Abstract
We recently showed that the activity of cerebellar Golgi cells can be powerfully modulated by stimulation of peripheral afferents, in a pattern different to local Purkinje cells. Here we have examined the pathways underlying these responses. Graded electrical stimulation of muscle and cutaneous nerves revealed that long-lasting depressions and short-lasting excitations of Golgi cells were evoked by stimulation of cutaneous nerves at stimulus intensities that activated large mechanoreceptive afferents, and grew as additional afferents were recruited. In contrast, none of the neurones responded to stimulation of muscle nerves at intensities that activated group I afferents, although about half responded with long-lasting depressions, but not excitations, to stimuli that recruited group II and III afferents. Selective lesions of the spinal dorsal columns did not affect either of these types of response. After lesions of one lateral funiculus in the lumbar cord the responses evoked by stimulation of the hindlimb contralateral to the lesion were reduced or abolished, leaving responses evoked by ipsilateral hindlimb afferents unaltered. Since both ipsi- and contralateral afferents generate responses in Golgi cells, the convergence from the two sides must occur supraspinally. It is difficult to reconcile these properties with any of the direct spinocerebellar pathways or spinoreticulocerebellar pathways that have been described. Instead, it is likely that the responses are evoked via the multimodal ‘wide dynamic range’ neurones of the anterolateral system. Golgi cell activity may thus be powerfully enhanced or depressed during arousal via the anterolateral system.
Analyses of information processing within the cerebellar cortex have largely focused on the precise spatial organization of mossy fibre inputs. In the rat, the best-studied region of cerebellar cortex in terms of input is Crus II in the posterior lobe, and in this region the granule cell layer comprises ‘patches’ with focally specific mossy fibre inputs (Shambes et al. 1978). Most of the patches in Crus II have both direct inputs from specific territories of the trigeminal nerve, and long loop cerebropontocerebellar inputs via the sensorimotor cortex conveying information from the same receptive field, which are reflected in the responses of granule cells and Purkinje cells (Bower & Woolston, 1983; Bower & Kassel, 1990; Morissette & Bower, 1996) and also Golgi cells (Vos et al. 1999; Holtzman et al. 2006). Recent work in our laboratory has shown that Golgi cells also commonly respond to afferent inputs with long-lasting depressions in firing rate which may also be accompanied by short-lasting excitations. Both types of response may occur alone (or together) and can be evoked from a very extensive, often bilateral receptive field. Since both types of response could be evoked singly or together within individual Golgi cells, each response type may be generated by independent neural mechanisms and thus may be carried by separate pathways in the spinal cord.
In comparison to the short-lasting excitations, the long-lasting depressions of Golgi cell firing have been shown to be the most powerful responses in terms of changes in overall spike discharge. Since they represent a reduction in spike output, they may be driven by intracortical inhibition or disfacilitation via excitatory mossy fibre afferents. In contrast, short-lasting excitations are likely to be driven by mossy fibres, either through direct contacts with Golgi cells (Palay & Chan-Palay, 1974) or via parallel fibre connections. The aim of the current study was to determine which types of peripheral afferents generate these responses and through which spinal ascending pathways. A multitude of potential pathways convey peripheral information to the cerebellum, including direct spinocerebellar pathways, that relay in the brainstem, and pathways that relay in the cerebral cortex (Bloedel & Courville, 1981; Ruigrok & Cella 1995). We used graded electrical stimulation of nerves containing muscle or principally cutaneous afferents to examine which afferents contribute to these responses. Different spinocerebellar pathways (both direct and indirect) convey different types of information (Bloedel et al. 1981), so this approach can provide evidence as to which ascending systems are involved. Different ascending pathways also have axons that ascend at different locations (Bloedel et al. 1981), so allied to nerve stimulation, selective lesions of different funiculi were used to examine the location of the ascending pathways in the spinal cord.
Methods
Experiments were performed on 23 adult rats (250–450 g, Wistar) under urethane anaesthesia (1000–1200 mg kg−1, i.p.) or pentobarbitone (40 mg kg−1i.p. initially, maintained as necessary with additional doses of 5 mg, sufficient to abolish withdrawal reflexes). All procedures were approved by the local ethical review panel of the University of Cambridge and by UK Home Office (Animals (Scientific Procedures) Act) regulations.
Surgery
Animals were fixed in a sterotaxic frame and core body temperature was maintained between 37.5 and 38°C using a servo-controlled blanket system. After removal of the attached muscles, Crus II of the cerebellar posterior lobe was exposed through a small burr hole in the occipital bone. The dura was left intact.
Recording
Electrode penetrations were made at angles perpendicular to the folial surface (typically 40–50 deg from vertical) with penetrations spanning a mediolateral axis extending approximately 4 mm lateral of the paravermal vein. The depth from the folial surface rarely exceeded 3 mm, allowing single unit recordings made from neurones located in lobules Crus Ic/IIa/b, locations confirmed by earlier studies using juxtacellular labelling (Holtzman et al. 2006).
Extracellular single neurone recordings were made with sharp (1 μm tip) stainless steel microelectrodes (impedance 2 MΩ). Microelectrode signals were amplified (gain, 10000), filtered (band-pass settings, 0.3–10 kHz for unit activity), and sampled (Cambridge Electronic Design 1401, Spike 2 software) at 25 kHz.
Analysis
On-line spike discrimination was used to allow immediate detection of alterations in unit behaviour and thence assist experimental decision-making. To assess responses in all experiments peristimulus time histograms (PSTHs) were made from at least 100 successive stimulus deliveries. Responses were assessed as described elsewhere (Holtzman et al. 2006), using PSTHs and cumulated summed differences (CUSUMs). Statistical significance tests on the PSTH data were made using the bins from the 200 ms period immediately preceding the stimulus as a control. For long-lasting depressions of Golgi cell activity, bins from the section of the PSTH 100–300 ms after the stimulus were compared with those from the control period using Student's t tests. Responses were considered to have occurred when the bin values were different at the P < 0.05 level (Holtzman et al. 2006). Analyses of the effects of the lesions on the long-lasting depressions were assessed from PSTHs and CUSUMs, as described above. Responses were considered to have been abolished if, after the lesion, the mean bin values from the period 100–300 ms after the stimulus were no longer significantly lower than the mean bin values for the 200 ms preceding the stimulus. In cases where the responses were not abolished, the ratios of mean bin values from the period 100–300 ms after the stimulus, and for the 200 ms before the stimulus, were used for group comparisons of the strength of the responses (paired t tests). For short-lasting excitations, the highest bin count within a window of 30 ms following the stimulus onset was used to quantify the response with reference to the number of stimuli used. The same protocol was applied when assessing the impact of spinal lesions on short lasting excitations, and under each condition, z tests were used to compare the response bin count with the counts in the bins used to establish the control period (–200 to 0 ms) to test for a significant response (judged if P < 0.01).
