.0 ± 0.7 pS (n = 4; Fig. 4F). From this estimate, the average maximum current in the presence of NO-711 (−325 ± 86 pA, n = 4) is mediated by approximately 1350 GABAC receptors, which may reflect the total number of GABAC receptors per terminal, and a GABAC mIPSC is mediated by approximately 42 receptors.
Discussion
The results of this study demonstrate that GABAA and GABAC receptors in BC terminals are activated independently by spontaneous GABA release and mediate currents with very different kinetics. GABAA mIPSCs are rapid and transient whereas GABAC mIPSCs decay slowly and give rise to a tonic current. GABAA mIPSCs exhibit no GABAC component, either in control conditions or following inhibition of GAT-1.
The results are consistent with a model in which GABAC receptors are excluded from GABAA synapses in BC terminals. GABAC receptors are therefore located at separate synapses and/or extrasynaptically. The strong punctate staining of GABAC receptor ρ subunits in BC terminals (Enz et al. 1996; Koulen et al. 1997, 1998; Fletcher et al. 1998) and the occurrence of fast-rising GABAC mIPSCs are most consistent with a synaptic localization. Conversely, the strong regulation of the GABAC current by GAT-1 would seem to suggest an extrasynaptic localization. However, due to the complete lack of desensitization of GABAC receptor currents (Hull et al. 2006), GABAC receptors within synapses would also be regulated by the activity of GABA transporters. Indeed, the rate of decay of the GABAC mIPSCs (τdecay ∼54 ms) may reflect the rate of clearance of GABA from the synaptic cleft by diffusion and uptake. This may explain some of the variability in τdecay between terminals (Fig. 4D).
The estimated γ values for GABAA and GABAC receptors in BC terminals (17 and 4 pS, respectively) are similar to values previously obtained from exogenous GABA application to isolated BCs. Estimates of γ for GABAA and GABAC were, respectively, 10 and 4 pS in hybrid bass BCs (Qian & Dowling, 1995) and 30 and 8 pS in rat BCs (Feigenspan & Bormann, 1994). It is interesting that the estimated γ of GABAA receptors mediating a tonic current in hippocampal neurons was ∼6 pS, significantly lower than that of GABAA receptors mediating fast mIPSCs in the same neurons (Bai et al. 2001). In BC terminals, the small γ of GABAC receptors appears to be compensated by a greater number of activated receptors per synapse, resulting in a similar quantal amplitude for GABAA and GABAC receptor synapses.
The apparent segregation of GABAA and GABAC receptors to different synapses in BC terminals will enable independent functioning and regulation of these kinetically distinct forms of inhibition. It will be interesting to determine whether particular classes of amacrine cell form only GABAA or GABAC receptor synapses. The specific roles of GABAA and GABAC receptor inhibition in retinal processing are at present unclear, although GABAC receptors are known to limit BC exocytosis during light responses. The prolonged time course of GABAC feedback inhibition is particularly suited to regulating sustained exocytosis from BCs (Vigh & von Gersdorff, 2005). GABAC receptors also have the potential to control regenerative potentials in BC terminals via effects on membrane conductance (Hull et al. 2006). The large slow oscillations in the GABAC tonic current observed in the present study suggest that membrane conductance may be continuously modulated by networked amacrine cell activity. By contrast, the rapid time course of the GABAA feedback current is suited to regulating phasic exocytosis from BCs. GABAA receptors have recently been shown to inhibit exocytosis from rod BCs during light responses, although to a lesser extent than GABAC receptors (Eggers & Lukasiewicz, 2006). Building on the current evidence for synaptic segregation of GABAA and GABAC receptors in BC terminals, further work will determine their mechanisms of regulation and specific functions in retinal processing.
Acknowledgments
This work was funded by a Medical Research Council Career Development Award. The author wishes to thank Drs Court Hull (University of California, San Diego, USA) and Henrique von Gersdorff (Vollum Institute, Oregon Health and Sciences University, Oregon, USA) for their work on related projects, Holger Taschenberger (Max Planck Institute for Biophysical Chemistry, Göttingen, Germany) for advice and provision of data analysis tools, and Nigel Cooper (Keele University, UK) for critical reading of the manuscript and assistance with data analysis.
