Abstract
The purpose of this study was to assess the influence of the work history of the inspiratory muscles upon the fatigue characteristics of the plantar flexors (PF). We hypothesized that under conditions where the inspiratory muscle metaboreflex has been elicited, PF fatigue would be hastened due to peripheral vasoconstriction. Eight volunteers undertook seven test conditions, two of which followed 4 week of inspiratory muscle training (IMT). The inspiratory metaboreflex was induced by inspiring against a calibrated flow resistor. We measured torque and EMG during isometric PF exercise at 85% of maximal voluntary contraction (MVC) torque. Supramaximal twitches were superimposed upon MVC efforts at 1 min intervals (MVCTI); twitch interpolation assessed the level of central activation. PF was terminated (Tlim) when MVCTI was <50% of baseline MVC. PF Tlim was significantly shorter than control (9.93 ± 1.95 min) in the presence of a leg cuff inflated to 140 mmHg (4.89 ± 1.78 min; P = 0.006), as well as when PF was preceded immediately by fatiguing inspiratory muscle work (6.28 ± 2.24 min; P = 0.009). Resting the inspiratory muscles for 30 min restored the PF Tlim to control. After 4 weeks, IMT, inspiratory muscle work at the same absolute intensity did not influence PF Tlim, but Tlim was significantly shorter at the same relative intensity. The data are the first to provide evidence that the inspiratory muscle metaboreflex accelerates the rate of calf fatigue during PF, and that IMT attenuates this effect.
Previous studies have demonstrated that fatiguing inspiratory muscle work elicits a sympathetically mediated vasoconstrictor response in resting limbs; the so-called ‘inspiratory muscle metaboreflex’ (Sheel et al. 2001). In addition, Harms et al. (2000) have demonstrated that the time to the limit of tolerance during very heavy cycling exercise is inversely related to the prevailing level of inspiratory muscle work. Most recently, Romer et al. (2005) have extended these observations by demonstrating that the magnitude of exercise-induced limb muscle fatigue is also inversely related to the prevailing level of inspiratory muscle work. These studies suggest that the work of the inspiratory muscles is capable of influencing exercise tolerance, and that this is probably mediated via its influence upon limb perfusion.
There is evidence that specific inspiratory muscle training (IMT) improves exercise tolerance in both healthy young people (McConnell & Romer, 2004) and patients with lung disease (Lotters et al. 2002). We have previously suggested that one of the mechanisms mediating this response may be an attenuation, abolition, or delay of the inspiratory muscle metaboreflex (McConnell & Romer, 2004). However, there is as yet no direct evidence of any influence of IMT upon the metaboreflex, and the current study was designed to overcome this shortfall.
We used an isolated human lower limb model to assess the influence of seven test conditions upon the rate of fatigue development of the limb during plantar flexion exercise (PF). Selected test conditions were repeated before and after a 4-week programme of pressure threshold IMT. Our main hypotheses were that the work history of the inspiratory muscles (i.e. the presence or absence of fatigue) would influence the rate of fatigue development during PF, and that IMT would ameliorate the influence of fatiguing inspiratory muscle work upon the rate of PF fatigue.
Methods
Participants
Eight participants (one male) took part in a series of seven experiments (one with only calf exercise; four with inspiratory loading followed by calf exercise, and two with different levels of leg blood flow occlusion during calf exercise). Mean ± standard deviation (s.d.) for age was 29 ± 6.3 years, stature 167 ± 6.1 cm and body mass 73 ± 13.1 kg. All took part in exercise at least three times per week for a minimum of 45 min. Participants were non-smokers and none had any history of cardiovascular or respiratory disease. All participants gave written informed consent and completed a health and safety questionnaire. Approval was obtained from the School Ethics Committee, and all procedures conformed to the standards laid down in the Declaration of Helsinki.
General design
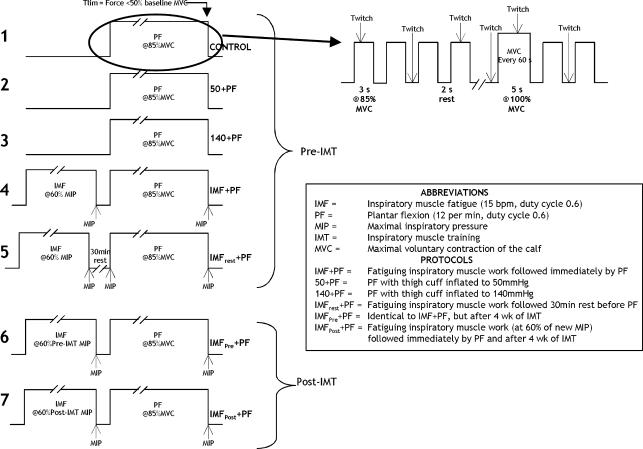
Participants undertook seven different protocols, which are illustrated in Fig. 1 (one participant failed to complete the IMFrest + PF protocol). Where possible, visits to the laboratory took place at a similar time of day. The first protocol (number 1) defined the control performance for calf plantar flexion (PF) exercise duration, which ceased when the fatigue criterion was met (Tlim, see later). Protocols 2 and 3 involved partial venous occlusion (50 mmHg and 140 mmHg, respectively) of the right thigh during right calf PF to Tlim, which was performed to test whether the PF Tlim was sensitive to changes in blood flow, and to assess the influence of occlusion upon physiological parameters. The remaining four protocols all involved inspiratory muscle loading prior to PF. Protocols 4 and 5 were administered before a 4-week period of IMT, while protocols 6 and 7 were administered following inspiratory muscle training (IMT). Protocol 4 involved a bout of fatiguing inspiratory muscle work (IMF), followed immediately by PF to Tlim (IMF + PF). This protocol was performed to assess the influence of IMF upon PF Tlim. Protocol 5 consisted of IMF followed by a 30 min rest, which was followed by PF exercise to Tlim (IMFrest + PF), which was performed to assess whether 30 min recovery of the inspiratory muscles abolished the influence of IMF. Protocols 6 and 7 were administered in a single-blind randomized order (four participants completed protocol 6 first, and four participants completed protocol 7 first). In one of these protocols the participants' pre-IMT IMF template was used (IMF at the same absolute intensity), and the loading was terminated at the time point that corresponded to Tlim in protocol 1 (control); IMF was followed immediately by PF (IMFpre + PF). This protocol was undertaken to compare the effects of an identical loading protocol undertaken before and after IMT. In the other protocol, a new IMF template was generated that took account of the improvement in inspiratory muscle function elicited by IMT (IMF at the same relative intensity). This was followed immediately by PF to Tlim (IMFpost + PF). This protocol was undertaken to assess whether a new, fatiguing IMF protocol induced changes similar to those observed in protocol 3 (IMF + PF). Throughout the four protocols involving inspiratory muscle loading (IMF), the only variable to be manipulated was the magnitude or timing of the inspiratory load administered. In addition, three participants undertook an additional session during which resting right femoral blood flow was measured during and following IMF.
Figure 1. Schematic diagram depicting the protocols implemented, as well as the pattern of muscle contraction, relaxation and superimposed twitch that was repeated until end test criteria were met.
Procedures
Maximal inspiratory pressure (MIP)
Maximal inspiratory mouth pressure (MIP; cmH2O) was recorded before a specific inspiratory muscle warm-up (RMU; Volianitis et al. 2001), immediately following the RMU, immediately following inspiratory muscle loading, and finally at the termination of PF exercise. The RMU was undertaken to minimize the number of MIP efforts required to attain a representative maximal value (Volianitis et al. 2001). MIP was measured at residual volume using a calibrated, portable hand-held mouth pressure meter (Micro Medical Ltd, Kent, UK) attached to a flanged mouthpiece and with a nose clip in place (PK Morgan Ltd, Gillingham, UK); the device was calibrated using a mercury manometer. As all participants had been previously habituated, the best MIP was recorded from a series of attempts resulting in three measurements within ±5% of one another (Volianitis et al. 2001).
