Abstract
Glutamate acts at central synapses via ionotropic (iGluR – NMDA, AMPA and kainate) and metabotropic glutamate receptors (mGluRs). Group I mGluRs are excitatory whilst group II and III are inhibitory. Inhibitory mGluRs also modulate peripherally the mechanosensitivity of gastro-oesophageal vagal afferents. Here we determined the potential of excitatory GluRs to play an opposing role in modulating vagal afferent mechanosensitivity, and investigated expression of receptor subunit mRNA within the nodose ganglion. The responses of mouse gastro-oesophageal vagal afferents to graded mechanical stimuli were investigated before and during application of selective GluR ligands to their peripheral endings. Two types of vagal afferents were tested: tension receptors, which respond to circumferential tension, and mucosal receptors, which respond only to mucosal stroking. The selective iGluR agonists NMDA and AMPA concentration-dependently potentiated afferent responses. Their corresponding antagonists AP-5 and NBQX alone attenuated mechanosensory responses as did the non-selective antagonist kynurenate. The kainate selective agonist SYM-2081 had minor effects on mechanosensitivity, and the antagonist UBP 302 was ineffective. The mGluR5 antagonist MTEP concentration-dependently inhibited mechanosensitivity. Efficacy of agonists and antagonists differed on mucosal and tension receptors. We conclude that excitatory modulation of afferent mechanosensitivity occurs mainly via NMDA, AMPA and mGlu5 receptors, and the role of each differs according to afferent subtypes. PCR data indicated that all NMDA, kainate and AMPA receptor subunits plus mGluR5 are expressed, and are therefore candidates for the neuromodulation we observed.
Glutamate is the major excitatory neurotransmitter in the central nervous system (CNS). In addition to mediating synaptic transmission it is also involved as a modulator at both pre- and postsynaptic sites. CNS transmission of vagal afferent signals in the nucleus of the tractus solitarius is largely glutamatergic, and is tightly regulated presynaptically via a number of glutamate receptors (Glaum & Miller, 1992, 1993, 1995; Foley et al. 1998; Liu et al. 1998; Chen et al. 2002). The range of glutamate receptors in the CNS includes ionotropic (NMDA, AMPA and kainate) receptors (iGluRs), which are ligand gated cation channels (Bleakman & Lodge, 1998), and metabotropic (group I, II, III) receptors (mGluRs), which are G-protein coupled receptors (Cartmell & Schoepp, 2000). mGluRs exist as eight subtypes: Group I mGluRs (mGluR1 and 5) mostly cause slow depolarization due to activation of phospholipase C. Group II (mGluR2 and 3), and group III (mGluR4, 6, 7 and 8) cause slow hyperpolarization via inhibition of adenylate cyclase and altered calcium and potassium currents (Schoepp et al. 1999; Cartmell & Schoepp, 2000). The three mGluR groups were initially designated based on similarities in their sequence and pharmacology. In addition to their function in the CNS, recently we showed that group II and III mGluRs modulate vagal afferents peripherally (Page et al. 2005b). Thus glutamate and selective group II and III agonists inhibited mechanosensitivity of gastro-oesophageal afferent endings, whilst a group III antagonist increased mechanosensitivity, indicating both exogenous and endogenous sources of glutamate were able to negatively modulate afferent endings.
Previously we had shown a similar inhibition of gastro-oesophageal afferents by GABAB receptor agonists (Page & Blackshaw, 1999; Partosoedarso et al. 2001); importantly this was associated with effects on gastro-oesophageal function in vivo (Blackshaw et al. 1999). The triggering of transient lower oesophageal sphincter (LOS) relaxations by gastric distension was markedly reduced by GABAB receptor agonists, which correspondingly reduced the incidence of gastro-oesophageal acid reflux in ferrets, dogs and humans, and in gastro-oesophageal reflux disease patients (Blackshaw et al. 1999; Lehmann et al. 1999; Lidums et al. 2000; Cange et al. 2002; van Herwaarden et al. 2002; Zhang et al. 2002). This has focused attention on peripheral vagal afferent endings as important potential targets for treatment of reflux disease. Similarly we found that the inhibitory effect of group III mGluR agonists on vagal afferents in vitro was conserved across species (Page et al. 2005b), and it translated into an inhibition of transient LOS relaxations in vivo (Frisby et al. 2005). Two important observations were made in the course of these studies on mGluRs. Firstly, the inhibitory effect of glutamate on vagal afferents was not seen unless iGluRs were blocked, suggesting a balance of (excitatory) iGluR and (inhibitory) mGluR actions. Secondly, a group I (mGluR5) antagonist potently inhibited transient LOS relaxations, but the mechanism for this effect remained undetermined. Therefore, although we had gained an understanding of the regulation of vagal afferents by inhibitory mGluRs, there remained major uninvestigated possibilities regarding their excitatory modulation by mGluRs and/or iGluRs.
Our aims in this study were to investigate systematically the iGluRs that may be involved in excitatory modulation of vagal afferents, and to determine the ability of mGluR5 to have peripheral effects by influencing vagal afferent mechanosensitivity in vitro. We also sought to determine if the relevant transcripts of iGluR and mGluR5 are expressed in the nodose ganglion, which contains the cell bodies of vagal afferents, and thus gain insight as to the subtypes that may be involved in modulating afferent function.
Methods
All studies were performed in accordance with the guidelines of and with the approval of the animal ethics committees of the Institute of Medical and Veterinary Science and The University of Adelaide.
RT-PCR analysis of iGluR and mGluR subunit expression in mouse nodose ganglia
Left and right nodose ganglia were removed from five male and female C57/BL6 mice aged 7–10 weeks after humane killing by CO2 inhalation. Ganglia were stored in RNAlater® (Qiagen, Australia) at −20°C and pooled for subsequent RNA extraction. RNA was isolated from nodose ganglia using homogenization and TRIzol reagent (Invitrogen, Australia), followed by isopropanol precipitation. Reverse transcription (RT) and polymerase chain reaction (PCR) was performed using a Qiagen one-step RT-PCR kit, with primers used as indicated in Table 1. RT-PCR reactions were performed using an alpha unit block (MJ Research, Waltham, MA, USA) attached to a PTC-200 Peltier thermal cycler (MJ Research, Waltham, MA, USA). Standard protocols were followed for reverse transcription, initial PCR activation, denaturing, annealing and extension as per the manufacturer's instructions. Control PCRs were performed by substituting RNA template with RNase-free water. Amplified products were resolved by 1.5–3% agarose gel electrophoresis in parallel with molecular mass markers and visualized via ethidium bromide staining.
Table 1.