Nerve stimulation experiments
In experiments in which peripheral nerves were stimulated electrically (n = 12/23), branches of the sciatic nerve in the left hindlimb were dissected free, cut and ligated. These were subsequently mounted on pairs of fine silver wires, fitted within a fine plastic tube for stimulation. Threading the nerve into a fine tube minimized the spread of current from the short branches dissected free to the main nerve trunks. The skin of the hindlimb was formed into a pool, which was filled with mineral oil to prevent the nerves from drying. In these experiments, the aim was to determine the threshold at which long-lasting depressions and short-lasting excitations of Golgi cell firing first appeared, and to examine the relative effectiveness of muscle and cutaneous nerves in evoking these responses. The muscle nerves taken were the longest branches to the hamstring muscles, biceps femoris, semitendinosus, semimembranosus and caudalis femoris (HAM), and the branches to the two heads of gastrocnemius, soleus and plantaris, all taken together (GS). The main (caudal) descending branch of the sural nerve (SUR), but excluding the peroneal communicating branch, was taken as a cutaneous nerve. The tibial nerve (TIB), comprising the medial and lateral plantar nerves, but excluding the deep posterior muscles tibialis posterior and the long flexors of the toes, is a mixed nerve, although the cutaneous fibres outnumber the muscle afferent fibres. Nerves were stimulated with square pulses, 0.2 ms in duration. Short trains of 3–6 stimuli (3–5 ms intervals) were delivered at rates of between 0.3 and 1 Hz. Stimulus intensities were expressed in terms of threshold for the most excitable fibres in the nerve (T), which was assessed using volleys recorded from silver ball electrodes placed on the dorsal surface of the spinal cord at the dorsal root entry (the dura was left intact). In the same experiments, pairs of percutaneous pins were inserted into the footpads of the right hindlimb and the forelimbs, to allow identification of neurones that responded with long-lasting depressions or short-lasting excitations, to provide confirmation that these cells were Golgi cells (see Holtzman et al. 2006 for details). These were stimulated with single square-pulse stimuli at intensities that evoked contractions of the local muscles (0.5–5 mA).
Spinal lesion experiments
In the experiments in which spinal lesions were made (n = 16/23), the low thoracic and upper lumbar segments of the spinal cord (approximately T9–L2) were exposed by laminectomy, leaving the dura intact. Spinal clamps on the dorsal processes of the L2 and more caudal vertebrae stabilized the cord. Peripheral afferents were activated by electrical stimulation delivered through percutaneous pins (modified 25 gauge hypodermic needles) which were inserted bilaterally into the vibrissal pads (ipsi Vib and co Vib), and the glabrous skin of the footpads of the forelimbs (FL), and hindlimbs (HL). Stimuli were delivered at intensities that activated local motoneurone axons. Ball electrode recordings from the dorsal columns of the low thoracic segments verified that these intensities were 2–5 times the threshold of the most excitable fibres. Golgi cells were selected which responded with long-lastin gdepressions of firing and/or short-lasting excitations following stimulation of the footpads of several limbs, including both hindlimb footpads. While maintaining recordings from the same neurone, small mechanical lesions of various funiculi of the spinal cord were made by teasing apart the fibres with watchmaker's forceps. After assessing responses from several inputs, subsequent lesions of other funiculi could be made and the responses reassessed. Since the neurones responded to stimulation of both forelimbs and hindlimbs bilaterally, responses to stimulation of these other inputs served as controls that the neurone discharge characteristics had not changed and that it remained responsive to somatosensory stimulation after the lesion. At the end of the experiment the animals were killed with anaesthetic overdose, perfused with 200 ml of saline, followed by 200 ml of fixative (4% paraformaldehyde in 0.1 m phosphate buffer), and the extent of the lesions was assessed histologically. Blocks of spinal cord containing the lesions were dissected and post-fixed for 3 days before being immersed in 30% sucrose in phosphate buffer until equilibrated. Frozen sections (80–100 μm) of the spinal cord were made, mounted serially and counterstained in methylene blue before dehydration.
Results
Identification of cells
Neurones were identified using criteria established by an earlier study in this laboratory. Briefly these included mean instantaneous firing frequencies below 28 Hz, median interspike intervals of 45 ms or greater, and modal interspike intervals 40 ms or greater (Holtzman et al. 2006). These criteria allow objective differentiation of Golgi cell firing patterns from those of Purkinje cells. Furthermore, all neurones included in this study responded with long-lasting depression responses following peripheral stimulation, an additional characteristic exclusive to Golgi cells in Crus I and II (Holtzman et al. 2006). In general terms, during experiments, relative depths below the pial surface were monitored continuously allowing for identification of granule cell layers in the most superficial parts of the electrode penetrations. Beyond this, Purkinje cell layers and the presence or absence of climbing fibre evoked field potentials (characteristic of the molecular layer using the type of electrodes in this study) were used to estimate the location of the recording electrode.
Long-lasting depressions and short-lasting excitations of Golgi cell activity are evoked by stimulation of low-threshold cutaneous afferents, but not by group I muscle afferents
Golgi cells were selected for analysis on the basis that they responded with long-lasting depressions alone, or accompanied by short-lasting excitations following electrical stimulation of afferents from the distal forelimb and/or contralateral hindlimb via percutaneous pins. Response onset latencies for long-lasting depressions were ∼50 ms for each of the limbs, and ∼12 and ∼17 ms for forelimb and hindlimb excitations, respectively (see Holtzman et al. 2006). All of the neurones also responded to stimulation of isolated nerves from the ipsilateral hindlimb. Stimulation of both cutaneous and muscle nerves evoked responses, but they were most readily evoked from nerves that contain cutaneous afferents (TIB and SUR) compared with the muscle nerves (GS and HAM).