Inspiratory muscle loading
Inspiratory muscle fatigue/loading (IMF) was achieved by participants breathing through a mouthpiece connected to a calibrated flow resistor with the nose occluded. Tidal volume (VT) was kept constant at 1.5 l, breathing frequency (fr) was 15 breaths min−1, mean inspiratory flow rate was 0.63 l s−1, target pressure was 60% of MIP and a duty cycle of 0.6 was adopted (3 s inspiration, 2 s expiration) (Sheel et al. 2001, 2002). Participants were instructed to follow a real-time pressure template that was displayed on an ‘oscilloscope’ chart on a laptop running a bespoke LabView 6 programme (National Instruments, Austin, TX, USA). The laptop also recorded the pressure template that was generated. Total inspiratory muscle work was calculated per protocol by summing the work done per breath (pressure × volume (cmH2O l), derived from the recorded pressure template). Standard respiratory tubing (diameter of 3.5 cm) was used to create an artificial dead space of 1 l, which was used to maintain end-tidal carbon dioxide close to resting levels.
A preliminary trial showed that the addition of the 1 l dead space to the IMF breathing circuit was insufficient to maintain complete isocapnia in all subjects; mean partial pressure of CO2 was 40.1 ± 4.28 mmHg at baseline and 28.5 ± 7.00 mmHg in the final minute of the IMF trial (P < 0.05); there was no significant difference across the trial. In human beings, acute severe hyperventilation elicits a decrease in calf vascular resistance and an increase in blood flow (Steurer et al. 1995; Coffman & Kelly, 1966). However, mild hypocapnia (∼30 mmHg) of the magnitude observed following our IMF task, fails to elicit changes in either forearm vascular resistance or blood pressure (Heistad & Wheeler, 1970). Furthermore, these authors also demonstrated that responses to lower body negative pressure were unaffected by this mild hypocapnia, suggesting that mild hypocapnia does not influence resting vessel tone, or reflex responses to baroreceptor stimulation.
Each minute participants were instructed to empty their lungs fully to residual volume and to produce a MIP using the mouth pressure meter. Participants then immediately recommenced the fatiguing/loading protocol. This sequence was repeated until participants failed to attain the pressure template target on three consecutive inspirations, following which they continued the protocol for a further minute (Sheel et al. 2001); thereafter a final MIP was recorded.
Specific inspiratory muscle training
A 4 week period of inspiratory muscle training was undertaken prior to the protocols 6 and 7. The IMT regimen consisted of 30 breaths twice daily (one set in the morning and one set in the evening). The initial load was set at 50% of MIP. The load was increased each week by the participants to ensure it continued to reflect ∼50% of their improving inspiratory muscle strength (Romer et al. 2002). Subjects adjusted the load themselves and were instructed to ensure that the load setting was such that they could only just complete 30 breaths. Each participant also completed a training diary so that IMT adherence and load adjustments could be monitored.
Blood flow
Following 10 min semisupine rest, blood flow was measured continuously (Echo Dopplersaote Megas GPX, Imotex, Italy) using the right femoral artery approximately 1 cm above the bifurcation to the superficial and deep femoral arteries (Radegran, 1997). A 7.5 Hz hand-held imaging probe with a variable Doppler frequency of 4.0–7.5 MHz was used to measure two-dimensional femoral artery diameter and blood velocity. Velocity was measured continuously, but each minute the recording mode was switched in order to make a measurement of vessel diameter. All measurements were recorded onto VHS videotape (TDK E-240TVED, TDK electronics, USA) for subsequent analysis. Insonation angle of the probe was <60 deg and the Doppler-shift spectra were corrected for this angulation using an on-screen cursor. Measurements were made at rest for 3 min, during the IMF task (60% MIP) and post-IMF for 3 min. Throughout the measurements, participants were seated upright on the dynamometer that was used for PF exercise (Biodex System 3 Pro, Biodex Medical Systems, New York) with the knee supported by the knee brace and the foot resting on the force transducer; the knee and foot being level (this position reflected that adopted during the calf exercise).
Blood flow was calculated as cross-sectional area (CSA) ([diameter/2] × 3.14) multiplied by the product of mean velocity time integral (VTI) multiplied by 60 s min−1 (blood flow = CSA × VTI × 60 s min−1 (Sheel et al. 2001)). Diameter showed no measurable changes throughout each trial and was assessed with the artery displayed along the longitudinal axis (B mode) at 1 min intervals by placing two on-screen cursors on each side of the arterial wall (Sheel et al. 2001). The VTI was calculated by integrating the total area under the outer envelope of the maximal velocity values of the blood velocity profile over the R–R interval. Thus, blood flow was calculated as mean flow per minute. Leg vascular conductance (LVC) was calculated subsequently by dividing mean blood flow per minute by average mean arterial pressure (MAP, see Blood pressure and heart rate) during the same minute (Romer et al. 2003).
Calf force measurement: voluntary and evoked
Isometric calf plantar flexion (PF) force was recorded using an isokinetic dynamometer (Biodex System 3 Pro, Biodex Medical Systems, New York). Torque measured by the dynamometer was interfaced to a data acquisition unit (Micro 1401, CED, UK), which sampled at 2 kHz. The analog to digital signal conversion rate was 100 Hz with an all-stop signal filter of 2 kHz (1902, CED, UK). The data acquisition unit and analog to digital filter were interfaced and recorded to a portable computer for a continuous graphical display of unrectified torque production, and analysed postexercise.
Isometic PF exercise was undertaken at an intensity of 85% of baseline maximal voluntary isometric PF contraction force (MVC) or MVC with superimposed twitch (see below, MVCTI), whichever was higher (see Fig. 1). Participants were seated, upright, with the knee at an angle of 90 deg and level with the height of the foot. The duty cycle was 0.6 (3 s contraction, 2 s relaxation) (Bigland-Ritchie et al. 1986) and was coordinated to a bespoke computer generated metronome. Eighty-five per cent MVC/MVCTI was marked on the force–trace screen of a portable computer (using Spike 2 version 5.04 software, Cambridge Electronic Design, UK) to provide visual feedback. Every 60 s, the duty cycle was temporarily suspended while a 5 s MVC with superimposed twitch was performed (MVCTI) (adapted from Bulow et al. 1995). Immediately after the MVCTI, participants re-coordinated with the metronome. This pattern was continued until participants reached their limit of tolerance and/or maintainable force fell below 50% MVC/MVCTI for three consecutive contractions (Schwendner et al. 1995).
Twitch interpolation was used to assess the degree of triceps surae voluntary activation (Bulow et al. 1995) (see Fig. 1). After cleaning the skin over the right gastrocenemius and soleus with alcohol pads, two large surface electrodes (13.5 cm × 8 cm, Dura-stick II, Chattanooga Group Inc, USA) were placed transcutaneously over the calf muscle and secured in place. In addition to being superimposed onto each MVC (∼3 s after commencement), twitches were stimulated in the resting muscle between PF voluntary contractions. Thus, twitches were superimposed every other contraction/relaxation cycle, so that twitch interpolation occurred alternately during resting and contraction phases. This pattern meant that between each superimposed twitch there was one whole cycle (contraction and relaxation) free from a superimposed twitch. (see Fig. 1). In addition, three resting twitches were given immediately before the start of calf exercise and immediately following its cessation.