Nucleotide primer sequences for RT-PCR identification of ionotropic receptor subunit and mGluR5 transcripts
| Receptor | Sequence | Predicted product size (bp) |
|---|---|---|
| GluR1 | Fwd: GGACCACAGAGGAAGGCATGATC | 358 |
| Rev: CAGTCCCAGCCCTCCAATC | ||
| GluR2 | Fwd: TGTGTTTGTGAGGACTACGGCA | 226 |
| Rev: GGATTCTTTGCCACCTTCATTC | ||
| GluR3 | Fwd: GCAGAGCCATCTGTGTTTGTGAGTT | 472 |
| Rev: AGTTTTGGGTGTTCTTTGTGAGTT | ||
| GluR4 | Fwd: GCAGAGCCGTCTGTGTTCACTAG | 220 |
| Rev: CGGCAAGGTTTACAGGAGTTCTT | ||
| GluR5 | Fwd: GCCCCTCTCACCATCACGTAT | 358 |
| Rev: TGGTCGATAGAGCCTTGGGCA | ||
| GluR6 | Fwd: TTCCTGAATCCTCTCTCCCCT | 259 |
| Rev: CACCAAATGCCTCCCACTATC | ||
| GluR7 | Fwd: GCAGAGTCAGGCCTGCTGGA | 300 |
| Rev: ACTCCACACCCCGACCTTCT | ||
| KA1 | Fwd: CCCATCGAGTCTGTGGATGA | 434 |
| Rev: CTGTGGTCCTCCTCCTTGGG | ||
| NMDAR1 | Fwd: GCTGTACCTGCTGGACCGCT | 210 |
| Rev: GCAGTGTAGGAAGCCACTATGATC | ||
| NR2A | Fwd: GCTACGGGCAGACAGAGAAG | 257 |
| Rev: GTGGTTGTCATCTGGCTCAC | ||
| NR2B | Fwd: GCTACAACACCCACGAGAAGAG | 314 |
| Rev: GAGAGGGTCCACGCTTTCC | ||
| NR2C | Fwd: AACCACACCTTCAGCAGCG | 464 |
| Rev: GACTTCTTGCCCTTGGTGAG | ||
| NR2D | Fwd: CGATGGCGTCTGGAATGG | 265 |
| Rev: AGATGAAAACTGTGACGGCG | ||
| NR3A | Fwd: CCGCGGGATGCCCTACTGTTC | 417 |
| Rev: CCAGTTGTTCATGGTCAGGAT | ||
| mGluR5 | Fwd: GTCCTTCTGTTGATCCTGTC | 118 |
| Rev: ATGCAGCATGGCCTCCACTC | ||
| β-ACTIN | Fwd: ATCATGTTTGAGACCTTCAACAC | 830 |
| Rev: TCTGCGCAAGTTAGGTTTTGTC |
In vitro mouse gastro-oesophageal vagal afferent preparation
Male and female C57/BL6 mice aged 7–10 weeks were killed by CO2 inhalation. The stomach, oesophagus with attached vagal nerves, heart and lungs were removed and placed in a modified Krebs' solution of the following compostion (mm): 118.1 NaCl, 4.7 KCl, 25.1 NaHCO3, 1.3 NaH2PO4, 1.2 MgSO4.7H2O, 1.5 CaCl2 1.0 citric acid, 11.1 glucose bubbled with 95% O2–5% CO2. The temperature was maintained at 4°C during dissection to prevent metabolic degradation. After further dissection the preparation was opened longitudinally along the oesophagus and greater curvature of the stomach whilst keeping the vagus nerves intact and removing connective tissue from them. The tissue was pinned out flat mucosa side up in a Perspex chamber (dimensions 6 cm × 2.5 cm × 1.2 cm) lined with Sylgard (Dow Corning) and perfused at a rate of 12 ml min−1 with carbogenated Krebs–bicarbonate buffer solution maintained at 34°C. The dorsal part of the stomach was removed in order that the tissue could be pinned out flat against the edge of the chamber adjacent to the nerve recording chamber. The nerves were extended into a separate chamber where a sliding wall with a mouse hole was able to separate the two chambers. The second chamber was 3.7 cm in diameter and 1.2 cm deep. The nerves were then bathed in paraffin oil and laid on a small glass platform. Under a dissecting microscope the nerve sheath was removed revealing the underlying nerve trunk. Fine forceps were used to tease apart the nerve trunk into 8–12 small bundles, and then one by one the nerve bundles were placed onto platinum recording electrodes. A reference electrode rested on the mirror surface in the bath in a small pool of Krebs' solution.
Characterization of gastro-oesophageal vagal afferent properties
Location of all types of vagal afferents along the oesophagus and stomach was determined by mechanical stimulation throughout the preparation with a brush, then more accurately with a fine calibrated von Frey hair. Accurate quantification of mechanical responsiveness varied according to the fibre type, as we have previously described (Page et al. 2002, 2004, 2005a,b). Mucosal receptors showed rapidly adapting responses to maintained pressure over the receptive field with a von Frey hair, but not to circumferential tension. The most reproducible stimulus-dependent response of these afferents to mucosal stimuli was evoked when the probe was moved at a rate of 5 mm s−1 rather than being stationary. A single test at each intensity of calibrated von Frey hair is prone to error due to the small size of the receptive field, and we therefore minimized experimental error by measuring the mean response to 10 standard strokes given at 1 s intervals, omitting the first and last from analysis because of increased associated error. The von Frey hairs are bent so an even force was applied to the receptive field for an entire stroke. This protocol was found to give highly reproducible data and was therefore used to assess the effects of GluR ligands on vagal afferents. Tension–response curves were also obtained for afferent fibres responsive to circular tension. Tension receptors were responsive to circular stretch and von Frey stroking, but were distinguished from mucosal receptors by their clear responsiveness to circular tension, which was slowly adapting. Tension stimuli were applied using a cantilever and claw system. Tension was applied via a thread attached to an unpinned point adjacent to the mechanoreceptive field. The thread was attached to the cantilever via a pulley and calibrated weights were applied for 1 min periods with 1 min rest in between weight applications. The response was measured as the mean discharge evoked, and subsequent responses during drug exposure are expressed as the percentage of the maximum control response (without drug addition), so that increases and decreases in responsiveness may be directly compared. Tension stimulus response relationships were assessed by applying weights to the cantilever system in the range of 1–5 g.