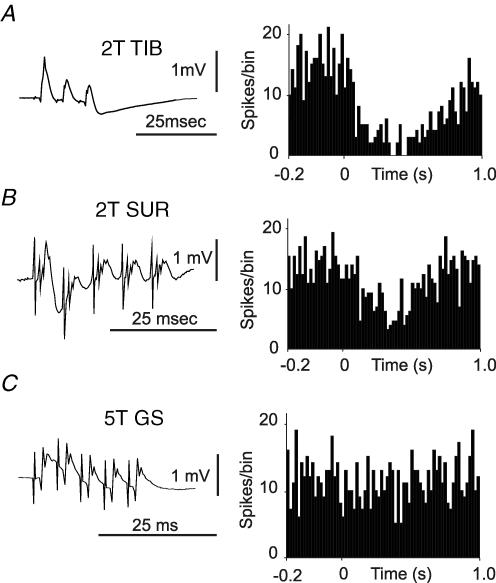
Examples of long-lasting depressions evoked by stimulation of TIB and SUR are shown in Fig. 1A and B, respectively. All of the neurones tested (n = 11) with stimulation of SUR, and all but one (12/13) of those tested with stimulation of TIB, responded with significant long-lasting depressions at stimulus intensities of less than twice threshold for the most excitable fibres in the nerve (2T). The lowest intensities at which responses were evoked were 1.25T for SUR and 1.3T for TIB. At these intensities only the large myelinated fibres, Aβ axons that innervate low-threshold cutaneous mechanoreceptors, should have been activated. These data therefore indicate that low-threshold mechanoreceptor axons contribute to the responses. The cord dorsum recordings support this conclusion: long-lasting depressions were evoked by stimuli that evoked submaximal early cord dorsum potentials (Fig. 1). Although responses were evoked by low-intensity stimulation, in all cases the responses became more pronounced as the stimulus intensity was increased above twice threshold, up to 5T, indicating that fibres recruited at these higher intensities also contributed to the responses.
Figure 1. Long-lasting depressions in a Golgi cells following stimulation of the tibial, sural and gastrocnemius-soleus nerves.
A and B, cord dorsum volleys recorded from the L2 segment, following stimulation of the tibial (TIB) and sural (SUR) nerves, respectively, plotted alongside the corresponding peristimulus time histograms (PSTHs) from an example Golgi cell. Repetitive stimulation of the gastrocnemius muscle (GS) nerve at 5T, shown in C, which evokes a substantial cord dorsum volley, failed to evoke a substantial response in this neurone although there was a small reduction in firing after the stimulus (P < 0.05, see Methods). Substantial responses were seen when this nerve was stimulated at 10T. PSTHs built from 100 stimuli, bin-size 10 ms.
In contrast to the effects of stimulating cutaneous nerves, stimulation of muscle nerves at intensities below twice threshold did not evoke responses in any of the Golgi cells tested (n = 15). Figure 1C shows an example Golgi cell where GS stimulation at 5T had little effect (a substantial long-lasting depression was evoked when the stimulus intensity was increased to 10T (not shown)). This was also clear from the cord dorsum records, which also showed substantial growth of a late component between stimulus intensities of 5T and 10T (data not shown). This component was at longer latency than the group I volley and was relatively long lasting, like the components evoked by cutaneous afferents. Similar later components were reliably seen at similar intensities in each experiment, suggesting that they relate consistently to a specific stimulus intensity, rather than reflecting current spread to activate cutaneous afferents in the main trunk of the sciatic nerve.
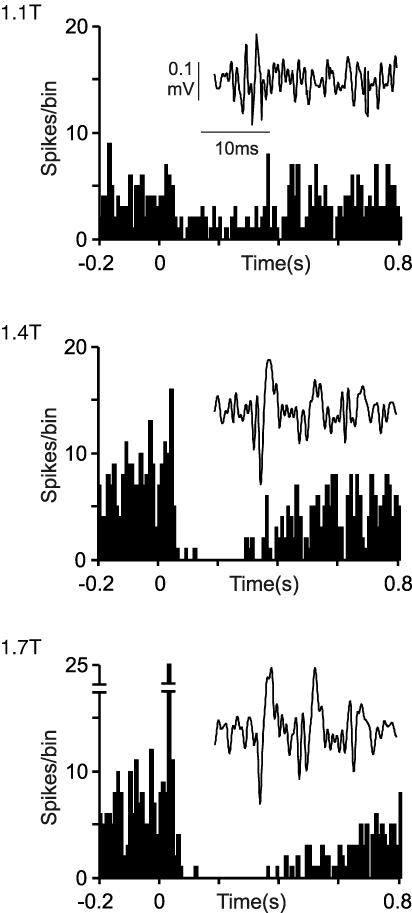
A similar analysis was undertaken for the short-lasting excitations for which example data are shown in Fig. 2, which shows the responses of a Golgi cell following stimulation of the SUR nerve. At stimulus intensities up to 1.3T, this neurone responded with long-lasting depressions, which grew with increasing stimulus intensity. Inset in each of the PSTHs are afferent volleys recorded from the main trunk of the sciatic nerve. As stimulus intensity increased above 1.4T, short-lasting excitations were evoked with increasing reliability. Similar results were obtained in five other neurones tested with SUR stimulation, but not with GS stimulation using stimulus intensities up to 20T. These observations suggest that similar to long-lasting depressions, short-lasting excitations are mediated by low-threshold principally Aβ mechanoreceptors, although in contrast they appear not to be influenced by the fibres carried in muscle nerves, such as the GS.
Figure 2. Short-lasting excitations and long-lasting depressions in a Golgi cell following stimulation of the sural nerve.
Top to bottom, PSTHs compiled from 100 stimuli at increasing intensities delivered to the SUR nerve. Inset in each PSTH are the cord dorsum volleys following three stimuli. Long-lasting depressions were evoked using stimuli of 1.1T and above, whereas short-lasting excitations were not evoked until 1.4T and above. Note that with increasing intensities the depressions grew stronger and the excitations more ‘precise’. PSTHs built from 100 stimuli, bin-size 10 ms.