The current amplitude (mA) of the electrical stimulator (DS7A, Digitimer, Hertfordshire, UK) was predetermined at the start of the testing sessions (280–300 mA) using a modified twitch-torque profile. This consisted of twitch increments of ∼40–50 mA every 60 s starting from 100 mA. This was continued until the torque produced failed to increase despite increasing current amplitude. The minimum amplitude required to elicit maximum torque was selected and verified with 2–3 further twitches (Scaglioni et al. 2002). Pulse width of the stimulus was set at 1000 μs, and voltage was set at a maximum of 400 V.
On analysis, the force data, which incorporated volitional and evoked force, were rectified and then smoothed (each 5 ms) to display mean force and allow the determination of central activation. The interpolated twitch was defined as the inflection in torque caused by superimposing a single twitch, above the plateau in voluntary torque (Fowles et al. 2000). Consequently, only the portion of the twitch above the ‘plateau’ was taken into account. If the twitch occurred when force was declining, a mean of the force from onset of the ‘plateau’ (the point where force ceased increasing), to the point immediately before the twitch was taken (this averaged ∼2–3 s). This was determined by measuring mean voluntary torque production (VT) during the plateau portion of the contraction and mean torque produced by the electrically evoked twitch (ET) that exceeded the VT. Interpolated twitch force was therefore ET minus VT. The degree of motor unit activation (%) was ([resting twitch/interpolated twitch]/resting twitch) × 100 (Fowles et al. 2000).
EMG
Differentiated EMG (mV; 1902 amplifier, CED, UK) was recorded from the right soleus and gastrocnemius throughout each protocol using bipolar electrodes (2 cm diameter; Arbo neonatal ECG electrodes, Kendall, Germany). The skin was cleaned using alcohol pads, following which one pair of electrodes was placed parallel over the belly of the gastrocnemius and one pair of electrodes was placed parallel to the muscle fibres on the inferior and lateral portion of the soleus (Cram & Kasman, 1998). A ground electrode was placed upon the bony aspect of the ankle. Each EMG electrode pair was separated by an interelectrode distance of 1 cm (de Luca, 1997).
Although EMG data were collected throughout MVCTI and submaximal contractions, EMG responses to twitch interpolation were not analysed. The EMG signals were subsequently digitized (1000× s−1; micro1401, CED, UK) and sampled at rate of 2 khz; a 20 Hz filter was used to eliminate DC shifts, mechanical artefact and extraneous electromyography provided by the body (Cram & Kasman, 1998). On analysis the raw EMG amplitude was rectified then smoothed as part of the root mean squared (RMS) process (time interval 5 ms) during each MVCTI effort (Cram & Kasman, 1998). The data were normalized by subtracting resting noise from the RMS.
Leg occlusion
Immediately before calf exercise, a standard thigh cuff was rapidly inflated using a hand pump around the mid-portion of the right thigh. Inflation pressure was maintained at either 50 mmHg (protocol 2; low level of occlusion) or 140 mmHg (protocol 3; moderate level of occlusion) and monitored throughout using a pressure dial connected to the cuff (adapted from (Cole & Brown, 2000). On termination of calf exercise and final MVCTI and twitches, the cuff was deflated immediately and loosened.
Blood pressure and heart rate
Mean arterial pressure and heart rate (fc) were recorded using an automated combined continuous monitor (CBM-700, COLIN, Scanmed, UK). A standard blood pressure cuff (12 cm width, circumference 23–33 cm) was placed around the top of the right arm positioned over the brachial artery. Blood pressure and fc were assessed each minute. Measurements were made throughout the active phases of all protocols, as well as for 3 min before PF and 3 min following PF cessation.
Ratings of perceived exertion
Ratings of perceived exertion (Borg 6–12 scale) were recorded during the final 10 s of each minute, immediately before MVCTI and at the end of calf exercise.
Data analysis
The seven conditions differed in duration both within and between participants; hence comparisons were made using isotime points (beginning, middle, end), as well as in a time-normalized format where time was normalized to the control protocol Tlim at 10% intervals for each participant.
A paired t test was used to compare differences in MIP pre- and post-RMU. Calf exercise duration and IMF duration were conducted using a one-way repeated measures ANOVA. Two-way (protocol × time) factorial ANOVAs with repeated measures were used to identify differences in MVC, EMG, central activation, RPE and MIP. Two sets of analysis were conducted on each variable: isotime and normalized time increments of 10% of control Tlim. Where applicable, Mauchly's test of sphericity was used to check for homogeneity of the covariance matrix. Where violated, the assumptions were corrected using Greenhouse–Geisser adjustments. Pairwise comparisons were made within the ANOVA using paired t tests with Bonferroni adjustments to modify the per family type I error rate per comparison. Results are expressed as mean ± standard deviation (s.d.) unless otherwise stated. In addition, planned, pairwise t tests with Bonferroni adjustments were used to compare differences in fc and MAP at the 10% time point between control and each of the four IMF protocols. An alpha level of 0.05 was set as a priori for statistical significance. All statistical analyses were conducted using version 14.0 SPSS for Windows (SPSS Inc, Chicago Illinois, USA).
Results
Maximal inspiratory pressure (MIP)
Effects due to respiratory muscle ‘warm-up’ (RWU) and inspiratory muscle training (IMT)
There was a significant difference between protocols (P = 0.01) and a significant interaction effect pre- versus post-RWU (P = 0.035)) (see Table 1).
Table 1.
Group mean MIP values at pre-RMU, baseline (post-RMU), post-IMF and post-PF (Tlim) for the four protocols (mean ±s.d.)
| Protocol | Pre-RMU | Baseline MIP (cmH2O) | Post-IMF | Post-PF |
|---|---|---|---|---|
| IMF + PF | 120 ± 31.7* | 135 ± 31.3 | 100 ± 26.0‡§. | 131 ± 28.6‡¶# |
| IMFrest + PF | 129 ± 34.1* | 137 ± 32.4 | 97 ± 30.8‡§. | 130 ± 33.3‡¶# |
| IMFpre + PF | 141 ± 29.1*† | 161 ± 32.0† | 130 ± 25.0‡ | 148 ± 27.3‡¶ |
| IMFpost+ PF | 141 ± 32.0*† | 163 ± 29.9† | 114 ± 23.4‡ | 138 ± 24.1‡¶ |
Significantly lower than baseline (P ≤ 0.01)
significantly higher compared to pre-IMT values (IMF + PF and IMFpre + PF, P ≤ 0.01)
significantly lower than baseline (P ≤ 0.01)
significantly higher than post-IMF (P ≤ 0.01)
significantly lower than IMFpre + PF for post-IMF values (P ≤ 0.01)
significantly lower than IMFpre + PF for post-PF values (P ≤ 0.05). During the IMFpre + PF protocol, the IMF load was equivalent to 60% of the pre-IMT MIP, which equated to 50% of the post-IMT MIP. During the IMFpost+ PF protocol, IMF load was equivalent to 60% of the post-IMT MIP.
Effects due to the inspiratory muscle fatigue task (IMF)
There was a significant difference between the four protocols (P = 0.001), and between time points (P = 0.000), as well as a significant interaction effect (P = 0.002). Table 1 summarizes the data in isotime form; the breathing task significantly reduced MIP for all protocols (P = 0.000), but MIP recovered during PF (P = 0.000). MIP also recovered following 30 min rest in the IMFrest + PF protocol (to 136 ± 30.7 cmH2O, which was not significantly different from baseline MIP).
In a subgroup of three subjects, femoral arterial blood flow and MAP were measured on a separate occasion to assess the influence of the IMF task upon leg vascular conductance. During the 3 min immediately prior to the IMF task, mean LVC was 7.75 ± 1.70 ml min−1 mmHg−1, falling to 6.86 ± 1.24 ml min−1 mmHg−1 during the final minute of the task (−11.5%), and 5.28 ± 2.73 ml min−1 mmHg−1 (−31.8%) during the first minute of recovery. LVC had returned towards baseline levels after 2 min of recovery (6.72 ± 3.35 ml min−1 mmHg−1).