Effects of GluR agonists and antagonists on mechanical sensitivity of vagal afferents
After mechanical sensitivity of gastro-oesophageal vagal afferents had been established, the effects of various agonists and antagonists on mechanical sensitivity were determined. Kynurenate (10−7–10−5m) was added to the superfusing Krebs' solution, and was allowed to equilibrate for 20 min, after which the stimulus–response curves were re-established. The agonists N-methyl d-aspartate (NMDA) (10−7–10−5m), α-amino-3-hydroxy-5-methyl-isoxazole-4-propionate (AMPA; 10−5–10−4m), and (25, 4R)-4-methylglutomic acid (SYM-2081; 10−6–10−5m) were added to the superfusion solution. The antagonists d(−)-2-amino-5-phosphono-pentanoic acid (AP-5) (10−6–10−5m), 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide (NBQX) (10−9–10−8m) and (s)-1-(2-amino-2-carboxyethyl)-2-(2-carboxybenzyl)pyrimidine-2,4-dione (UBP 302; 10−7–10−5m) were added to a ring placed on the preparation over the receptive field. Concentrations used were based on published potencies (Wilding & Huettner, 1996; Bleakman & Lodge, 1998; Bleakman et al. 1999; Yamakura & Shimoji, 1999; Lynch & Guttmann, 2001; Cosford et al. 2003). The non-selective iGluR antagonist kynurenate was tested at concentrations of 10−7–10−4m in three pilot experiments which demonstrated concentration-dependent effects and ultimately complete abolition of firing; ranges around the estimated EC50 were subsequently chosen for this study. Drug addition to the ring was followed by a 10 min equilibration period before stimulus response curves were once again established immediately after removal of the ring. Equilibration periods were necessary for complete penetration of the drug into all layers of the tissue. Preliminary data indicated that effects of drugs were maintained for approximately 10 min after removal of the ring. Time control experiments (n = 10) showed there was no significant change in mechanical responses over a comparable time period. Five of nine experiments involving NMDA were performed in the absence of nifedipine and all other experiments were performed in the presence of nifedipine (10−6m), in order to limit the effects of drugs secondary to smooth muscle responses. Three additional experiments were performed using suspended circular-orientated rings to investigate length–tension relationships. No change in these were seen with any of the agonists used at maximum concentration. Also no effect was seen on smooth muscle responses to electrical field stimulation in separate experiments (M.E. O'Callaghan, unpublished observations). Statistical analysis of the electrophysiological stimulus–response curves was by two-way analysis of variance unless otherwise indicated, where P < 0.05 is considered significant.
Data recording and analysis
Afferent impulses were amplified with a biological amplifier (DAM 50; World Precision Instruments, Sarasota, FL, USA), filtered (Band pass filter-932; CWE Inc., Ardmore, PA, USA) and monitored using an oscilloscope (DL 1200A, Yokogawa, Tokyo). Single units were discriminated on the basis of action potential shape, duration and amplitude using Spike software (Cambridge Electronic Design, Cambridge, UK).
Drugs
Stock solutions of all drugs were kept frozen and diluted to their final concentrations in Krebs' solution on the day of the experiment. NMDA, AP-5, NBQX, UBP 302 and SYM-2081 were all obtained from Tocris (Tocris, Bristol, UK). MTEP was obtained from Astrazeneca (Astrazeneca, Molndal, Sweden) and kynurenate and AMPA were obtained from Sigma (Sigma, Castle Hill, NSW, Australia).
Results
Expression of glutamate receptor subunits in vagal (nodose) afferents
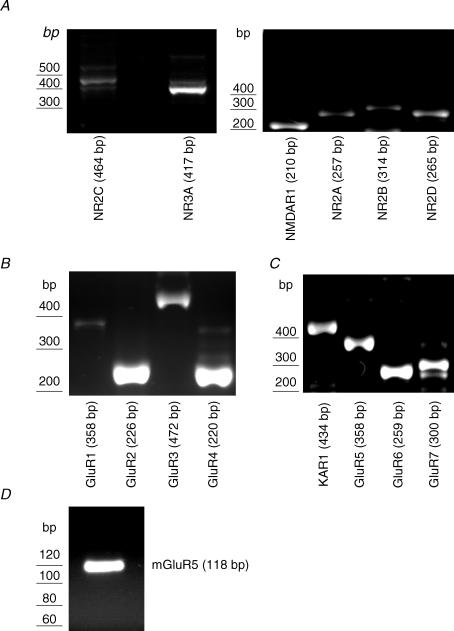
We were able to identify the expression of a number of transcripts corresponding to iGluR subunits and mGluR5 in mouse nodose ganglia. Electrophoresis of PCR amplification products revealed single bands, corresponding to the predicted sizes of all of the NMDA receptor subunits investigated (NMDAR1, NR2A, NR2B, NR2C, NR2D and NR3A; Fig. 1A). RT-PCR also revealed the expression of all AMPA receptor subunits (GluR1, GluR2, GluR3 and GluR4; Fig. 1B) and all kainate receptor subunits (KA1, GluR5, GluR6 and GluR7: Fig. 1C). Expression of mGluR5 was also evident (Fig. 1D) in mouse nodose ganglion.
Figure 1. Detection by RT-PCR of iGluR and mGluR subunits in mouse nodose ganglion.
A, all six NMDA receptor subunits (NMDAR1, NR2A-D, NR3A) were detected with the correct product size for the target. B, all subunits of the AMPA receptor, GluR1–4, were detected, with strong signals for GluR2 and GluR4. C, all subunits of the kainate receptor (KA1, GluR5–7) were detected, along with mGluR5 (D). See Table 1 for primers used to detect GluR subtypes.
Electrophysiological studies
To determine the role of peripheral iGluR and mGluR in regulating mechanosensitivity, we examined the responses of single afferent fibres using an in vitro gastro-oesophageal vagal afferent recording preparation. The effects of specific agonists and antagonists directed at iGluR and mGluR are illustrated in Figs 2–6. Single afferent fibres were classified into mucosal or tension receptors depending on their response to mechanical stimulation. All afferent endings were located in the distal oesophagus or on the lower oesophageal sphincter. Increasing forces of von Frey hair stroking (10–1000 mg) applied to the mucosa excited both mucosal and tension receptors in a graded manner; however, mucosal receptors were distinct from tension receptors in that they were unresponsive to circumferential tension (Page et al. 2002). Tension receptors elicited a graded increase in the number of action potentials in response to applied load (1–5 g).
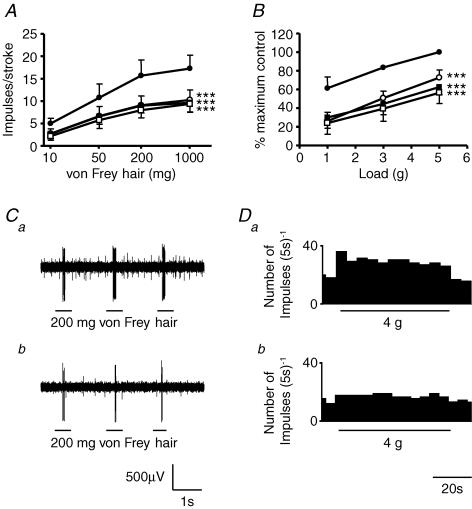
Figure 2. The effect of kynurenate on mouse gastro-oesophageal vagal afferents.
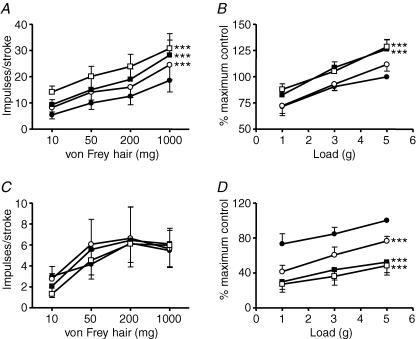
Stimulus–response functions of mucosal (A, n = 5) and tension receptors (B, n = 6) to mucosal stroking and circumferential tension, respectively. The responses are before (•) and after exposure to kynurenate (0.1 μm (○), 0.3 μm (▪) and 1 μm (□)). Asterisks indicate significant difference from control using a two-way ANOVA (***P < 0.001). C, original recording of a mucosal receptor response to mucosal stroking with a 200 mg von Frey hair before (a) and after (b) exposure to kynurenate (1 μm). D, original recording of a tension receptor response to circumferential tension using a 4 g weight before (a) and after (b) exposure to kynurenate (1 μm).