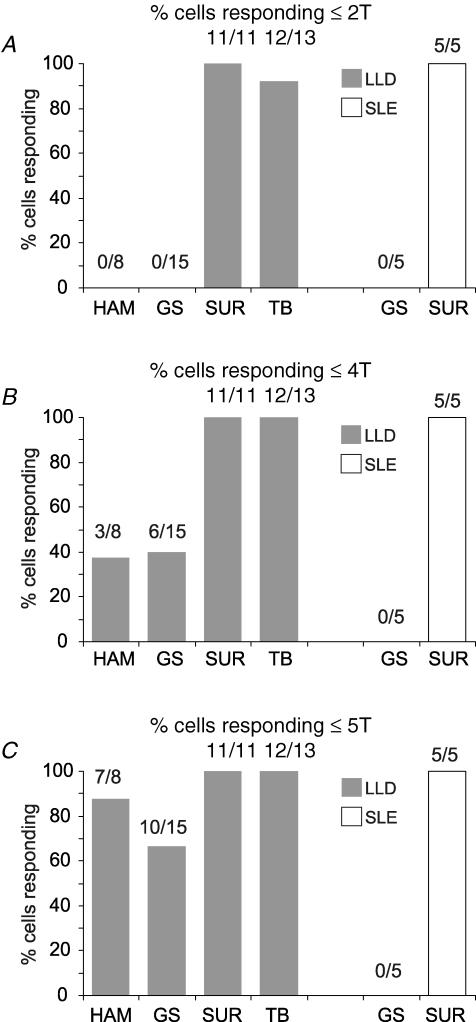
Grouped data from the nerve stimulation studies are shown in Fig. 3. The proportions of Golgi cells responding with long-lasting depressions or short-lasting excitations to stimuli of 2T, 4T and 5T are shown in Fig. 3A–C, with the numbers of neurones tested indicated above each column. While none of the neurones responded to stimulation of muscle nerves at 2T (HAM and GS), approximately 40% responded to stimuli at 4T with long-lasting depressions but not short-lasting excitations, and more to stimuli at 5T. All neurones responded with depressions to stimulation of muscle nerves at 10T (not shown). In contrast, almost all of the neurones responded to stimulation of the nerves containing cutaneous afferents (SUR, 11/11 depressions, 5/5 excitations; TIB, 12/13 depressions) at intensities of 2T and below. The difference between the incidence of responses to stimulation of the muscle and cutaneous nerve afferents at twice threshold was highly significant (χ2 test, P < 0.001).
Figure 3. Responses of Golgi cells to stimulation at different intensities: grouped data.
The histograms show the proportions of Golgi cells tested in which significant responses were evoked at the stimulus intensity indicated, i.e. long-lasting depressions or short-lasting excitations. None of the neurones tested responded to stimulation of the muscle nerves (hamstring (HAM) and GS) at 2T, although almost all responded to SUR or TIB stimulation with long-lasting depressions and/or short-lasting excitations. Stimulation of the muscle nerves HAM and GS at 4T evoked long-lasting depressions in about one-third of the Golgi cells, and this proportion increased again as the intensity was raised to 5T. In contrast, none of the neurones showed short-lasting excitations following muscle nerve stimulation, even at intensities up to 20T.
Spinal cord lesions
Dorsal column lesions do not affect long-lasting depression or short-lasting excitation Golgi cell responses
Initially we made lesions of the dorsal columns to elucidate the ascending pathways associated with the long-lasting depressions of Golgi cell activity. In six experiments, long-lasting depressions in single Golgi cells were examined both before and after large lesions that interrupted the entire dorsal column, and extended into the dorsal horn grey matter. None of these lesions produced a measurable change in cell response. In three experiments, lesions of the dorsal columns were also ineffective in changing short-lasting excitations. These observations make it unlikely that the dorsal columns carry the ascending afferents involved in generating either of the Golgi cell responses.
Lateral funiculus lesions abolish or attenuate Golgi cell responses evoked from the contralateral hindlimb
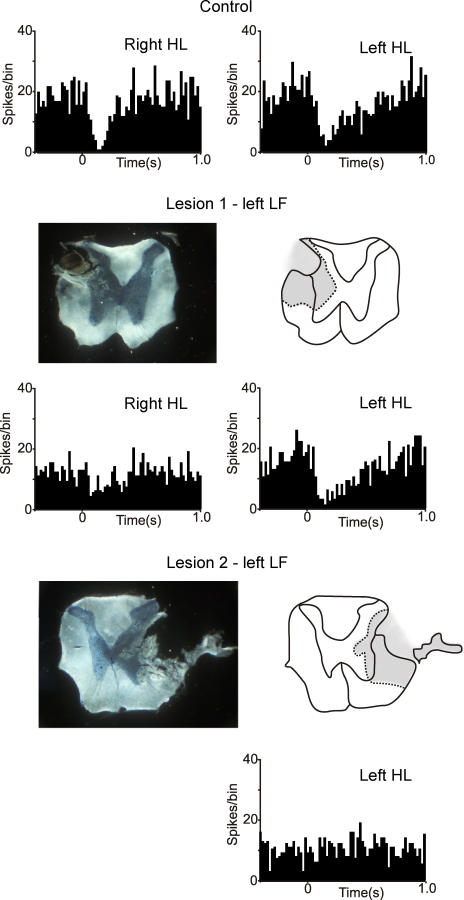
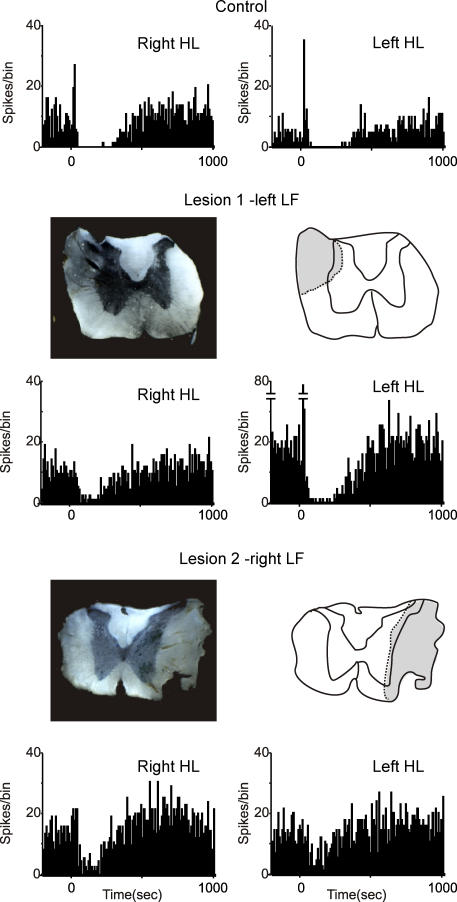
Since the dorsal columns do not appear to be involved in generating the Golgi cell responses, lesions of other parts of the white matter were made in subsequent experiments. In contrast to lesions of the dorsal columns, lesions of the lateral funiculi (LF) did substantially affect both types of response. An illustration of the results from one experiment is shown in Fig. 4. In this case, the activity of the same Golgi cell was recorded before and after a lesion of the left LF, and a subsequent lesion of the right LF. The initial recordings (PSTHs at the top) show long-lasting depressions evoked by stimulation of the right hindlimb and left hindlimb, respectively, with the spinal cord intact. The first lesion removed the dorsal part of the left LF, sparing some of the most ventral fibres. The line drawing shows the greatest extent of the lesion, based on inspection of the series of sections through the lesion site. Following this lesion, the response to stimulation of the right hindlimb (contralateral to the lesion) was substantially reduced: prelesion the Golgi cell firing was depressed by 85% over the period 100–300 ms after the stimulus; after the lesion the depression was reduced to 43%.