Total work completed during IMF tasks
There was no difference between any of the trials in terms of the total amount of work done (P = 0.510). The IMFpost + PF trial was shorter than the other three trials; thus, the work done per minute was greater (Table 2).
Table 2.
Group mean values for total inspiratory muscle work completed and time to task failure during the IMF trials preceding each of the four protocols (mean ±s.d.)
| Protocol | Inspiratory muscle work (cmH2O l) | Time to task failure (min) |
|---|---|---|
| IMF + PF | 1786 ± 566 | 5.60 ± 1.39 |
| (944–2541) | (3.52–8.56) | |
| IMFrest + PF | 1821 ± 629 | 5.22 ± 1.39 |
| (1255–2898) | (3.25–7.34) | |
| IMFpre + PF | 1719 ± 402 | 5.25 ± 1.08 |
| (1065–2232) | (3.25–7.00) | |
| IMFpost+ PF | 2172 ± 1783 | 4.74 ± 1.46 |
| (918–6347) | (2.38–7.22) |
n = 8 except IMFrest + PF where n = 6. Range in parentheses. During the IMFpre + PF protocol, the IMF load was equivalent to 60% of the pre-IMT MIP, which equated to 50% of the post-IMT MIP. During the IMFpost+ PF protocol, IMF load was equivalent to 60% of the post-IMT MIP.
Performance and perceptual changes
Data are expressed in two forms; firstly, as a protocol isotime (beginning, middle, end); secondly, with respect to time at normalized time points corresponding to 10–100% of the control Tlim duration for each subject. The latter accounted for differences in absolute Tlim between subjects. Statistical comparison of the latter data could only be made up to the 40% control Tlim time point, which was the final point at which data were recorded for all protocols, i.e. the duration of the shortest protocol.
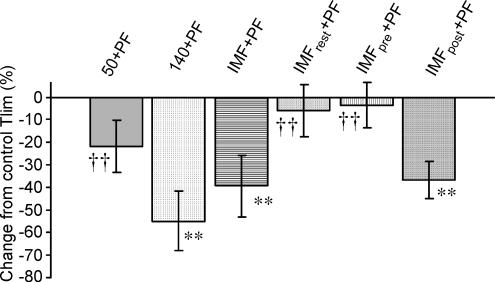
Plantar flexion Tlim
The time taken for MVC force to decrease by 50% from baseline differed significantly between protocols (P = 0.000; see Fig. 2). The longest Tlim was the control protocol (9.93 ± 1.95 min), which was significantly longer than 140 + PF (4.89 ± 1.78 min; P = 0.006), IMF + PF (6.28 ± 2.24 min; P = 0.009) and IMFpost + PF (6.33 ± 1.67; P = 0.001). There was no difference in the Tlim between the control, 50 + PF (7.77 ± 1.91 min), IMFrest + PF (9.55 ± 1.88 min) or IMFpre + PF (9.52 ± 1.94 min). Similarly there was no difference between 140 + PF, IMFpre + PF and IMFpost+ PF protocols. The 50 + PF protocol was only different from 140 mmHg (P = 0.030).
Figure 2. Tlim as a percentage change from the control Tlim for each of the experimental protocols.
** = significantly different from control; †† = significantly different from 140 + PF (P ≤ 0.01).
Ratings of perceived exertion (RPE)
Expressed in isotime (beginning, middle, end), there was no difference in RPE between protocols, but RPE did increase over time (P = 0.000). There were also no differences between protocols when data were normalized with respect to time 10–100% of control Tlim duration.
Muscle property changes
Maximal voluntary contraction force profile
There was a significant effect of time (P = 0.01) within protocols for isotime data (see Table 3).
Table 3.
Group mean MVCTI at each isotime point for each protocol (mean ±s.d.)
| Isotime (N) | |||
|---|---|---|---|
| Protocol | Baseline | Mid | End |
| Control | 293 ± 66.90 | 208 ± 42.65* | 143 ± 35.44*† |
| 50 + PF | 269 ± 80.38 | 201 ± 62.60* | 136 ± 34.55*† |
| 140 + PF | 281 ± 54.85 | 166 ± 54.85* | 116 ± 38.13*† |
| IMF + PF | 303 ± 63.54 | 217 ± 40.19* | 152 ± 27.81*† |
| IMFrest + PF | 298 ± 53.36 | 234 ± 57.85* | 150 ± 38.50*† |
| IMFpre + PF | 300 ± 67.82 | 237 ± 82.18* | 134 ± 27.10*† |
| IMFpost+ PF | 304 ± 54.72 | 204 ± 40.84* | 140 ± 24.12*† |
Significantly different from base (P ≤ 0.01)
significantly different from midpoint (P ≤ 0.01). No significant difference was observed between protocols (P ≥ 0.05).
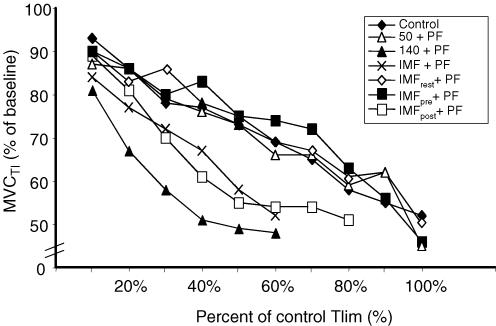
For the time-normalized data there was a clear tendency for MVCTI to decline in the 140 + PF, IMF + PF and IMFpost+ PF protocols, and for this to be greater than for other protocols (see Fig. 3). There was a significant effect of time for all protocols (P = 0.005).
Figure 3. Mean percentage changes from baseline MVCTI.
Significance levels are not depicted on the figure for clarity of presentation; there was a significant effect of time for all protocols (P = 0.005).
EMG
There was no significant difference between protocols when mean gastrocnemius EMG (gEMG) was expressed at isotime (P = 0.447), but there was a progressive reduction throughout the PF exercise (P = 0.000). There were also no significant differences between protocols when the time-normalized data were compared across the 10–40% time points (P = 0.751), or within protocols across time (P = 0.421).
Similar changes were observed for soleus EMG (sEMG), i.e. there was no difference between conditions at the isotime points (P = 0.214), but there was a progressive reduction over time during each protocol (P = 0.026). No significant difference was observed between protocols when the time-normalized data were compared up to the 40% time point (P = 0.73), but in contrast to gEMG, there was a significant effect of time upon sEMG within protocols (P = 0.020).
Central activation
When expressed in isotime, there were no significant differences between protocols (P = 0.851), but there was a significant effect of time within protocols (P = 0.009). When time-normalized data were compared at the 10–40% time points, there was no significant effect of protocol (P = 0.644) or time (P = 0.266) upon central activation.
Muscle twitch-resting amplitude (RTA)
Twitch potentiation was observed during the first minute, hence all comparisons of twitch data are expressed as percentage change from minute 1 of the protocol, rather than percentage change from baseline. This normalization also helped to overcome the wide daily variation in mean RTA.
When compared at isotimes there was no significant difference between protocols (P = 0.245), but there was a significant difference across time within protocols (P = 0.029). (see Table 4). Statistical comparison of time-normalized data up to 40% control Tlim revealed no significant difference between (P = 0.060) or within protocols (P = 0.805).
Table 4.