Figure 6. The effect of MTEP on mouse gastro-oesophageal vagal afferents.
Stimulus-response functions of mucosal (A; n = 9) and tension receptors (B; n = 5) to mucosal stroking and circumferential tension, respectively. The responses are before (•) and after exposure to MTEP (1 μm (○), 3 μm (▪), 10 μm (□) and 30 μm (▴)). Asterisks indicate significant difference from control using a two-way ANOVA (**P < 0.01; ***P < 0.001).
Effects of non-selective iGluR blockade on vagal afferents
The effect of kynurenate on mouse mucosal and tension receptor responses to mechanical stimulation is illustrated in Fig. 2. Kynurenate (10−7–10−6m) significantly inhibited mechanosensitivity of both mucosal and tension receptors in a concentration-dependent manner with maximal effects at 10−6m (P < 0.001). These data prompted further investigation of the role of endogenous glutamate at subtypes of ionotropic glutamate receptors.
Effects of NMDA receptor ligands on vagal afferents
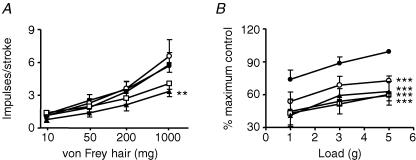
The effect of the NMDA receptor agonist NMDA on mouse mucosal and tension receptor responses to mechanical stimulation is illustrated in Fig. 3A and B. NMDA 10−9–10−7m had no significant effect on sensitivity of tension receptors to applied load although a trend for potentiation of afferent sensitivity was observed. In individual cases where NMDA caused a clear increase in sensitivity of tension receptors, original responses were restored after 10 min washout, possibly suggesting subgroups of responders and non-responders. These may be comparable to two populations of tension-responsive fibres we found in ferrets which showed differing pharmacology (Page et al. 2005b). Effects of NMDA on responses to tension were similar to their responses to local stimulation with von Frey hairs in two additional experiments (data not shown). NMDA significantly potentiated mucosal receptor responses to mucosal stroking (P < 0.001, Fig. 3B). These effects of NMDA were also reversible upon washout with normal Krebs' solution. A subset of experiments observing the effect of NMDA on mechanical sensitivity of afferents was performed in the absence of nifedipine, but all remaining experiments were performed with nifedipine (10−6m) in the superfusing Krebs solution in order to block smooth muscle contraction. Effects of NMDA were unaffected by this treatment indicating likelihood of a direct effect of the drug at nerve endings.
Figure 3. The effect of NMDA and AP-5 on mouse gastro-oesophageal vagal afferents.
Stimulus–response functions of mucosal (A, n = 6) and tension receptors (B, n = 9) to mucosal stroking and circumferential tension, respectively. The responses are before (•) and after exposure to NMDA (0.1 μm (○), 1 μm (▪) and 10 μm (□)). Stimulus–response functions of mucosal (C, n = 5) and tension receptors (D, n = 6) before (•) and after exposure to AP-5 (1 μm (▪) and 10 μm (□)). Asterisks indicate significant difference from control using a two-way ANOVA (**P < 0.01, ***P < 0.001).
The effects of the competitive NMDA receptor AP-5 on mucosal and tension receptor sensitivity to mechanical stimulation is illustrated in Fig. 3C and D. AP-5 (10−6–10−5m) significantly and concentration-dependently reduced the responses of mucosal receptors to mucosal stroking (P < 0.001) and responses of tension receptors to circumferential tension (P < 0.001).
Effects of AMPA/kainate receptor ligands on vagal afferents
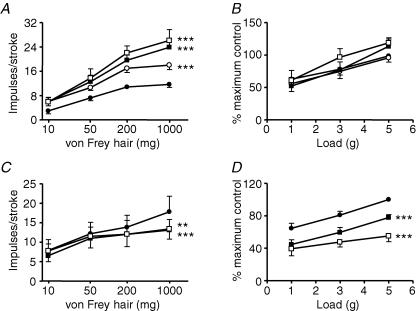
The effect of the selective agonist AMPA on mouse mucosal and tension receptors is illustrated in Fig. 4A and B. AMPA (10−5–10−4m; P < 0.001) concentration-dependently and significantly increased the response of mucosal receptors to mucosal stroking. AMPA at this concentration range also significantly (P < 0.001) increased the response of tension receptors to circumferential tension. The effect of AMPA was completely reversed on wash-out and baseline mechanosensitivity returned. Selective kainate receptor agonism with SYM-2081 at doses effective in other systems (10−6–10−5m; Bleakman et al. 1999) had no effect on sensitivity of tension receptor responses to circular tension, but potentiated slightly the responses of mucosal receptors (Fig. 5A and B). The kainate receptor antagonist UBP 302 (10−7–10−5m) had no effect on either afferent type (Fig. 5C and D). The AMPA/kainate receptor antagonist NBQX (10−9–10−8m, Fig. 4C and D)significantly reduced the response of tension receptorsto circumferential tension (P < 0.0001), but not that of mucosal receptors to von Frey hairs.
Figure 4. The effect of AMPA and NBQX on mouse gastro-oesophageal vagal afferents.
Stimulus–response functions of mucosal (A, n = 5) and tension receptors (A, n = 7) to mucosal stroking and circumferential tension, respectively. The responses are before (•) and after exposure to AMPA (10 μm (○), 30 μm (▪) and 100 μm (□)). Stimulus–response functions of mucosal (C, n = 4) and tension receptors (D, n = 5) before (•) and after exposure to NBQX (1 nm (○), 3 nm (▪) and 10 nm (□)). Asterisks indicate significant difference from control using a two-way ANOVA (***P < 0.001).
Figure 5. The effect of SYM 2081 and UBP 302 on mouse gastro-oesophageal vagal afferents.
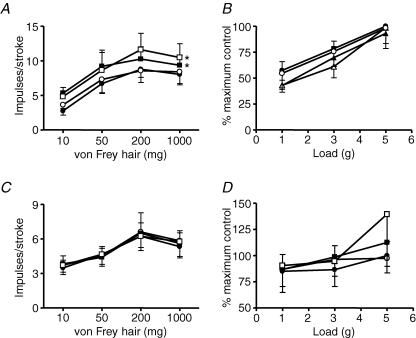
Stimulus–response functions of mucosal (A, n = 5) and tension receptors (B, n = 5) to mucosal stroking and circumferential tension, respectively. The responses are before (•) and after exposure to SYM 2081 (1 μm (○), 3 μm (▪) and 10 μm (□)). Stimulus–response functions of mucosal (C, n = 5) and tension receptors (D, n = 5) before (•) and after exposure to UBP 302 (0.1 μm (○), 1 μm (▪) and 10 μm (□)). Asterisks indicate significant difference from control using a two-way ANOVA (***P < 0.05).