Figure 4. Effects of lateral funiculi lesions on Golgi cell long-lasting depressions.
The PSTHs show the responses of a Golgi cell with the cord intact (top row), after a lesion of the left lateral funiculi (LF; lesion 1, middle row) and after a more extensive lesion of the right LF (lesion 2; bottom row). Lesion 1 had little effect on the responses evoked by stimulation of the left hindlimb (HL; ipsilateral to the lesion) or the left FL, but reduced the responses to the right HL (by about 50%). Lesion 1 included the dorsal part of the LF, but spared some of the ventral part. After lesion 2, which removed all of the LF, the response evoked from the left HL (contralateral to lesion 2) was abolished. PSTHs built from 100 stimuli, bin-size 20 ms.
In the same experiment a second lesion was made in the right LF, slightly rostral to the first lesion. Lesion 2 was more extensive and removed the entire LF. After this lesion, the response to stimulation of the left hindlimb was abolished, shown in the PSTH at the bottom of Fig. 4. Throughout the experiment, the responses evoked from the left forelimb were monitored as ‘internal controls’ and these remained unchanged by either lesion (data not shown).
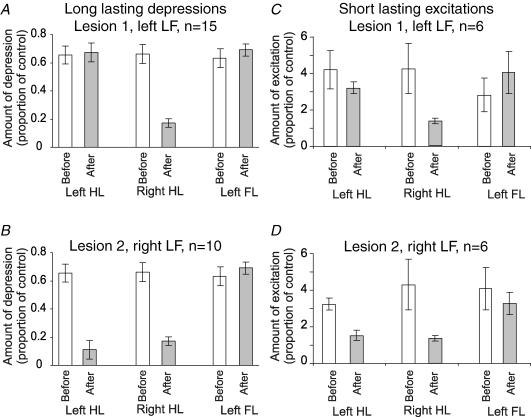
In all 15 experiments where a unilateral LF lesion was made, long-lasting Golgi cell depressions evoked by stimulation of the hindlimb contralateral to the lesion were reduced, and in five cases the response was abolished (the bin counts between 100 and 300 ms after the stimulus were not significantly different to those in the control period before the stimulus, see Methods). Grouped data were also analysed for comparison, and are illustrated in Fig. 6A and B. Considering long-lasting depression, stimulation of either hindlimb or the forelimbs before any lesion evoked responses where the firing was reduced by 60–70% over the period 100–300 ms after the stimulus (Fig. 6A). Following lesion 1 (left LF), the mean depression for the right HL was significantly attenuated to a value close to 17% (Fig. 6A, shaded bars; P < 0.001, paired t test), whereas the responses from the other limbs remained unchanged. In 10 of these experiments, a second lesion was subsequently made in the right LF (lesion 2): an example is shown in Fig. 4 (lesion 2), and grouped data are shown in Fig. 6B. In all cases, the responses evoked from the left hindlimb were substantially reduced by this lesion, and in two cases they were abolished. The reduction in size of these responses was highly significant (P < 0.002, paired t test).
Figure 6. Effects of LF lesions on Golgi cell responses: grouped data.
A and C, mean responses of Golgi cells before (open bars) and after a lesion of the left LF (shaded bars). Responses to the left hindlimb and forelimb (left HL, left FL) were unaltered, but the responses to stimulation of the right hindlimb (right HL) were significantly attenuated (P < 0.001, paired t test). B and D, effects of a subsequent lesion of the right LF, after which not all of the Golgi cells remained discriminable. In this case the responses evoked by stimulation of the left HL were significantly reduced (P < 0.002, paired t test), while the responses to stimulation of forelimb afferents remained unaltered.
In six experiments, a similar approach was taken to Golgi cells that responded with short-lasting excitations (as well as long-lasting depressions). In all cases, lesions of the LF were effective in attenuating or abolishing short-lasting excitations evoked from the contralateral hindlimb. Using the same convention as for Fig. 4, data from an example Golgi cell with hindlimb evoked short-lasting excitations are illustrated in Fig. 5. Both hindlimbs (and the ipsilateral forelimb, not shown) evoked short-lasting excitations and long-lasting depressions before the lesions (top row of PSTHs). Following a small lesion of the left LF, the short-lasting excitation evoked from the right hindlimb was abolished and, as expected, the long-lasting depression was attenuated. Note that the response from the left hindlimb was unaltered by this lesion (middle row of PSTHs). In the same experiment, a second lesion was performed on the right LF, resulting in extensive destruction of the LF. Following this lesion, the short-lasting excitation from the left hindlimb was abolished, and the long-lasting depression was much reduced. As before, left forelimb responses remained unaffected by either lesion (not shown).
Figure 5. Effects of LF lesions on Golgi cell short-lasting excitations.
Using the same convention as Fig. 4, the responses of a single Golgi cell are shown in the PSTHs. Stimuli to each of the limbs evoked responses consisting of short-lasting excitations accompanied by long-lasting depressions. Lesion 1 (dorsal portion of the left LF) attenuated the long-lasting depression from the right HL and also abolished the short-lasting excitation. Lesion 2 (right LF) was more extensive and achieved a greater attenuation of the long-lasting depression evoked from the left HL, again with abolishment of the short-lasting excitation. Neither lesion affected the responses from the forelimb. PSTHs built from 100 stimuli, bin-size 10 ms.
Grouped data for the short-lasting excitations are plotted in Fig. 6C and D relating to the short-lasting excitations before and after lesions of the LF on either side. Hindlimb-evoked excitations, measured as the peak bin height, were usually in the range of three- to sixfold greater than the control period of firing (see histograms). Statistical significance of a short-lasting excitation was measured using z tests to compare the peak bin height to the individual bin values of the period of control firing, and a value of P < 0.05 was taken to be significant. Grouped data for lesions of the left LF show a significant reduction in the size of the excitations evoked from the right hindlimb (Fig. 6, shaded bars, P < 0.001, paired t test) with no significant change in the excitations evoked from the left hindlimb or the left forelimb (same side as lesion). In comparison, lesions of the right LF (lesion 2) significantly reduced excitations evoked from the left hindlimb (P < 0.005, paired t test).
In this experimental design, the responses evoked from the forelimbs (depressions and excitations) provided controls that the Golgi cells remained responsive: all neurones continued to respond to stimulation of forelimb afferents and the sizes of these responses were unaltered.