Group mean RTA at each isotime point between each protocol: expressed as percentage change from minute 1 (mean ±s.d.)
| Isotime (%) | |||
|---|---|---|---|
| Protocol | Mid | End | Post |
| Control | −0.1 ± 18.09 | −5.9 ± 15.58 | −10.93 ± 14.23* |
| 50 + PF | −4.1 ± 26.15 | −4.8 ± 26.97 | −1.9 ± 27.99* |
| 140 + PF | −5.9 ± 14.57 | −10.3 ± 21.60 | −10.4 ± 14.39* |
| IMF + PF | 3.9 ± 16.05 | 6.4 ±−17.8 | −15.7 ± 17.53* |
| IMFrest + PF | 14.6 ± 22.50 | −0.7 ± 16.76 | −3.9 ± 18.44* |
| IMFpre + PF | 3.1 ± 7.65 | −3.8 ± 18.29 | −9.1 ± 10.10* |
| IMFpost+ PF | 10.5 ± 22.25 | 10.5 ± 38.19 | 1.2 ± 32.49* |
Significantly different from mean RTA at minute 1 (P ≤ 0.05). No significant difference was observed between protocols, but there was a significant effect of time within protocols (P = 0.029).
Muscle twitch-interrelationships
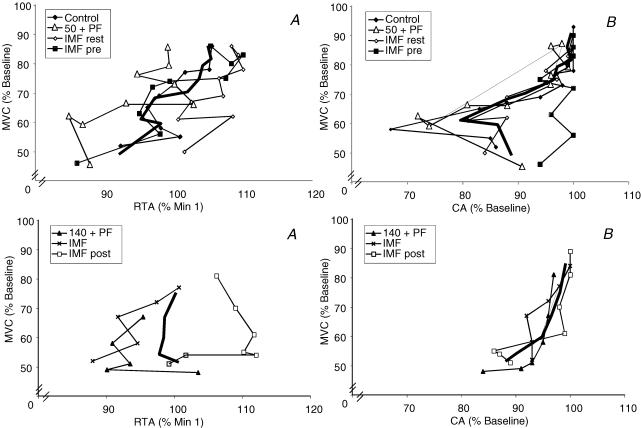
The interrelationship of MVC and RTA is shown in Fig. 4A (left panels). The RTA was very labile, but it is apparent that in the protocols in which Tlim was indistinguishable from control (upper left panel) that a decline in MVC was associated with a decline in RTA. Similarly, in the protocols with significant shortening of Tlim relative to control (lower left panel), a decline in MVC is generally associated with a decline in RTA, although the response in the IMFpost protocol is ambiguous, as is the final data point for the 140 + PF protocol.
Figure 4. Interrelationship of maximal voluntary contraction force (MVC) with resting twitch amplitude (RTA) and central activation (CA), respectively.
Top panel represents data from protocols in which Tlim was not significantly different from control; bottom panel represents data from protocols in which Tlim was significantly shorter than control. Bold line = average. Significance levels are not depicted on the figure for clarity of presentation, See text for details.
The interrelationship of MVC and CA are shown in Fig. 4B (right panels). Since CA is derived using RTA, the data are also quite labile, but in the protocols in which Tlim was indistinguishable from control (upper right panel) a decline in MVC was associated with a decline in CA. Similarly, in the protocols with significant shortening of Tlim relative to control (lower right panel), a decline in MVC is generally associated with a decline in CA.
Cardiovascular changes
Mean arterial pressure
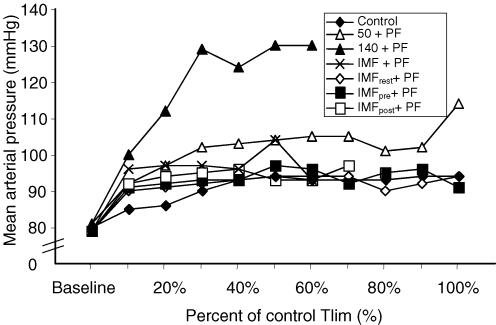
When compared at isotimes, a significant difference was observed between (P = 0.035) and within protocols (P = 0.021), but there was no interaction effect of protocol and time. Mid- and End time points were significantly higher than Baseline and Post for all protocols (P ≤ 0.01). The 140 + PF and 50 + PF protocols were significantly higher than all other protocols (P ≤ 0.05), and 140 + PF was also significantly higher than the 50 + PF protocol (P = 0.002).
Comparison of time-normalized data revealed no significant difference between (P = 0.237) or within protocols (P = 0.474) (see Fig. 5). During the 140 + PF protocol, MAP was significantly greater at every time point compared to all other protocols.
Figure 5. Average mean arterial pressure (MAP) recorded throughout the each protocol.
Significance levels are not depicted on the figure for clarity of presentation, see text for details.
Planned pairwise t tests on the MAP at the 10% time increment (with Bonferroni correction; P < 0.0125) indicated a tendency for higher values of MAP at this point for protocols that were preceded immediately by inspiratory muscle work. After Bonferroni correction, this difference was significant between control and IMFpost + PF (84.8 ± 7.8 versus 92.4 ± 6.86 mmHg, P = 0.004), but not between control and IMF + PF (95.6 ± 14.05 mmHg, P = 0.046), or IMFrest + PF (90.3 ± 13.5 mmHg, P = 0.0179), or IMFpre + PF (91.1 ± 11.0 mmHg, P = 0.044).
Heart rate
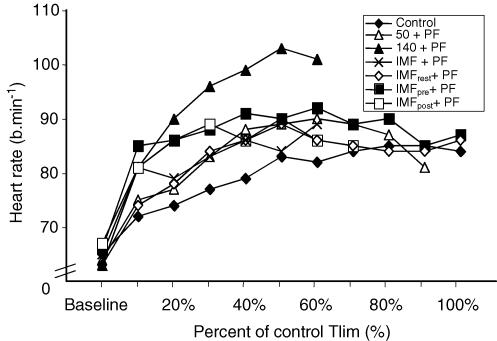
When expressed in isotime, no significant difference was observed between protocols (P = 0.093), but fc increased significantly with time (P = 0.001). Mid and End time points were significantly higher than Baseline and Post for all protocols (P ≤ 0.05). Post was significantly higher than Baseline for all protocols ((P ≤ 0.05).
There was no significant difference between conditions (P = 0.277) or within (P = 0.096) protocols for the time-normalized data (10–40% of control Tlim), However, fc demonstrated a time-dependent increase for all protocols (see Fig. 6).
Figure 6. Average heart rate recorded throughout each protocol.
Significance levels are not depicted on the figure for clarity of presentation, see text for details.
Planned pairwise t tests on the fc at the 10% time increment (with Bonferroni correction; P < 0.0125) indicated a tendency for higher values of fc at this point for protocols that were preceded immediately by inspiratory muscle work. However, after Bonferroni correction there was no significant difference in the fc between control (71.9 ± 8.03 beats min−1) IMF + PF (80.6 ± 16.8 beats min−1, P = 0.0124), IMFrest + PF (74.3 ± 13.36 beats min−1, P = 0.288), IMFpre + PF (84.9 ± 16.32 beats min−1, P = 0.018), or IMFpost+ PF (80.9 ± 12.54 beats min−1, P = 0.016).
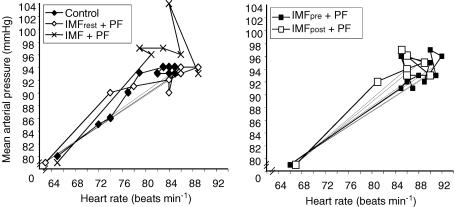
The interrelationship of MAP and fc is depicted in Fig. 7. The left panel depicts data for pre-IMT protocols (excluding the occlusion trials), while the right panel depicts data for the post-IMT protocols. The MAP appears to be more closely regulated under control conditions than during any other protocol, and is indistinguishable from IMFrest + PF. In contrast, during the IMF + PF protocol, MAP is higher for a given fc, relative to control. For the two post-IMT protocols (right panel), there is a tendency for MAP to be lower at a given fc for the IMFpre + PF protocol compared with IMFpost+ PF.