Effects of mGluR5 antagonist on vagal afferents
The effects of the selective mGluR5 antagonist MTEP on mouse mucosal and tension receptors is illustrated in Figs 5A and B. 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine (MTEP; 10−6–3 × 10−5m) significantly inhibited sensitivity of mucosal receptors to mucosal stroking (10–1000 mg; P < 0.01 at 3 × 10−5m). MTEP (10−6–3 × 10−5m) also significantly inhibited mechanical sensitivity of tension receptors to circumferential tension in a concentration-dependent manner (P < 0.0001 all concentrations). Therefore the potency of MTEP on mucosal receptors appeared less than on tension receptors because significance was only seen at the highest concentration applied (3 × 10−5m, P < 0.01).
Discussion
A number of conceptual advances arise from the findings of this study. First our data indicate that mainly two classes of iGluR – AMPA and NMDA – plus mGluR5 are involved in the peripheral excitatory modulation of vagal afferent mechanosensitivity. These peripheral glutamatergic effects are in addition to the established role of glutamate in the synaptic transfer of mechano- and chemosensitive input from gastrointestinal vagal afferents to the CNS. Second, in addition to augmentation of mechanosensitivity by iGluR agonists, we showed inhibition of mechanosensitivity by iGluR antagonists, indicating that endogenous glutamate is most likely involved in peripheral modulation. Third, our previous study using a similar approach demonstrated inhibitory modulation of vagal afferent mechanical sensitivity via group III mGluRs (Page et al. 2005b). Together with the findings of the present study it therefore emerges that there is a counter-balance of excitatory and inhibitory influences of glutamate, involving mainly AMPA, NMDA and mGlu5 receptors in excitation and group II and III mGluRs in inhibition. Fourth, although our pharmacological investigation of excitatory modulation in this study clearly demonstrated involvement of NMDA, AMPA and mGluR5 receptors, it did not provide evidence for a major role of kainate receptors, despite the fact that kainate receptor mRNAs are expressed in the nodose ganglion. Therefore expression of a receptor by vagal afferents is not necessarily correlated with a functional role. Fifth, our data demonstrate that each receptor has a slightly different functional role on the two different subtypes of primary afferents. Sixth, our findings on MTEP provide evidence that its potent inhibitory effect on triggering of transient LOS relaxation after gastric distension may be mediated peripherally. This would suggest a peripheral site for therapeutic intervention in gastro-oesophageal reflux disease. Finally, our findings on iGluR indicate a role for these receptors in the vagal system similar to their role in the spinal innervation of the colon, where it is already evident that they may underlie behavioural responses to colonic distension. Each of the above concepts is specifically addressed below.
Peripheral role for excitatory glutamate receptors
Excitatory glutamate receptors are expressed in the caudal brainstem with iGluRs in particular being implicated in the signalling of gastric distension, in swallowing, transient lower oesophageal sphincter relaxations, emetic signalling, and satiety (Berthoud & Neuhuber, 2000; Partosoedarso & Blackshaw, 2000; Furukawa et al. 2001; Lehmann & Branden, 2001), suggesting a role specifically in afferent transmission from the periphery to the CNS, particularly in areas of gastro-oesophageal vagal afferent terminations in the NTS. The present study indicates their role in peripheral modulation of vagal afferent signalling may be just as important as their role at central sites. Our data in mouse complement and extend those of previous studies in ferret and rat, which suggested excitatory actions of glutamate on vagal afferents (Sengupta et al. 2004; Page et al. 2005b), although guinea pig afferent endings in contrast lack functional receptors for glutamate and GABA (Zagorodnyuk et al. 2002, 2003). Our data are compatible with the observation that glutamate receptors undergo bidirectional axonal transport in both central and peripheral branches of the vagus nerve (Cincotta et al. 1989). Expression of mGluR and subtypes of iGluR has been demonstrated in vagal afferent cell bodies of several species with transportation of receptors towards peripheral endings (Shigemoto et al. 1992; Hay et al. 2000; Hoang & Hay, 2001; Chang et al. 2003; Page et al. 2005b), suggesting a peripheral function. Glutamate receptors are also expressed and function in peripheral terminals of sensory spinal afferents innervating the skin (Coggeshall & Carlton, 1998; deGroot et al. 2000; Carlton, 2001; Carlton et al. 2001), and may be involved in hyperalgesia associated with inflammation (Carlton & Coggeshall, 1999; Du et al. 2001). NMDA receptors have been implicated in signalling painful distension of the colon, as peripheral administration of an ionotropic receptor antagonist, memantine, caused a reduction in afferent and behavioural responses to colorectal distension (McRoberts et al. 2001). However memantine is a non-selective ligand and may have effects other than at NMDA receptors.
Positive and negative modulation of mechanosensitivity by glutamate
Our previous in vitro experiments in ferret indicated there may be a balance of excitatory and inhibitory glutamatergic control of mechanosensitivity in which glutamate simultaneously activates both iGluRs and mGluRs (Page et al. 2005b). There was no effect of glutamate in the absence of drug, but in the presence of the non-selective iGluR antagonist kynurenate, a potent inhibitory effect of glutamate was revealed that was mimicked by selective group II and group III mGluR agonists. In vivo observations in the rat indicated similarly that iGluR may be involved in excitatory modulation of vagal afferents (Sengupta et al. 2004). It remained to be determined which subtypes of iGluR were involved and the potential for involvement of excitatory group I mGluRs remained undetermined. The observations of our present and previous studies clearly indicate a balance in control of vagal afferent mechanosensitivity with group II and III mGluRs mediating ongoing inhibition and iGluRs plus mGluR5 mediating potentiation. More specifically, effects of the selective agonists and antagonists for the AMPA, NMDA and kainate receptors on vagal afferent mechanosensitivity suggest AMPA and NMDA receptors are mainly involved in the excitatory side of the balance, and kainate receptors appear to play a minor role.
Endogenous glutamate plays a role in modulation of vagal afferents
The inhibitory effects of iGluR and mGluR selective antagonists on mechanosensitivity indicate the removal of a tonic stimulation, and therefore an endogenous action of glutamate. The question arises as to the likely source of endogenous glutamate. We speculate that vagal afferents are themselves likely to be responsible for the release of glutamate both centrally, as well as in the periphery. Glutamate is detected in approximately 60% of nodose ganglion cells and on vagal afferent fibres originating from the nodose ganglion (Schaffar et al. 1997). The peripheral endings of vagal afferents contain the vesicular glutamate transporter VGLUT2 (Raab & Neuhuber, 2003), which is also widespread in the enteric nervous system (Kirchgessner, 2001) and is necessary for glutamate release. There are several possible sources of glutamate other than vagal afferents but in close proximity to their endings such as contracting muscle and from the diet – both luminal and circulating (Graham et al. 2000). The effects of excess endogenous glutamate in our study may account for the lack of effect of NMDA on tension receptor mechanosensitivity, in contrast with the potent inhibition of mechanosensitivity by an NMDA receptor antagonist. An excess of endogenous glutamate may also explain why a kainate receptor agonist had little effect in this study and why a group I mGluR agonist had no effect in our previous study (Page et al. 2005b). On the other hand, endogenous activation of AMPA receptors may be surmountable by exogenous AMPA.
