Abstract
There is increasing evidence that synapse function depends on interactions with glial cells, namely astrocytes. Studies on specific neurons of the central nervous system (CNS) indicated that glial signals also control synapse development, but it remained unclear whether this is a general principle that applies to other neuronal cell types. To address this question, we developed new methods to immunoisolate neurons from different brain regions of postnatal mice and to culture them in a chemically defined medium. Electrophysiological recordings and immunocytochemical staining revealed vigorous synaptogenesis in hippocampal and cerebellar neurons, but not in retinal ganglion cells (RGCs) in the absence of glial cells. Co-culture with glia promoted synapse formation in RGCs as indicated by a strong increase in the incidence and frequency of action potential-independent miniature synaptic currents, but showed no such effects in hippocampal or cerebellar neurons. On the other hand, glial signals promoted the efficacy of excitatory synapses in all regions as indicated by an increase in the size of spontaneous synaptic events in cerebellar cultures and of miniature synaptic currents in hippocampal neurons and RGCs. Inhibitory synaptic currents remained largely unaffected by glia. Our results indicate that in the mammalian CNS, the way that glial signals promote the development of excitatory synapses depends on the type of neuron.
There is increasing evidence that glial cells regulate the function of synapses (Haydon, 2001; Colomar & Robitaille, 2004; Fellin & Carmignoto, 2004; Hertz & Zielke, 2004; Newman, 2004; Oliet et al. 2004; Volterra & Steinhauser, 2004; Allen & Barres, 2005; Perea & Araque, 2005), but it is less clear, whether they also control their formation. One approach to address this question involves primary cultures of neurons that can be highly purified and maintained under defined, glia-free conditions. Two types of neurons, namely RGCs (Meyer-Franke et al. 1995) and motoneurons (Hanson et al. 1998) that can be immunoisolated from postnatal rats, require glia-derived factors to form numerous and efficient synapses in vitro (Pfrieger & Barres, 1997; Mauch et al. 2001; Nagler et al. 2001; Ullian et al. 2001, 2004b; Christopherson et al. 2005; Goritz et al. 2005). These studies, together with evidence from other preparations including neuromuscular junctions of Rana pipiens in vivo (Reddy et al. 2003) and primary cultures of hippocampal neurons from embryonic (Yang et al. 2003; Hama et al. 2004; Elmariah et al. 2005) or newborn rats (Mazzanti & Haydon, 2003) and of spinal cord neurons from Xenopus embryos (Peng et al. 2003), prompted the hypothesis that glia-derived signals control synaptogenesis (Slezak & Pfrieger, 2003; Ullian et al. 2004a; Feng et al. 2005). To test the generality of this hypothesis, we established protocols to immunoisolate and culture neurons from different brain regions of postnatal mice. These culture preparations allowed for the first time direct comparison of the relevance of glial factors for synapse development in different brain regions under standardized conditions.
Methods
Immunoisolation and culture of CNS neurons from postnatal mice
Postnatal Balb/c mice (7 days old; animal facility, Faculty of Medicine, Universite Louis Pasteur, Strasbourg; Elevage Janvier, Le Genest Saint Isle, France) were killed by decapitation according to institutional guidelines. Hippocampi and cerebella were dissected, cut in small pieces and incubated (5% CO2, 37°C) for 60 min in Earle's buffered salt solution (EBSS) (Gibco/Invitrogen, Cergy-Pontoise, France) containing 200 U ml−1 papain (Worthington Biochemical Corporation, Lakewood, NJ, USA), 200 U ml−1 DNAse (Sigma, St-Quentin Fallavier, France), 1.5 mm CaCl2, 1 mm MgSO4 and 0.5 mm EDTA (Sigma). To isolate RGCs, retinae from the same animals were incubated for 45 min at 37°C in D-PBS (Gibco/Invitrogen) containing 160 U ml−1 papain and 200 U ml−1 DNAse. The tissues were then sequentially triturated in D-PBS containing 0.15% trypsin inhibitor (Roche Diagnostics, Meylan, France), 650 U ml−1 DNAse and 1:75 rabbit anti-rat macrophage antibody (Sigma) to remove microglial cells. Cells were spun down (800 g for 13 min), resuspended in 1% trypsin inhibitor in D-PBS, spun down again and then resuspended in D-PBS containing 0.02% bovine serum-albumin (fraction V; Sigma). For immunopanning, one (hippocampus, cerebellum) or two (RGCs) subtraction plates (150 mm diameter Petri-dishes; Falcon; BD Biosciences/VWR, Fontenay sous Bois, France) and one selection plate (100 mm diameter Petri-dish) were incubated for > 12 h at 4°C with 10 μg ml−1 secondary antibody in 50 mm Tris-HCl (pH 9.5) (for subtraction: goat anti-rabbit IgG; for selection: hippocampus–cerebellum, goat anti-rat IgG; for RGCs, goat anti-mouse IgG (Jackson Immunoresearch Laboratories/Beckman Coulter, Marseille, France)). After washing for three times with PBS, selection plates were covered with 0.2% bovine serum albumin (fraction V; in D-PBS) and incubated for > 4 h (cerebellum, RGCs) or > 12 h (hippocampus) at room temperature with 0.2 μg ml−1 primary antibody (hippocampus, cerebellum: rat IgG anti-L1 clone 324, Chemicon/Euromedex, Mundolsheim, France; RGCs: mouse IgM anti-Thy1.2, MCA01, Serotec, Cergy Saint-Christophe, France) and then washed with D-PBS. The cell suspensions were filtered through a nylon mesh (Nitex 20 μm, Tetko/Sefar Filtration, Rüschlikon, Switzerland) and incubated on subtraction plates for 30 min. The supernatant was filtered and incubated on the selection plate (hippocampus, cerebellum: 60 min, RGCs: 45 min). Non-adherent cells were thoroughly washed off and bound cells were released by washing with 0.02% BSA (hippocampus, cerebellum) or by trypsination (RGCs: 12 000 U ml−1 in EBSS for 10 min in 5% CO2 at 37°C). Following washing or inactivation of trypsin by 30% fetal calf serum (Gibco/Invitrogen), cells were spun down and resuspended in culture medium.
After determination of cell counts (haemocytometer using tryptan blue staining, Sigma), cells were plated at indicated densities. For electrophysiological recordings and immunocytochemical staining, neurons were plated at 600 cells mm−2 in a small circle (10 mm diameter) centred on tissue culture plates (35 mm diameter; Falcon, BD Biosciences) coated with 5 μg ml−1 poly d-lysine (MW ∼40 kDa; Sigma) with (hippocampus) or without (cerebellum, RGCs) 10 μg ml−1 laminin (Sigma). For viability (3 h) and survival assays at 3 days in vitro (DIV), cells were plated on 96-well plates at 5000 cells per well in indicated culture media. For the proliferation assay, cerebellum cells were plated at 1000 cells mm−2 in 24-well culture plates (Falcon, BD Bioscience). Cells were cultured in Neurobasal medium (Gibco/Invitrogen) supplemented with (all from Sigma, except where indicated) pyruvate (1 mm), glutamine (2 mm; Gibco/Invitrogen), N-acetyl-l-cysteine (60 μg ml−1), putrescine (16 μg ml−1), selenite (40 ng ml−1), bovine serum albumin (100 μg ml−1; fraction V, crystalline grade), streptomycin (100 μg ml−1), penicillin (100 U ml−1), triiodothyronine (40 ng ml−1), holotransferrin (100 μg ml−1), insulin (5 μg ml−1) and progesterone (62 ng ml−1). This medium is referred to as minimally supplemented medium (MSM). To support neuronal survival, this medium was further supplemented with B27 (1:50, Gibco/Invitrogen), brain-derived neurotrophic factor (BDNF; 25 ng ml−1; PeproTech, London, UK), ciliary neurotrophic factor (CNTF; 10 ng ml−1; PeproTech) and forskolin (10 μm; Sigma). This medium is referred to as fully supplemented medium (FSM). For co-cultures, neurons were plated with glial cells from the respective region except for RGCs, which were cultured with cortical glia (Mauch et al. 2001). For treatment with soluble glial factors, GCM was obtained from primary cultures of mouse hippocampal, cerebellar and cortical glial cells according to a standard protocol (Pfrieger & Barres, 1997). Briefly, papain-digested and triturated tissue from the different brain regions of 7-day-old mice was cultured in PDL-coated tissue culture flasks (25 cm2, TPP/VWR) in a medium containing (all Gibco/Invitrogen) DMEM, heat-inactivated fetal calf serum (10%), penicillin (100 units ml−1), streptomycin (100 μg ml−1), glutamine (2 mm) and sodium pyruvate (1 mm). After 1 week, culture flasks were washed with PBS, and glial cells were cultured in MSM. Three times a week, half of the GCM was harvested and replaced by fresh MSM. GCM was spun down (5 min at 3000 g) to remove cellular debris and added to 1-day-old cultures (1.7 ml GCM to 2 ml culture medium). For some experiments, cholesterol (5 μg ml−1 from a 1000-fold ethanolic stock solution; Sigma) was added to 1-day-old cultures of RGCs. To some hippocampal cultures, recombinant murine tumour necrosis factor alpha (100 ng ml−1; R & D Systems, Lille, France) was added at 4 DIV for 48 h.