Figure 7. Average heart rate and mean arterial pressure recorded throughout each protocol.
Discussion
The data support the notion that the work history of the inspiratory muscles influences the time to fatigue of the calf muscles during plantar flexion exercise (Tlim). Prior fatigue of the inspiratory muscles hastened fatigue of the plantar flexors; the accompanying pattern of physiological changes was very similar to that observed when limb blood flow was restricted mechanically using a thigh cuff inflated to 140 mmHg. Further, after 4 weeks of IMT, an identical bout of inspiratory muscle work undertaken prior to plantar flexion resulted in no change in the plantar flexor Tlim. These data are the first to demonstrate that prior fatigue of the inspiratory muscles influences limb performance during subsequent exercise, and that IMT abolishes this response. They also provide strong support for the notion that an ergogenic influence of IMT upon whole-body exercise performance may be underpinned, at least in part, by preservation of limb blood flow.
Having established that our model was sensitive to the influence of changes in limb perfusion, it was our intention to create a number of protocol scenarios in which the work history of the inspiratory muscles varied. This enabled us to compare the physiological repercussions of performing PF exercise immediately after the inspiratory muscle fatigue task (IMF) with those observed when the inspiratory muscles had either been rested for 30 min, trained, or exercised at a different relative (but identical absolute) intensity. As intended, the IMF task resulted in significant inspiratory muscle fatigue during both of the pre-IMT tests (IMF + PF and IMFrest + PF; MIP fell 26% and 29%, respectively), as well as after IMT during the protocol in which the inspiratory load was adjusted to the same relative intensity (MIP fell 31%), i.e. 60% of the post-IMT MIP. When the inspiratory muscles were rested for 30 min prior to the PF exercise, MIP recovered so that it was not significantly different from the pre-IMF value. Further, a significantly lower level of fatigue was observed (19%) when the IMF task was undertaken at the same absolute intensity post-IMT as had been undertaken pre-IMT, i.e. 60% of the pre-IMT MIP (50% of the post-IMT MIP). Thus, we achieved our objective of commencing PF exercise under conditions of differing inspiratory muscle function.
Our inspiratory muscle fatigue task was based upon those shown previously by Sheel et al. (2001, 2002) and Romer et al. (2003) to evoke an inspiratory muscle metaboreflex. We confirmed the efficacy of our protocol in a subgroup of three subjects who showed an average reduction in LVC of 32% in the first minute after cessation of the task. This is consistent with the magnitude of the maximum change reported by Romer et al. (24%) and Sheel et al. (∼34%) (Sheel et al. 2001; Romer et al. 2003) following IMF tasks with slightly differing duty cycles, but the same inspiratory load characteristics. Thus, we are confident that our protocol was sufficiently challenging to evoke an inspiratory muscle metaboreflex. However, the temporal features of the metaboreflex justify comment, since previous studies have shown a restoration of LVR/LVC within 30–60 s of the cessation of the IMF task when the task is followed by rest (Sheel et al. 2001, 2002). When rest follows the cessation of inspiratory muscle work, the restoration of the baroreflex set-point to resting levels will probably result in a rapid withdrawal of vasomotor outflow and a swift restoration of LVC, despite the persistence of a dissipating signal from the muscle metaborecptors (Shoemaker et al. 2000). The time dependency of muscle metaboreflex effects upon limb vascular resistance (Sheel et al. 2001, 2002) implies that it takes time before these effects exert their full influence upon sympathetic vasomotor outflow. Evidence suggests that it is the maturation of the input from muscle metaboreceptors that provokes an increase in vasomotor outflow (Pawelczyk et al. 1992; Sheel et al. 2002). The time course of the fc and MAP response in the control protocol was consistent with the gradual development of a metaboreflex input from the calf. However, if PF exercise is immediately superimposed upon a dissipating signal from the inspiratory metaboreceptors, the medullary centres will presumably be subjected immediately to new central command and mechanoreceptor feedback from the plantar flexors. Thus, it is possible that the commencement of PF exercise may have led to a swift restoration of the exercise pressor response, and a persistence of limb vasoconstriction. This is consistent with our observations of a more rapid onset of increases in heart rate and MAP under the IMF + PF and IMFpost+ PF conditions.
In addition, the interrelationship of the fc and MAP responses (Fig. 7) suggests that under conditions of pre-existing inspiratory metaboreflex activation, elevated vasoconstrictor activity may be present, since MAP was higher for a given fc. It was also interesting to note the greater variability of the MAP response under conditions of pre-existing inspiratory metaboreflex activation (Fig. 7), which suggests that the ability to regulate MAP may not be as fine as it is under control conditions, possibly because of an abrupt increase in the baroreceptor set-point. The temporal interrelationships discussed above warrant further investigation, as does the time course of the putative baroreceptor set-point resetting during exercise that accompanies or follows fatiguing inspiratory muscle work.
We believe that our paradigm has a number of advantages over that used in previous studies by Dempsey and colleagues, who have observed changes in muscle sympathetic nerve activity (St Croix et al. 2000; Derchak et al. 2002), leg vascular resistance (LVR) or conductance (LVC) and limb blood flow in human limbs during inspiratory and/or expiratory loading (Sheel et al. 2001; Derchak et al. 2002). In their study of the role of the metaboreflex during plantar flexion exercise, Romer et al. (2003) observed a confounding influence of concurrent respiratory loading upon calf EMG, such that changes in LVC were masked by the increase in calf activity. This influence required ‘correction’ of the blood flow data to compensate for the changes in calf activation. For this reason, we implemented the fatiguing inspiratory muscle work immediately prior to the PF exercise. Also, our approach excluded any potential influence of central respiratory motor output, breathing pattern, intrathoracic/intra-abdominal pressure changes and discomfort induced by the breathing task per se. Despite these methodological differences, our data are consistent with those of Romer et al. (2003) who noted a decrease in LVC when fatiguing inspiratory muscle work accompanied PF exercise.
In the present study, when an identical inspiratory muscle work task was completed after a period of IMT, there was a significant reduction in the severity of inspiratory muscle fatigue (IMF + PF = 26% cf. IMFpre + PF = 19%), and no influence of the IMF task upon PF Tlim. These observations are the first to suggest that IMT can increase the work threshold required to elicit the inspiratory muscle metaboreflex. However, it is interesting to note that the absolute reduction in MIP induced by the IMF task was around 30 cmH2O for both the IMF + PF and IMFpre + PF tests. This suggests that acute changes in the static strength of the inspiratory muscles are independent of the inspiratory muscle metaboreflex, since fatigue (a decrease in MIP) was seen under both the IMF + PF and IMFpre + PF conditions; however, Tlim was only curtailed in the case of the former. This is consistent with evidence that the inspiratory muscle metaboreflex is evoked by accumulation of intramuscular metabolic by-products (Sheel et al. 2002; Rodman et al. 2003) and that the reflex has threshold qualities (Sheel et al. 2002). It is therefore reasonable to suppose that after a period of IMT, the accumulation of metabolites during the original IMF task was insufficient to exceed the threshold required to evoke the metaboreflex, but that the muscle work was sufficient to produce an impairment in MIP. Similarly, when 30 min rest was permitted between the termination of the IMF task and the commencement of the PF exercise, the effects of the metaboreflex dissipated.