Glutamate receptor subtypes have varying effects on subtypes of primary afferents
Selective agonists and antagonists to iGluR receptors and mGluR5 receptors were effective on responses of both tension sensitive and mucosal receptors to mechanical stimulation. Whilst ligands generally had highly potent effects on vagal afferents, there was some discrepancy in the effectiveness of ligands on tension or mucosal receptors. For example NMDA was more potent on mucosal receptors, and MTEP was more potent on tension receptors, suggesting that NMDA receptors may be more highly expressed by mucosal receptor cell bodies, and mGluR5 may be expressed more by tension receptors, although localization of receptors on individual afferent populations ending in different layers of gut has not yet been feasible.
All subtypes of GluR are expressed in nodose ganglion
Expression of mRNA encoding all mGluRs and some subtypes of iGluR in nodose ganglia has been demonstrated in several species (Shigemoto et al. 1992; Hoang & Hay, 2001; Chang et al. 2003; Page et al. 2005b). This study confirms expression in mouse of all iGluR subunits and mGluR5, which from previous observations in ferret we expect to be transported to peripheral endings. All NMDA receptors function as heteromeric assemblies composed of two NR1 subunits conjugated with two or three NR2 or NR3 subunits. A particular subunit composition in afferent fibres is probably important for both nociception and mechanical sensitivity as subunit composition may alter conductance, affinity for glutamate, magnesium block and calcium permeability (Carlton, 2001; Marvizon et al. 2002). Two types of NMDA receptors are suggested to occur in sensory neurones, those expressing an NR1 subunit conjugated with NR2B subunits, which are present in both A and C fibres, and those conjugated with NR2D subunits, present only in A fibres. Although this study confirmed the expression of all NMDA receptor subunits, how they assemble in individual vagal afferent neurones is difficult to determine with available methods.
This study provided evidence of expression of all subunits of AMPA receptors in the mouse nodose ganglion, which provides a molecular correlate of the functional effects of the AMPA agonist and antagonist. Although all AMPA receptor subunits are not required for full function of the receptor, it is often found that all are expressed in a given brain region (Bleakman & Lodge, 1998).
All subtypes of kainate receptors were expressed in the nodose ganglion; however, there was evidence for only a relatively minor functional role on gastro-oesophageal vagal afferent endings. It is therefore possible that most kainate receptors are expressed more abundantly on afferents innervating regions other that stomach and oesophagus. Our results may also suggest that whilst receptor transcripts may be expressed in the nodose ganglion, their protein products may exhibit poor functional coupling on peripheral vagal afferent endings.
Actions on vagal afferents may underlie the potential therapeutic effects of mGluR5 antagonists in reflux disease
We have shown that mGluR5 antagonists inhibit triggering of transient LOS relaxations by gastric distension in conscious ferret and dog models (Frisby et al. 2005; Jensen et al. 2005). This inhibition was shown to be associated with inhibition of gastro-oesophageal reflux, demonstrating the potential for therapeutic intervention with mGluR5 in treatment of gastro-oesophageal reflux disease. An important question raised by these findings was whether effects of MTEP were mediated peripherally or centrally. The data from the present study indicate that at least part of the effect is mediated peripherally by reducing sensitivity of gastro-oesophageal vagal afferents to distension. Peripherally directed treatments are an attractive option for gastro-oesophageal reflux disease owing to the reduced likelihood of CNS side-effects, which are likely to be associated with antagonism of mGlu5 receptors in the CNS (Frisby et al. 2005; Jensen et al. 2005). The results of this study therefore provide a good rationale for the development of peripherally restricted mGluR5 antagonists.
Roles for glutamate receptors in the vagal and spinal sensory system
Anatomical studies in skin have provided evidence for expression of GluR on afferent endings, including mGluR5 and NMDA receptors (Carlton et al. 1995, 2001; Coggeshall & Carlton, 1998; Bhave et al. 2001; Carlton, 2001). Functional studies indicate these receptors may be involved in controlling excitability in a way similar to the one shown here for vagal afferents. Thus peripherally applied agonists increase and antagonists decrease either the responsiveness of afferent fibres or the behavioural response of the animal to mechanical stimuli. A similar finding was made in viscera, in which the non-selective ionotropic antagonist memantine reduced afferent and behavioural responses to colorectal distension (McRoberts et al. 2001). It is not yet known what role is played by each major subtype of GluR in spinal afferents or if there are opposing influences of positively and negatively coupled receptors. Therefore it is possible that there are many similarities between vagal and spinal afferents in the actions of glutamate. An interesting discrepancy between vagal and spinal afferents, however, is the presence of VGLUT2 in vagal cell bodies and endings (Tong et al. 2001; Raab & Neuhuber, 2003), which was not seen in their dorsal root ganglion counterparts (Morris et al. 2005), and may in turn suggest a greater role for activation of glutamate autoreceptors on vagal afferents than on spinal afferents.
In conclusion, this study in conjunction with a previous one from our group, has completed a comprehensive characterization of the influence of all major GluR subtypes on vagal afferent mechanosensitivity. There is clearly scope for both excitatory and inhibitory modulation of afferent sensitivity by glutamate from both exogenous and endogenous sources, providing a balance to achieve normal vagal afferent function. This balance is clearly possible to manipulate pharmacologically. How it may be altered in disease states we hope will be the subject of our continuing investigations.
Acknowledgments
L.A.B. was supported by a National Health and Medical Research Council of Australia Senior Research Fellowship. This work was supported by NHMRC Australia grant number 104814, and by AstraZeneca Research and Development Mölndal.