These observations indicate that the pathway(s) for both long-lasting depressions and short-lasting excitations are crossed and ascend in the lateral funiculus. Some circumstantial evidence suggests that a substantial component of the long-lasting depression pathway ascends in the ventral part of the lateral funiculus. In these experiments, relatively large lesions were needed to abolish the long-lasting depressions compared with more superficial lesions required to abolish the short-lasting excitations. Such lesions were made from the exposed dorsal part of the LF, and were progressively enlarged during the experiment until the responses were obviously reduced. In five of the experiments, initial superficial mechanical lesions restricted to the dorsal part of the LF produced relatively modest reductions in responses evoked from the contralateral hindlimb, but did not abolish long-lasting depressions. However, subsequent lesions that extended deeper into the lateral funiculus to include the more ventral fibres had much larger effects. Figure 4 also shows some evidence of this: the initial lesion (1) left some ventrally located fibres of the left LF intact and, although the response to stimulation of the right hindlimb was substantially reduced (by more than 50%) a long-lasting depression was still present. Lesion 2 removed the entire right LF, leaving only the ventral funiculus intact, and abolished the response evoked from the left hindlimb. Our conclusion from these data is that the ascending fibres for long-lasting depressions are distributed across the lateral funiculus, whereas the ascending fibres for short-lasting excitations may be located more superficially.
Discussion
Pathways underlying the long-lasting depressions
Data from our nerve stimulation experiments suggest that afferents of different electrical thresholds converge in the pathway that underlies the responses of Golgi cells. Both types of responses were readily evoked by stimulation of low-threshold (stimulation at <2T) cutaneous afferents, which were likely to have been Aβ mechanoreceptors. However, the growth of the long-lasting depressions with stronger stimuli suggests that higher threshold afferents from cutaneous nerves also contribute. Stimuli which activated group I muscle afferents alone did not evoke any responses, and stimuli which activated both group I and the majority of group II muscle afferents (4T) were also ineffective (see Riddell & Hadian, 1998 for a discussion of the relative thresholds of afferent fibres of rat peripheral nerves to electrical stimulation). However, stimulation of muscle afferents at strengths that would begin to activate group III muscle afferents (5T and above) did evoke long-lasting depressions, suggesting that information from these afferents converges on the pathway. Some long-lasting components of the cord dorsum potentials appeared at these higher stimulus intensities, and we cannot completely exclude that the stimuli may have spread to cutaneous afferents in the parent nerve.
Our results from lesion experiments show that the ascending pathways responsible for both types of Golgi cell response are not located in the dorsal columns but are instead crossed pathways that seem to be distributed within the dorsal and ventral portions of the lateral funiculus.
Taking these two sets of findings together, we can rule out some of the candidate pathways for mediating the Golgi cell responses. There are two major direct spinocerebellar pathways, i.e. the dorsal and ventral spinocerebellar tracts (DSCT and VSCT), and several smaller indirect pathways (see Matsushita et al. 1979). Several of these pathways ascend ipsilaterally, including the DSCT arising from Clarke's column cells (see Bloedel et al. 1981), the dorsal horn neurones in midlumbar segments that relay group II afferent information (Edgley & Jankowska, 1988), and the dorsal horn neurones in lower lumbar segments that relay integrated group I information (Aoyama et al. 1988). We can rule these pathways out as mediators of the Golgi cell responses on the grounds that they have axons that ascend ipsilaterally and that the neurones giving rise to them are powerfully affected by group I or II proprioceptive afferents. Two substantial crossed pathways have been described, the VSCT, which arises from neurones from the lateral border of the spinal grey and scattered through lamina VII of the L4–L6 segments, and several groups of neurones from the sacral segments, including cells from Stilling's nucleus (see Edgley & Grant, 1991). However, based on three lines of evidence, we think it unlikely that the Golgi cell responses could have been mediated by these neurones. First, the neurones contributing to these pathways have input from group I proprioceptive afferents, and cutaneous inputs to them are not conspicuous (Lundberg & Weight, 1971; Edgley & Grant, 1991). Second, the fibres of these pathways occupy the dorsal part of the lateral funiculus (see Yamada et al. 1991), whereas a considerable proportion of the fibres mediating long-lasting depressions of Golgi cell firing are likely to be located in the ventral part of the lateral funiculus. However, anecdotally, the fibres carrying short-lasting excitations may be chiefly located in the dorsal portion of the lateral funiculus. Third, the Golgi cells in this study were located in Crus II, a region of cerebellar cortex that is generally reported not to receive the terminals of direct spinocerebellar pathways (Matsushita, 1988; Matsushita & Yaginuma, 1989).
Indirect spinocerebellar pathways have also been described, many of which convey highly convergent information from much of the body, and a pathway of this type is more likely to mediate the long-lasting depressions of Golgi cell activity. The best studied of the indirect pathways is the spinoreticulocerebellar pathway through the lateral reticular nucleus (LRN) in the medulla (Bloedel et al. 1981; Ito, 1984). Neurones in the LRN are activated from wide receptive fields by cutaneous afferents and proprioceptors, including higher threshold muscle afferents (Ekerot, 1990a,b). The projection from the LRN to the cerebellar cortex has been studied principally in the cat, and although the system shares some properties with the pathway that generates the long-lasting depressions of Golgi cell firing, there are also some inconsistencies. First, barbiturates have been reported to suppress the widely convergent inputs to LRN neurons (Crichlow & Kennedy, 1967). These findings suggest that the LRN may not be involved in mediating Golgi cell responses, as widely convergent bilateral receptive fields are readily observed in Golgi cells under a variety of anaesthetics, including barbiturates (Holtzman et al. 2006). Second, the target regions of cerebellar cortex for the electrophysiological actions of the LRN in the cat are the ‘classical spinal receiving areas of the cerebellum’ (Clendenin et al. 1974), and neuroanatomical studies support this projection (Matsushita & Ikeda, 1976). Detailed neuroanatomical tracing of single axons in the rat has shown a widespread termination of LRN fibres in the cerebellar cortex (Wu et al. 1999), but terminals were absent in Crus Ic/IIa/b, where the Golgi cells in this study were recorded. Furthermore, a major ascending input to LRN neurones is from the ascending bilateral ventral flexor reflex tract (bVFRT; see Rosen & Scheid, 1973; Clendenin et al. 1974; Ekerot, 1990a,b). The neurones giving rise to this pathway have axons that cross the midline to ascend in the ventral part of the lateral funiculus. However, the bVFRT neurones from the lumbosacral cord of the cat are characterized by bilateral inputs, and this is inconsistent with the finding that Golgi cell responses from each limb are mediated by pathways in the contralateral lateral funiculus. In addition, the convergence onto bVFRT neurones in the cat includes substantial group I and II proprioceptive fibre components (Rosen & Scheid, 1973), which are not apparent in the Golgi cell responses.