In seeking an explanation for our finding of a curtailment of Tlim following fatiguing inspiratory muscle work, it is important to exclude the possibility that this originated from a change in calf muscle blood flow due to a reduction in perfusion pressure, which has been shown to exacerbate muscle fatigue (Wright et al. 1999). Since there was no evidence that the MAP response was impaired following the protocols in which Tlim was curtailed, we are confident that the shorter Tlim during the IMF + PF and IMFpost+ PF protocols was attributable to a reduction in blood flow secondary to increased vasoconstriction, not to a reduction in driving pressure.
Another potential flaw in our study would be if the changes in MVCTI that formed the basis of our criterion for the determination of Tlim were due to a decline in subject motivation, or were a response to muscle discomfort. We attempted to account for this by measuring the degree of central activation and the resting muscle twitch amplitude (RTA) throughout all protocols. Unfortunately, due to the effects of potentiation and other factors, RTA proved to be a very labile quantity, and our data were more variable than we had anticipated (see Fig. 4A). However, there is clear evidence of genuine peripheral fatigue, particularly under the two protocols in which ‘real’ IMF was induced, as well as during mechanical occlusion at 140 mmHg, and there was a significant reduction in RTA immediately after cessation of PF. Figure 4A illustrates separately the interrelationship of MVCTI and RTA for the protocols in which Tlim was unchanged relative to control (50 + PF, IMFrest + PF, IMFpre + PF) or curtailed (140 + PF; IMF + PF and IMFpost+ PF). Similarly, the interrelationship of MVCTI and central activation (CA) for the unchanged, and curtailed Tlim protocols are illustrated in Fig. 4B. If withdrawal of voluntary effort had been the primary cause of the fall in MVCTI that led to the termination of PF, then we would expect to see this reflected in the level of CA for the protocols in which Tlim was curtailed. However, CA remained high throughout all three curtailed protocols, falling only during the second half of each; it was also accompanied by a progressive decline in MVCTI; in other words, MVCTI was already on an almost linear downward trajectory before there was any evidence of a reduction in CA. There was also no significant difference observed between protocols in the level of CA at any time point.
The EMG data also support our belief that the time-dependent reduction in MVCTI was due to genuine fatigue. With a flexed knee position (as in the current study) the predominant force producer is the soleus muscle (Sale et al. 1982; Cresswell et al. 1995); this is because soleus cross-sectional area is greater (Sale et al. 1982), and gastrocnemius length is shorter (Cresswell et al. 1995). Thus, the soleus was likely to have been the major contributor to force production during the PF task. Both gEMG and sEMG declined progressively, indicating the development of fatigue (Kawakami et al. 2000; Patikas et al. 2002), and there was no difference between protocols in this response. However, the reduction in EMG at the end of the protocols was greatest in the soleus (43% versus 29%). Had the observed reductions in MVCTI and EMG been due to a reduction in voluntary effort, then we would predict that the fall in sEMG and gEMG would be similar in magnitude. Our data are therefore consistent with well-established observations that the pattern of fatigue in the plantar flexors is one of a combination of central and peripheral influences (Kawakami et al. 2000; Boerio et al. 2005).
Since PF exercise of the type undertaken in this experiment would clearly have induced considerable local metabolite production, another important question that arises is why didn't local vasodilator effects within the exercising muscle overwhelm the descending sympathetic vasoconstrictor influence? We can only speculate upon this question, but it has been shown previously that even during maximal cycling exercise, the inspiratory muscle metaboreflex overrides local vasodilator influences, and induces vasoconstriction (Harms et al. 1997, 1998). In addition, Secher & Volianitis (2006) have argued that the predominant mechanism by which MAP is defended during rest and exercise is via modulation of vascular conductance, even when there is reserve capacity to increase cardiac output. The necessity to maintain a restraining influence upon functional hyperaemia is self-evident, since failure to do so would result in a catastrophic fall in MAP. Thus, implicitly, the potency of sympathetic reflex control of limb vasoconstriction must exceed that of the local vasodilatory influences. Hence, in the face of a pre-existing increase in sympathetic vasomotor outflow following fatiguing inspiratory muscle work, and a rapidly evolving, and perhaps ‘potentiated’ metaboreflex input from the exercising calf during PF, it is perhaps not surprising that the local vasodilatory influence was overwhelmed. Indeed, there is now ample evidence that muscle metaboreflexes emanating from the upper limbs are sufficiently potent to induce vasoconstriction in the exercising legs. For example, intense handgrip or forearm exercise reduced calf blood flow during plantar flexion (Saito et al. 1992; Kagaya et al. 1994), and the response had the same threshold properties as have been observed for the inspiratory muscles (Saito et al. 1992). Thus, the inspiratory muscle metaboreflex appears to have very similar properties to that of limb muscles.
Summary
Our data are consistent with those of previous investigators who have demonstrated that elevating inspiratory muscle work during heavy exercise exacerbates leg fatigue (Romer et al. 2005). The data are the first to provide direct evidence that in the absence of any limitation to cardiac output, the inspiratory muscle metaboreflex reduces exercising limb blood flow and accelerates fatigue. Furthermore, they are the first to demonstrate that IMT attenuates the vasomotor changes induced by the metaboreflex response to an inspiratory muscle work task at the same absolute intensity, but not at the same relative intensity. These data are consistent with repeated observations that IMT improves exercise tolerance in healthy young adults, and patients, and suggest that this arises in part because of an attenuation or delay in the vasomotor changes induced by the inspiratory muscle metaboreflex.
Acknowledgments
The authors are most grateful for the helpful comments of Drs Lee Romer and Stefanos Volianitis during the preparation of our manuscript, and to Dr Alex Nowicky for his expert technical guidance. AKM acknowledges a beneficial interest in the POWERbreathe inspiratory muscle trainer in the form of a royalty share on licence income to the University of Birmingham. AKM provides consultancy services to Gaiam Ltd.