References
- Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- Bhave G, Karim F, Carlton SM, Gereau RW., 4th Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat Neurosci. 2001;4:417–423. doi: 10.1038/86075. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Staunton E, Lehmann A, Dent J. Inhibition of transient LES relaxations and reflux in ferrets by GABA receptor agonists. Am J Physiol Gastrointest Liver Physiol. 1999;277:G867–G874. doi: 10.1152/ajpgi.1999.277.4.G867. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Lodge D. Neuropharmacology of AMPA and kainate receptors. Neuropharmacology. 1998;37:1187–1204. doi: 10.1016/s0028-3908(98)00139-7. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Ogden AM, Ornstein PL, Hoo K. Pharmacological characterization of a GluR6 kainate receptor in cultured hippocampal neurons. Eur J Pharmacol. 1999;378:331–337. doi: 10.1016/s0014-2999(99)00478-1. [DOI] [PubMed] [Google Scholar]
- Cange L, Johnsson E, Rydholm H, Lehmann A, Finizia C, Lundell L, Ruth M. Baclofen-mediated gastro-oesophageal acid reflux control in patients with established reflux disease. Aliment Pharmacol Ther. 2002;16:869–873. doi: 10.1046/j.1365-2036.2002.01250.x. [DOI] [PubMed] [Google Scholar]
- Carlton SM. Peripheral excitatory amino acids. Curr Opin Pharmacol. 2001;1:52–56. doi: 10.1016/s1471-4892(01)00002-9. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Coggeshall RE. Inflammation-induced changes in peripheral glutamate receptor populations. Brain Res. 1999;820:63–70. doi: 10.1016/s0006-8993(98)01328-6. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL, Coggeshall RE. Localization and activation of glutamate receptors in unmyelinated axons of rat glabrous skin. Neurosci Lett. 1995;197:25–28. doi: 10.1016/0304-3940(95)11889-5. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL, Coggeshall RE. Localization of metabotropic glutamate receptors 2/3 on primary afferent axons in the rat. Neuroscience. 2001;105:957–969. doi: 10.1016/s0306-4522(01)00238-x. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Chang HM, Liao WC, Lue JH, Wen CY, Shieh JY. Upregulation of NMDA receptor and neuronal NADPH-d/NOS expression in the nodose ganglion of acute hypoxic rats. J Chem Neuroanat. 2003;25:137–147. doi: 10.1016/s0891-0618(02)00101-1. [DOI] [PubMed] [Google Scholar]
- Chen CY, Ling Eh EH, Horowitz JM, Bonham AC. Synaptic transmission in nucleus tractus solitarius is depressed by Group II and III but not Group I presynaptic metabotropic glutamate receptors in rats. J Physiol. 2002;538:773–786. doi: 10.1113/jphysiol.2001.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cincotta M, Beart PM, Summers RJ, Lodge D. Bidirectional transport of NMDA receptor and ionophore in the vagus nerve. Eur J Pharmacol. 1989;160:167–171. doi: 10.1016/0014-2999(89)90668-7. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Carlton SM. Ultrastructural analysis of NMDA, AMPA, and kainate receptors on unmyelinated and myelinated axons in the periphery. J Comp Neurol. 1998;391:78–86. doi: 10.1002/(sici)1096-9861(19980202)391:1<78::aid-cne7>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- Cosford ND, Tehrani L, Roppe J, Schweiger E, Smith ND, Anderson J, Bristow L, Brodkin J, Jiang X, McDonald I, Rao S, Washburn M, Varney MA. 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: a potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity. J Med Chem. 2003;46:204–206. doi: 10.1021/jm025570j. [DOI] [PubMed] [Google Scholar]
- Du J, Koltzenburg M, Carlton SM. Glutamate-induced excitation and sensitization of nociceptors in rat glabrous skin. Pain. 2001;89:187–198. doi: 10.1016/s0304-3959(00)00362-6. [DOI] [PubMed] [Google Scholar]
- Foley CM, Moffitt JA, Hay M, Hasser EM. Glutamate in the nucleus of the solitary tract activates both ionotropic and metabotropic glutamate receptors. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1858–R1866. doi: 10.1152/ajpregu.1998.275.6.R1858. [DOI] [PubMed] [Google Scholar]
- Frisby CL, Mattsson JP, Jensen JM, Lehmann A, Dent J, Blackshaw LA. Inhibition of transient lower esophageal sphincter relaxation and gastroesophageal reflux by metabotropic glutamate receptor ligands. Gastroenterology. 2005;129:995–1004. doi: 10.1053/j.gastro.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Furukawa N, Hatano M, Fukuda H. Glutaminergic vagal afferents may mediate both retching and gastric adaptive relaxation in dogs. Auton Neurosci. 2001;93:21–30. doi: 10.1016/S1566-0702(01)00322-8. [DOI] [PubMed] [Google Scholar]
- Glaum SR, Miller RJ. Metabotropic glutamate receptors mediate excitatory transmission in the nucleus of the solitary tract. J Neurosci. 1992;12:2251–2258. doi: 10.1523/JNEUROSCI.12-06-02251.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaum SR, Miller RJ. Metabotropic glutamate receptors depress afferent excitatory transmission in the rat nucleus tractus solitarii. J Neurophysiol. 1993;70:2669–2672. doi: 10.1152/jn.1993.70.6.2669. [DOI] [PubMed] [Google Scholar]
- Glaum SR, Miller RJ. Presynaptic metabotropic glutamate receptors modulate ω-conotoxin-GVIA-insensitive calcium channels in the rat medulla. Neuropharmacology. 1995;34:953–964. doi: 10.1016/0028-3908(95)00076-i. [DOI] [PubMed] [Google Scholar]
- Graham TE, Sgro V, Friars D, Gibala MJ. Glutamate ingestion: the plasma and muscle free amino acid pools of resting humans. Am J Physiol Endocrinol Metab. 2000;278:E83–E89. doi: 10.1152/ajpendo.2000.278.1.E83. [DOI] [PubMed] [Google Scholar]
- deGroot J, Zhou S, Carlton SM. Peripheral glutamate release in the hindpaw following low and high intensity sciatic stimulation. Neuroreport. 2000;11:497–502. doi: 10.1097/00001756-200002280-00014. [DOI] [PubMed] [Google Scholar]
- Hay M, Hoang CJ, Hasser EM, Price EM. Activation of metabotropic glutamate receptors inhibits synapsin I phosphorylation in visceral sensory neurons. J Membr Biol. 2000;178:195–204. doi: 10.1007/s002320010027. [DOI] [PubMed] [Google Scholar]
- van Herwaarden MA, Samsom M, Rydholm H, Smout AJ. The effect of baclofen on gastro-oesophageal reflux, lower oesophageal sphincter function and reflux symptoms in patients with reflux disease. Aliment Pharmacol Ther. 2002;16:1655–1662. doi: 10.1046/j.1365-2036.2002.01325.x. [DOI] [PubMed] [Google Scholar]
- Hoang CJ, Hay M. Expression of metabotropic glutamate receptors in nodose ganglia and the nucleus of the solitary tract. Am J Physiol Heart Circ Physiol. 2001;281:H457–H462. doi: 10.1152/ajpheart.2001.281.1.H457. [DOI] [PubMed] [Google Scholar]