References
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acidA receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- De Koninck Y, Mody I. Noise analysis of miniature IPSCs in adult rat brain slices: properties and modulation of synaptic GABAA receptor channels. J Neurophysiol. 1994;71:1318–1335. doi: 10.1152/jn.1994.71.4.1318. [DOI] [PubMed] [Google Scholar]
- Dong CJ, Werblin FS. Temporal contrast enhancement via GABAC feedback at bipolar terminals in the tiger salamander retina. J Neurophysiol. 1998;79:2171–2180. doi: 10.1152/jn.1998.79.4.2171. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Boycott BB. Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci. 1966;166:80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Werblin FS. Organization of retina of the mudpuppy, Necturus maculosus. I. Synaptic structure. J Neurophysiol. 1969;32:315–338. doi: 10.1152/jn.1969.32.3.315. [DOI] [PubMed] [Google Scholar]
- Du JL, Yang XL. Subcellular localization and complements of GABAA and GABAC receptors on bullfrog retinal bipolar cells. J Neurophysiol. 2000;84:666–676. doi: 10.1152/jn.2000.84.2.666. [DOI] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. GABAA, GABAC and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells. J Physiol. 2006;572:215–225. doi: 10.1113/jphysiol.2005.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enz R, Brandstatter JH, Wassle H, Bormann J. Immunocytochemical localization of the GABAC receptor ρ subunits in the mammalian retina. J Neurosci. 1996;16:4479–4490. doi: 10.1523/JNEUROSCI.16-14-04479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, Bormann J. Differential pharmacology of GABAA and GABAC receptors on rat retinal bipolar cells. Eur J Pharmacol. 1994;288:97–104. doi: 10.1016/0922-4106(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Fletcher EL, Koulen P, Wassle H. GABAA and GABAC receptors on mammalian rod bipolar cells. J Comp Neurol. 1998;396:351–365. doi: 10.1002/(sici)1096-9861(19980706)396:3<351::aid-cne6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Frech MJ, Backus KH. Characterization of inhibitory postsynaptic currents in rod bipolar cells of the mouse retina. Vis Neurosci. 2004;21:645–652. doi: 10.1017/S0952523804214134. [DOI] [PubMed] [Google Scholar]
- Hartveit E. Reciprocal synaptic interactions between rod bipolar cells and amacrine cells in the rat retina. J Neurophysiol. 1999;81:2923–2936. doi: 10.1152/jn.1999.81.6.2923. [DOI] [PubMed] [Google Scholar]
- Hull C, Li GL, von Gersdorff H. GABA transporters regulate a standing GABAC receptor-mediated current at a retinal presynaptic terminal. J Neurosci. 2006;26:6979–6984. doi: 10.1523/JNEUROSCI.1386-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A, Suzuki S, Pinto LH, Tachibana M. Membrane currents and pharmacology of retinal bipolar cells: a comparative study on goldfish and mouse. Comp Biochem Physiol C. 1991;98:115–127. doi: 10.1016/0742-8413(91)90188-y. [DOI] [PubMed] [Google Scholar]
- Koulen P, Brandstatter JH, Enz R, Bormann J, Wassle H. Synaptic clustering of GABAC receptor ρ-subunits in the rat retina. Eur J Neurosci. 1998;10:115–127. doi: 10.1046/j.1460-9568.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- Koulen P, Brandstatter JH, Kroger S, Enz R, Bormann J, Wassle H. Immunocytochemical localization of the GABAC receptor rho subunits in the cat, goldfish, and chicken retina. J Comp Neurol. 1997;380:520–532. doi: 10.1002/(sici)1096-9861(19970421)380:4<520::aid-cne8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz PD, Shields CR. Different combinations of GABAA and GABAC receptors confer distinct temporal properties to retinal synaptic responses. J Neurophysiol. 1998;79:3157–3167. doi: 10.1152/jn.1998.79.6.3157. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz PD, Werblin FS. A novel GABA receptor modulates synaptic transmission from bipolar to ganglion and amacrine cells in the tiger salamander retina. J Neurosci. 1994;14:1213–1223. doi: 10.1523/JNEUROSCI.14-03-01213.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K, Hasegawa J, Tachibana M. Modulation of excitatory synaptic transmission by GABAC receptor-mediated feedback in the mouse inner retina. J Neurophysiol. 2001;86:2285–2298. doi: 10.1152/jn.2001.86.5.2285. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Cull-Candy S, Farrant M. Differences in synaptic GABAA receptor number underlie variation in GABA mini amplitude. Neuron. 1997;19:697–709. doi: 10.1016/s0896-6273(00)80382-7. [DOI] [PubMed] [Google Scholar]
- Palmer MJ, Taschenberger H, Hull C, Tremere L, von Gersdorff H. Synaptic activation of presynaptic glutamate transporter currents in nerve terminals. J Neurosci. 2003;23:4831–4841. doi: 10.1523/JNEUROSCI.23-12-04831.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Dowling JE. GABAA and GABAC receptors on hybrid bass retinal bipolar cells. J Neurophysiol. 1995;74:1920–1928. doi: 10.1152/jn.1995.74.5.1920. [DOI] [PubMed] [Google Scholar]
- Sagdullaev BT, McCall MA, Lukasiewicz PD. Presynaptic inhibition modulates spillover, creating distinct dynamic response ranges of sensory output. Neuron. 2006;50:923–935. doi: 10.1016/j.neuron.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Shen W, Slaughter MM. Multireceptor GABAergic regulation of synaptic communication in amphibian retina. J Physiol. 2001;530:55–67. doi: 10.1111/j.1469-7793.2001.0055m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields CR, Tran MN, Wong RO, Lukasiewicz PD. Distinct ionotropic GABA receptors mediate presynaptic and postsynaptic inhibition in retinal bipolar cells. J Neurosci. 2000;20:2673–2682. doi: 10.1523/JNEUROSCI.20-07-02673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Silver RA, Cull-Candy SG. Estimated conductance of glutamate receptor channels activated during EPSCs at the cerebellar mossy fiber-granule cell synapse. Neuron. 1993;11:279–289. doi: 10.1016/0896-6273(93)90184-s. [DOI] [PubMed] [Google Scholar]
- Vigh J, von Gersdorff H. Prolonged reciprocal signaling via NMDA and GABA receptors at a retinal ribbon synapse. J Neurosci. 2005;25:11412–11423. doi: 10.1523/JNEUROSCI.2203-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H, Koulen P, Brandstatter JH, Fletcher EL, Becker CM. Glycine and GABA receptors in the mammalian retina. Vision Res. 1998;38:1411–1430. doi: 10.1016/s0042-6989(97)00300-3. [DOI] [PubMed] [Google Scholar]
- Zhang J, Jung CS, Slaughter MM. Serial inhibitory synapses in retina. Vis Neurosci. 1997;14:553–563. doi: 10.1017/s0952523800012219. [DOI] [PubMed] [Google Scholar]
- Zhang J, Slaughter MM. Preferential suppression of the ON pathway by GABAC receptors in the amphibian retina. J Neurophysiol. 1995;74:1583–1592. doi: 10.1152/jn.1995.74.4.1583. [DOI] [PubMed] [Google Scholar]