References
- Bigland-Ritchie B, Furbush F, Woods JJ. Fatigue of intermittent submaximal voluntary contractions: central and peripheral factors. J Appl Physiol. 1986;61:421–429. doi: 10.1152/jappl.1986.61.2.421. [DOI] [PubMed] [Google Scholar]
- Boerio D, Jubeau M, Zory R, Maffiuletti NA. Central and peripheral fatigue after electrostimulation-induced resistance exercise. Med Sci Sports Exerc. 2005;37:973–978. [PubMed] [Google Scholar]
- Bulow PM, Norregaard J, Mehlsen J, Danneskiold-Samsoe B. The twitch interpolation technique for study of fatigue of human quadriceps muscle. J Neurosci Meth. 1995;62:103–109. doi: 10.1016/0165-0270(95)00062-3. [DOI] [PubMed] [Google Scholar]
- Coffman JD, Kelly P. Hyperventilation and human calf blood flow. Am J Physiol. 1966;211:1255–1260. doi: 10.1152/ajplegacy.1966.211.5.1255. [DOI] [PubMed] [Google Scholar]
- Cole MA, Brown MD. Response of the human triceps surae muscle to electrical stimulation during varying levels of blood flow restriction. Eur J Appl Physiol. 2000;82:39–44. doi: 10.1007/s004210050649. [DOI] [PubMed] [Google Scholar]
- Cram JR, Kasman GS. Introduction to Surface Electromyography. USA: Aspen Publishers Inc; 1998. [Google Scholar]
- Cresswell AG, Loscher WN, Thorstensson A. Influence of gastrocnemius muscle length on triceps surae torque development and electromyographic activity in man. Exp Brain Res. 1995;105:283–290. doi: 10.1007/BF00240964. [DOI] [PubMed] [Google Scholar]
- Derchak PA, Sheel AW, Morgan BJ, Dempsey JA. Effects of expiratory muscle work on muscle sympathetic nerve activity. J Appl Physiol. 2002;92:1539–1552. doi: 10.1152/japplphysiol.00790.2001. [DOI] [PubMed] [Google Scholar]
- Fowles JR, Sale DG, MacDougall JD. Reduced strength after passive stretch of the human plantarflexors. J Appl Physiol. 2000;89:1179–1188. doi: 10.1152/jappl.2000.89.3.1179. [DOI] [PubMed] [Google Scholar]
- Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol. 1997;82:1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Hanson P, Dempsey JA. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol. 1998;85:609–618. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- Harms CA, Wetter TJ, St Croix CM, Pegelow DF, Dempsey JA. Effects of respiratory muscle work on exercise performance. J Appl Physiol. 2000;89:131–138. doi: 10.1152/jappl.2000.89.1.131. [DOI] [PubMed] [Google Scholar]
- Heistad DD, Wheeler RC. Effect of acute hypoxia on vascular responsiveness in man. I. Responsiveness to lower body negative pressure and ice on the forehead. II. Responses to norepinephrine and angiotensin. 3. Effect of hypoxia and hypocapnia. J Clin Invest. 1970;49:1252–1265. doi: 10.1172/JCI106338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagaya A, Saito M, Ogita F, Shinohara M. Exhausting handgrip exercise reduces the blood flow in the active calf muscle exercising at low intensity. Eur J Appl Physiol Occup Physiol. 1994;68:252–257. doi: 10.1007/BF00376774. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Amemiya K, Kanehisa H, Ikegawa S, Fukunaga T. Fatigue responses of human triceps surae muscles during repetitive maximal isometric contractions. J Appl Physiol. 2000;88:1969–1975. doi: 10.1152/jappl.2000.88.6.1969. [DOI] [PubMed] [Google Scholar]
- Lotters F, van Tol B, Kwakkel G, Gosselink R. Effects of controlled inspiratory muscle training in patients with COPD: a meta-analysis. Eur Resp J. 2002;20:570–577. doi: 10.1183/09031936.02.00237402. [DOI] [PubMed] [Google Scholar]
- de Luca CJ. The use of surface electromyography in biomechanics. J Appl Biomech. 1997;13:135–163. [Google Scholar]
- McConnell AK, Romer LM. Respiratory muscle training in healthy humans: resolving the controversy. Int J Sports Med. 2004;25:284–293. doi: 10.1055/s-2004-815827. [DOI] [PubMed] [Google Scholar]
- Patikas D, Michailidis C, Bassa H, Kotzamanidis C, Tokmakidis S, Alexiou S, Koceja DM. Electromyographic changes of agonist and antagonist calf muscles during maximum isometric induced fatigue. Int J Sports Med. 2002;23:285–289. doi: 10.1055/s-2002-29079. [DOI] [PubMed] [Google Scholar]
- Pawelczyk JA, Hanel B, Pawelczyk RA, Warberg J, Secher NH. Leg vasoconstriction during dynamic exercise with reduced cardiac output. J Appl Physiol. 1992;73:1838–1846. doi: 10.1152/jappl.1992.73.5.1838. [DOI] [PubMed] [Google Scholar]
- Radegran G. Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. J Appl Physiol. 1997;83:1383–1388. doi: 10.1152/jappl.1997.83.4.1383. [DOI] [PubMed] [Google Scholar]
- Rodman JR, Henderson KS, Smith CA, Dempsey JA. Cardiovascular effects of the respiratory muscle metaboreflexes in dogs: rest and exercise. J Appl Physiol. 2003;95:1159–1169. doi: 10.1152/japplphysiol.00258.2003. [DOI] [PubMed] [Google Scholar]
- Romer LM, Haverkamp HC, Lovering AT, Pegelow DF, Dempsey JA. Effect of inspiratory muscle work on exercise-induced quadriceps muscle fatigue in healthy humans. J Physiol. 2005;571:425–439. doi: 10.1113/jphysiol.2005.099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer LM, McConnell AK, Jones DA. Effects of inspiratory muscle training on time-trial performance in trained cyclists. J Sports Sci. 2002;20:547–562. doi: 10.1080/026404102760000053. [DOI] [PubMed] [Google Scholar]
- Romer LM, Miller JD, Pegelow DF, Jaques AJ, Dempsey JA. Effects of combined inspiratory and expiratory muscle work on limb vascular conductance and blood flow. Med Sci Sports Exerc. 2003;35:S4. [Google Scholar]
- Saito M, Kagaya A, Ogita F, Shinohara M. Changes in muscle sympathetic nerve activity and calf blood flow during combined leg and forearm exercise. Acta Physiol Scand. 1992;146:449–456. doi: 10.1111/j.1748-1716.1992.tb09446.x. [DOI] [PubMed] [Google Scholar]
- Sale D, Quinlan J, Marsh E, McComas AJ, Belanger AY. Influence of joint position on ankle plantarflexion in humans. J Appl Physiol. 1982;52:1636–1642. doi: 10.1152/jappl.1982.52.6.1636. [DOI] [PubMed] [Google Scholar]
- Scaglioni G, Ferri A, Minetti AE, Martin A, Van Hoecke J, Capodaglio P, Sartorio A, Narici MV. Plantar flexor activation capacity and H reflex in older adults: adaptations to strength training. J Appl Physiol. 2002;92:2292–2302. doi: 10.1152/japplphysiol.00367.2001. [DOI] [PubMed] [Google Scholar]
- Schwendner KI, Mikesky AE, Wigglesworth JK, Burr DB. Recovery of dynamic muscle function following isokinetic fatigue testing. Int J Sports Med. 1995;16:185–189. doi: 10.1055/s-2007-972989. [DOI] [PubMed] [Google Scholar]
- Secher NH, Volianitis S. Are the arms and legs in competition for cardiac output? Med Sci Sports Exerc. 2006 doi: 10.1249/01.mss.0000230343.64000.ac. in press. [DOI] [PubMed] [Google Scholar]
- Sheel AW, Derchak PA, Morgan BJ, Pegelow DF, Jacques AJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J Physiol. 2001;537:277–289. doi: 10.1111/j.1469-7793.2001.0277k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheel AW, Derchak PA, Pegelow DF, Dempsey JA. Threshold effects of respiratory muscle work on limb vascular resistance. Am J Physiol Heart Circ Physiol. 2002;282:H1732–H1738. doi: 10.1152/ajpheart.00798.2001. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Herr MD, Sinoway LI. Dissociation of muscle sympathetic nerve activity and leg vascular resistance in humans. Am J Physiol Heart Circ Physiol. 2000;279:H1215–H1219. doi: 10.1152/ajpheart.2000.279.3.H1215. [DOI] [PubMed] [Google Scholar]
- St Croix CM, Morgan BJ, Wetter TJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J Physiol. 2000;529:493–504. doi: 10.1111/j.1469-7793.2000.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steurer J, Kaplan V, Vetter W, Bollinger A, Hoffmann U. Local blood flux in skin and muscle during voluntary hyperventilation in healthy controls and patients with hyperventilation syndrome. Int J Microcirc Clin Exp. 1995;15:277–282. doi: 10.1159/000179075. [DOI] [PubMed] [Google Scholar]
- Volianitis S, McConnell AK, Jones DA. Assessment of maximum inspiratory pressure. Prior submaximal respiratory muscle activity (‘warm-up’) enhances maximum inspiratory activity and attenuates the learning effect of repeated measurement. Respiration. 2001;68:22–27. doi: 10.1159/000050458. [DOI] [PubMed] [Google Scholar]
- Wright JR, McCloskey DI, Fitzpatrick RC. Effects of muscle perfusion pressure on fatigue and systemic arterial pressure in human subjects. J Appl Physiol. 1999;86:845–851. doi: 10.1152/jappl.1999.86.3.845. [DOI] [PubMed] [Google Scholar]