- Jensen J, Lehmann A, Uvebrant A, Carlsson A, Jerndal G, Nilsson K, Frisby C, Blackshaw LA, Mattsson JP. Transient lower esophageal sphincter relaxations in dogs are inhibited by a metabotropic glutamate receptor 5 antagonist. Eur J Pharmacol. 2005;519:154–157. doi: 10.1016/j.ejphar.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL. Glutamate in the enteric nervous system. Curr Opin Pharmacol. 2001;1:591–596. doi: 10.1016/s1471-4892(01)00101-1. [DOI] [PubMed] [Google Scholar]
- Lehmann A, Antonsson M, Bremner-Danielsen M, Flardh M, Hansson-Branden L, Karrberg L. Activation of the GABAB receptor inhibits transient lower esophageal sphincter relaxations in dogs. Gastroenterology. 1999;117:1147–1154. doi: 10.1016/s0016-5085(99)70400-2. [DOI] [PubMed] [Google Scholar]
- Lehmann A, Branden L. Effects of antagonism of NMDA receptors on transient lower esophageal sphincter relaxations in the dog. Eur J Pharmacol. 2001;431:253–258. doi: 10.1016/s0014-2999(01)01442-x. [DOI] [PubMed] [Google Scholar]
- Lidums I, Lehmann A, Checklin H, Dent J, Holloway RH. Control of transient lower esophageal sphincter relaxations and reflux by the GABAB agonist baclofen in normal subjects. Gastroenterology. 2000;118:7–13. doi: 10.1016/s0016-5085(00)70408-2. [DOI] [PubMed] [Google Scholar]
- Liu Z, Chen CY, Bonham AC. Metabotropic glutamate receptors depress vagal and aortic baroreceptor signal transmission in the NTS. Am J Physiol Heart Circ Physiol. 1998;275:H1682–H1694. doi: 10.1152/ajpheart.1998.275.5.H1682. [DOI] [PubMed] [Google Scholar]
- Lynch DR, Guttmann RP. NMDA receptor pharmacology: perspectives from molecular biology. Curr Drug Targets. 2001;2:215–231. doi: 10.2174/1389450013348434. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, McRoberts JA, Ennes HS, Song B, Wang X, Jinton L, Corneliussen B, Mayer EA. Two N-methyl-D-aspartate receptors in rat dorsal root ganglia with different subunit composition and localization. J Comp Neurol. 2002;446:325–341. doi: 10.1002/cne.10202. [DOI] [PubMed] [Google Scholar]
- McRoberts JA, Coutinho SV, Marvizon JC, Grady EF, Tognetto M, Sengupta JN, Ennes HS, Chaban VV, Amadesi S, Creminon C, Lanthorn T, Geppetti P, Bunnett NW, Mayer EA. Role of peripheral N-methyl-D-aspartate (NMDA) receptors in visceral nociception in rats. Gastroenterology. 2001;120:1737–1748. doi: 10.1053/gast.2001.24848. [DOI] [PubMed] [Google Scholar]
- Morris JL, Konig P, Shimizu T, Jobling P, Gibbins IL. Most peptide-containing sensory neurons lack proteins for exocytotic release and vesicular transport of glutamate. J Comp Neurol. 2005;483:1–16. doi: 10.1002/cne.20399. [DOI] [PubMed] [Google Scholar]
- Page AJ, Blackshaw LA. GABAB receptors inhibit mechanosensitivity of primary afferent endings. J Neurosci. 1999;19:8597–8602. doi: 10.1523/JNEUROSCI.19-19-08597.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Martinez-Salgado C, Wemmie JA, Brennan TJ, Symonds E, Omari T, Lewin GR, Welsh MJ, Blackshaw LA. The ion channel ASIC1 contributes to visceral but not cutaneous mechanoreceptor function. Gastroenterology. 2004;127:1739–1747. doi: 10.1053/j.gastro.2004.08.061. [DOI] [PubMed] [Google Scholar]
- Page AJ, Martin CM, Blackshaw LA. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol. 2002;87:2095–2103. doi: 10.1152/jn.00785.2001. [DOI] [PubMed] [Google Scholar]
- Page AJ, Slattery JA, O'Donnell TA, Cooper NJ, Young RL, Blackshaw LA. Modulation of gastro-oesophageal vagal afferents by galanin in mouse and ferret. J Physiol. 2005a;563:809–819. doi: 10.1113/jphysiol.2004.075291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Young RL, Martin CM, Umaerus M, O'Donnell TA, Cooper NJ, Coldwell JR, Hulander M, Mattsson JP, Lehmann A, Blackshaw LA. Metabotropic glutamate receptors inhibit mechanosensitivity in vagal sensory neurons. Gastroenterology. 2005b;128:402–410. doi: 10.1053/j.gastro.2004.11.062. [DOI] [PubMed] [Google Scholar]
- Partosoedarso ER, Blackshaw LA. Roles of central glutamate, acetylcholine and CGRP receptors in gastrointestinal afferent inputs to vagal preganglionic neurones. Auton Neurosci. 2000;83:37–48. doi: 10.1016/S0165-1838(00)00109-0. [DOI] [PubMed] [Google Scholar]
- Partosoedarso ER, Young RL, Blackshaw LA. GABAB receptors on vagal afferent pathways: peripheral and central inhibition. Am J Physiol Gastrointest Liver Physiol. 2001;280:G658–G668. doi: 10.1152/ajpgi.2001.280.4.G658. [DOI] [PubMed] [Google Scholar]
- Raab M, Neuhuber WL. Vesicular glutamate transporter 2 immunoreactivity in putative vagal mechanosensor terminals of mouse and rat esophagus: indication of a local effector function? Cell Tissue Res. 2003;312:141–148. doi: 10.1007/s00441-003-0721-5. [DOI] [PubMed] [Google Scholar]
- Schaffar N, Rao H, Kessler JP, Jean A. Immunohistochemical detection of glutamate in rat vagal sensory neurons. Brain Res. 1997;778:302–308. doi: 10.1016/s0006-8993(97)01058-5. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Petersen J, Peles S, Shaker R. Response properties of antral mechanosensitive afferent fibers and effects of ionotropic glutamate receptor antagonists. Neuroscience. 2004;125:711–723. doi: 10.1016/j.neuroscience.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Ohishi H, Nakanishi S, Mizuno N. Expression of the mRNA for the rat NMDA receptor (NMDAR1) in the sensory and autonomic ganglion neurons. Neurosci Lett. 1992;144:229–232. doi: 10.1016/0304-3940(92)90756-w. [DOI] [PubMed] [Google Scholar]
- Tong Q, Ma J, Kirchgessner AL. Vesicular glutamate transporter 2 in the brain-gut axis. Neuroreport. 2001;12:3929–3934. doi: 10.1097/00001756-200112210-00015. [DOI] [PubMed] [Google Scholar]
- Wilding TJ, Huettner JE. Antagonist pharmacology of kainate- and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-preferring receptors. Mol Pharmacol. 1996;49:540–546. [PubMed] [Google Scholar]
- Yamakura T, Shimoji K. Subunit- and site-specific pharmacology of the NMDA receptor channel. Prog Neurobiol. 1999;59:279–298. doi: 10.1016/s0301-0082(99)00007-6. [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Costa M, Brookes SJ. Mechanotransduction by intraganglionic laminar endings of vagal tension receptors in the guinea-pig oesophagus. J Physiol. 2003;553:575–587. doi: 10.1113/jphysiol.2003.051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, D'Antona G, Brookes SJ, Costa M. Functional GABAB receptors are present in guinea pig nodose ganglion cell bodies but not in peripheral mechanosensitive endings. Auton Neurosci. 2002;102:20–29. doi: 10.1016/s1566-0702(02)00183-2. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Lehmann A, Rigda R, Dent J, Holloway RH. Control of transient lower oesophageal sphincter relaxations and reflux by the GABAB agonist baclofen in patients with gastro-oesophageal reflux disease. Gut. 2002;50:19–24. doi: 10.1136/gut.50.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]