Fewer studies have examined the LRN in the rat, and not all of those that have been done have focused on the cerebellar projection. Those that have focused on this projection have not investigated all subnuclei of the LRN, in particular the poorly understood linear nucleus of the LRN (Ruigrok & Cella, 1995). However, there are very substantial projections to the region of the LRN in the rat from crossed pathways ascending in the ventral part of the lateral funiculus. These include large neurones located in the ventral grey matter (which presumably include bVFRT neurones), but also include very many dorsal horn neurones, that make up the anterolateral system (Menetrey et al. 1983). This system, and specifically neurones located in the contralateral spinal dorsal horn that can be antidromically activated from the LRN, includes many multireceptive neurones that are activated by both innocuous and noxious cutaneous inputs, as well as muscle inputs. These are usually described as ‘wide dynamic range’ neurones (Menetrey et al. 1984) and form a convergent pathway with input from cutaneous mechanoreceptors and higher threshold afferents, but not group I proprioceptors, which is crossed and ascends in the lateral funiculus, including the ventral part. If this pathway contacts cerebellar-projecting neurones, then this could mediate the Golgi cell responses.
There are, however, other potential routes through which this system could influence cerebellar afferents, including spinoreticular projections to other brainstem structures, such as the parabrachial nucleus and other arousal related structures (Sotgiu, 1987; Gauriau & Bernard, 2002). The long latency of the long-lasting depression responses (see Holtzman et al. 2006) could permit a pathway with several supraspinal relays to mediate the responses.
The long-lasting depressions might result from a withdrawal of excitatory mossy fibre/parallel fibre input and/or direct inhibitory influences on the Golgi cells. There are a number of sites at which this might occur. As discussed previously (Holtzman et al. 2006), this might reflect cerebellar cortical processing, for example Lugaro cells inhibit Golgi cells (Dieudonne & Dumoulin, 2000) as may metabotropic glutamate receptor activation (Watanabe & Nakanishi, 2003). Consistent with a disfacilitation (i.e. inhibition of an excitatory mossy fibre input) is the observation that many LRN neurones are inhibited by stimulation of peripheral afferents (Ekerot, 1990a,b). An intriguing possibility is also that the long-lasting depressions may represent disfacilitation at a spinal level: a consistent finding was that one class of dorsal horn spinoreticular neurone that projects to the LRN fires continuously at a relatively high rate, but falls silent in response to activation of peripheral afferents, including innocuous cutaneous stimulation (e.g. Figs 7 and 8 in Menetrey et al. 1984).
Functional implications
Golgi cells have been considered to act as negative feedback gain controllers (Marr, 1969; Albus, 1971; De Schutter et al. 2000). Mossy fibres excite Golgi cells both indirectly via parallel fibres and directly via the en marron synapses of mossy fibres, and their inhibitory output modulates the transmission between afferent mossy fibres and the excitatory granule cells within the glomerulus (Eccles et al. 1967; Ito, 1984). Our current knowledge of Golgi cell behaviour suggests that they are not ideally suited to perform as gain controllers (De Schutter et al. 2000; Holtzman et al. 2006). The situation is also complicated by the long-lasting consequences of activity at the Golgi cell output synapses, which result from GABA spillover (Rossi & Hamann, 1998).
Given that both types of Golgi cell response may be mediated through spinoreticular or other ascending components of the anterolateral system, which is important in peripherally mediated arousal (e.g. Perl, 1984), transmission in the mossy-fibre–granule-cell system may be modulated by arousal, and may be influenced by inputs from widely separated areas of the body. There has been substantial recent interest in the increased metabolic activity in the cerebellum in arousal, generated by noxious stimulation, seen in conscious humans using functional magnetic resonance imaging. The possibility that an ascending arousal system targets Golgi cells in Crus II of the posterior lobe is particularly interesting in view of the evidence from functional magnetic resonance imaging that the same cerebellar region in humans shows increased metabolic activity in response to noxious lower limb stimulation (Dimitrova et al. 2003).
Acknowledgments
Supported by the BBSRC
References
- Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61. [Google Scholar]
- Aoyama M, Hongo T, Kudo N. Sensory input to cells of origin of uncrossed spinocerebellar tract located below Clarke's column in the cat. J Physiol. 1988;398:233–257. doi: 10.1113/jphysiol.1988.sp017040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloedel JR, Courville JC. Handbook of Physiology, section 2, The Nervous System, Motor Control. part 2. II. Bethesda, MD, USA: American Physiological Society; 1981. Cerebellar afferent systems; pp. 735–830. [Google Scholar]
- Bower JM, Kassel J. Variability in tactile projection patterns to cerebellar folia crus IIA of the Norway rat. J Comp Neurol. 1990;302:768–778. doi: 10.1002/cne.903020409. [DOI] [PubMed] [Google Scholar]
- Bower JM, Woolston DC. Congruence of spatial organization of tactile projections to granule cell and Purkinje cell layers of cerebellar hemispheres of the albino rat: vertical organization of cerebellar cortex. J Neurophysiol. 1983;49:745–766. doi: 10.1152/jn.1983.49.3.745. [DOI] [PubMed] [Google Scholar]
- Clendenin M, Ekerot CF, Oscarsson O, Rosen I. The lateral reticular nucleus in the cat. I. Mossy fibre distribution in cerebellar cortex. Exp Brain Res. 1974;21:473–486. doi: 10.1007/BF00237166. [DOI] [PubMed] [Google Scholar]
- Crichlow EC, Kennedy TT. Functional characteristics of neurons in the lateral reticular nucleus with reference to localized cerebellar potentials. Exp Neurol. 1967;18:141–153. doi: 10.1016/0014-4886(67)90036-2. [DOI] [PubMed] [Google Scholar]
- De Schutter E, Vos B, Maex R. The function of cerebellar Golgi cells revisited. Prog Brain Res. 2000;124:81–93. doi: 10.1016/s0079-6123(00)24009-0. [DOI] [PubMed] [Google Scholar]
- Dieudonne S, Dumoulin A. Serotonin-driven long-range inhibitory connections in the cerebellar cortex. J Neurosci. 2000;20:1837–1848. doi: 10.1523/JNEUROSCI.20-05-01837.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova A, Kolb FP, Elles HG, Maschke M, Forsting M, Diener HC, Timmann D. Cerebellar responses evoked by nociceptive leg withdrawal reflex as revealed by event-related FMRI. J Neurophysiol. 2003;90:1877–1886. doi: 10.1152/jn.00053.2003. [DOI] [PubMed] [Google Scholar]
- Eccles J, Ito M, Szentagothai J. The Cerebellum as a Neuronal Machine. Berlin: Springer-Verlag; 1967. [Google Scholar]
- Edgley SA, Grant GM. Inputs to spinocerebellar tract neurones located in stilling's nucleus in the sacral segments of the rat spinal cord. J Comp Neurol. 1991;305:130–138. doi: 10.1002/cne.903050112. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. Information processed by dorsal horn spinocerebellar tract neurones in the cat. J Physiol. 1988;397:81–97. doi: 10.1113/jphysiol.1988.sp016989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot CF. The lateral reticular nucleus in the cat. VIII. Excitatory and inhibitory projection from the bilateral ventral flexor reflex tract (bVFRT) Exp Brain Res. 1990a;79:129–137. doi: 10.1007/BF00228881. [DOI] [PubMed] [Google Scholar]
- Ekerot CF. The lateral reticular nucleus in the cat. VI. Excitatory and inhibitory afferent paths. Exp Brain Res. 1990b;79:109–119. doi: 10.1007/BF00228879. [DOI] [PubMed] [Google Scholar]
- Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87:251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- Holtzman T, Rajapaksa T, Mostofi A, Edgley SA. Different responses of rat cerebellar Purkinje cells and Golgi cells evoked by widespread convergent sensory inputs. J Physiol. 2006;574:491–507. doi: 10.1113/jphysiol.2006.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. The Cerebellum and Neural Control. New York: Raven Press; 1984. [Google Scholar]
- Lundberg A, Weight F. Functional organization of connexions to the ventral spinocerebellar tract. Exp Brain Res. 1971;12:295–316. doi: 10.1007/BF00237922. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M. Spinocerebellar projections from the lowest lumbar and sacral-caudal segments in the cat, as studied by anterograde transport of wheat germ agglutinin–horseradish peroxidase. J Comp Neurol. 1988;274:239–254. doi: 10.1002/cne.902740208. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Hosoya Y, Ikeda M. Anatomical organization of the spinocerebellar system in the cat, as studied by retrograde transport of horseradish peroxidase. J Comp Neurol. 1979;184:81–106. doi: 10.1002/cne.901840106. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Ikeda M. Projections from the lateral reticular nucleus to the cerebellar cortex and nuclei in the cat. Exp Brain Res. 1976;24:403–421. doi: 10.1007/BF00235006. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Yaginuma H. Spinocerebellar projections from spinal border cells in the cat as studied by anterograde transport of wheat germ agglutinin-horseradish peroxidase. J Comp Neurol. 1989;288:19–38. doi: 10.1002/cne.902880103. [DOI] [PubMed] [Google Scholar]
- Menetrey D, de Pommery J, Besson JM. Electrophysiological characteristics of lumbar spinal cord neurons backfired from lateral reticular nucleus in the rat. J Neurophysiol. 1984;52:595–611. doi: 10.1152/jn.1984.52.4.595. [DOI] [PubMed] [Google Scholar]
- Menetrey D, Roudier F, Besson JM. Spinal neurons reaching the lateral reticular nucleus as studied in the rat by retrograde transport of horseradish peroxidase. J Comp Neurol. 1983;220:439–452. doi: 10.1002/cne.902200406. [DOI] [PubMed] [Google Scholar]
- Morissette J, Bower JM. Contribution of somatosensory cortex to responses in the rat cerebellar granule cell layer following peripheral tactile stimulation. Exp Brain Res. 1996;109:240–250. doi: 10.1007/BF00231784. [DOI] [PubMed] [Google Scholar]
- Palay SL, Chan-Palay V. Cerebellar Cortex: Cytology and Organisation. Berlin: Springer-Verlag; 1974. [Google Scholar]
- Perl E. Pain and nociception. In: Darian-Smith I, editor. Handbook of Physiology, section 1, The Nervous System. Vol. 3. Bethesda, MD, USA: American Physiological Society; 1984. pp. 915–975. [Google Scholar]
- Riddell JS, Hadian M. Topographical organization of group II afferent input in the rat spinal cord. J Comp Neurol. 1998;394:357–373. doi: 10.1002/(sici)1096-9861(19980511)394:3<357::aid-cne7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Rosen I, Scheid P. Patterns of afferent input to the lateral reticular nucleus of the cat. Exp Brain Res. 1973;18:242–255. doi: 10.1007/BF00234595. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M. Spillover-mediated transmission at inhibitory synapses promoted by high affinity alpha6 subunit GABA(A) receptors and glomerular geometry. Neuron. 1998;20:783–795. doi: 10.1016/s0896-6273(00)81016-8. [DOI] [PubMed] [Google Scholar]
- Ruigrok T, Cella F. In: Precerebellar Nuclei and Red Nucleus. The Rat Nervous System. Paxinos G, editor. San Diego, CA, USA: Academic Press; 1995. pp. 277–388. [Google Scholar]
- Shambes GM, Gibson JM, Welker W. Fractured somatotopy in granule cell tactile areas of rat cerebellar hemispheres revealed by micromapping. Brain Behav Evol. 1978;15:94–140. doi: 10.1159/000123774. [DOI] [PubMed] [Google Scholar]
- Sotgiu ML. The effects of periaqueductal gray and nucleus raphe magnus stimulation on the spontaneous and noxious-evoked activity of lateral reticular nucleus neurons in rabbits. Brain Res. 1987;414:219–227. doi: 10.1016/0006-8993(87)90002-3. [DOI] [PubMed] [Google Scholar]
- Vos BP, Volny-Luraghi A, De Schutter E. Cerebellar Golgi cells in the rat: receptive fields and timing of responses to facial stimulation. Eur J Neurosci. 1999;11:2621–2634. doi: 10.1046/j.1460-9568.1999.00678.x. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Nakanishi S. mGluR2 postsynaptically senses granule cell inputs at Golgi cell synapses. Neuron. 2003;39:821–829. doi: 10.1016/s0896-6273(03)00530-0. [DOI] [PubMed] [Google Scholar]
- Wu HS, Sugihara I, Shinoda Y. Projection patterns of single mossy fibers originating from the lateral reticular nucleus in the rat cerebellar cortex and nuclei. J Comp Neurol. 1999;411:97–118. doi: 10.1002/(sici)1096-9861(19990816)411:1<97::aid-cne8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Yamada J, Shirao K, Kitamura T, Sato H. Trajectory of spinocerebellar fibers passing through the inferior and superior cerebellar peduncles in the rat spinal cord: a study using horseradish peroxidase with pedunculotomy. J Comp Neurol. 1991;304:147–160. doi: 10.1002/cne.903040111. [DOI] [PubMed] [Google Scholar]