Survival and proliferation assay
The neuronal survival rate was determined by the live/dead assay (Molecular Probes/Invitrogen) according to the manufacturer's instructions. Calcein, ethidium and bisbenzimide fluorescence were viewed on an inverted microscope (Axiovert 135TV; Zeiss, Göttingen, Germany) using suitable filter sets (Omega Optical/PhotoMed GmbH, Seefeld, Germany) and digitized by an air-cooled monochrome CCD camera (Sensicam, PCO Computer Optics, Kehlheim, Germany). The percentage of living cells was analysed by a custom-written routine (Labview; National Instruments, Le Blanc-Mesnil, France). For the proliferation assay, cells were incubated 3 h (n = 2) and 21 h (n = 2) after plating for 6 h in culture medium with 5-bromo-2-deoxyuridine (BrdU; 10 μm; Sigma), fixed, immunostained (mouse anti-BrdU; clone BU33; 1:1000; Sigma) and viewed on the same set-up as described above.
Electrophysiological recordings
Recordings of spontaneous postsynaptic currents were performed and analysed as described earlier (Nagler et al. 2001; Goritz et al. 2005). The intracellular recording solution contained (mm, all Sigma): 100 potassium gluconate, 10 KCl, 10 EGTA and 10 Hepes adjusted to pH 7.3 with KOH. The extracellular solution contained (mm, all Sigma): 120 NaCl, 3 CaCl2, 2 MgCl2, 5 KCl and 10 Hepes adjusted to pH 7.4 with NaOH. Analysis of postsynaptic currents was performed automatically by custom-written Labview routines. The frequency of excitatory postsynaptic currents (EPSCs) was determined from inward currents recorded at −70 mV holding potential. EPSCs could be distinguished from inhibitory postsynaptic currents (IPSCs), which were also inwardly directed at −70 mV due to their faster time course (Fig. 2): half-widths of EPSCs ranged from 0.8 to 6 ms whereas those from IPSCs ranged between 6 and 40 ms. The frequency of IPSCs was determined from outward currents recorded at −30 mV. All neurons tested were electrically excitable as indicated by the presence of voltage-activated sodium currents in response to depolarizing voltage steps to 0 mV holding potential. Miniature EPSCs (mEPSCs) and IPSCs (mIPSCs), which are due to action potential-independent transmitter release, were recorded in the presence of tetrodotoxin (1 μm; Sigma).
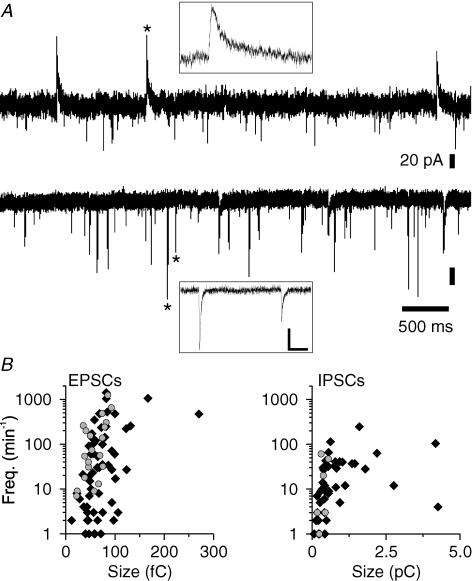
Figure 2. Presence of functional excitatory and inhibitory synapses in glia-free cultures of hippocampal neurons immunoisolated from postnatal mice.
A, recordings of spontaneous synaptic activity from hippocampal neurons at −30 mV (top trace) and at −70 mV (bottom trace). Prior to recordings, neurons were immunoisolated and cultured for 7 days in FSM. Asterisks label individual synaptic currents shown in boxes with different time and amplitude scales (scale bars, 40 pA; 20 ms). B, scatter plots of frequencies and sizes (charge transfer) of sEPSCs (left) and sIPSCs (right) in individual hippocampal neurons (black diamonds, randomly selected cells; grey circles, pyramidal neurons).
Immunocytochemistry
Immunocytochemical staining was carried out using standard procedures (Nagler et al. 2001). To determine the purity of neuronal preparations, cells were stained 24 h after plating with combinations of cell-type specific antibodies: for oligodendrocytes, mouse anti 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase; 1:500; Sigma) and mouse anti-O4 (oligodendrocyte lineage; 1:20; Roche Diagnostics); for astrocytes, rabbit anti-S100β (astrocytes; 1:1000; Swant, Bellinzona, Switzerland) and rabbit anti-glial fibrillary acid protein (GFAP; astrocytes; 1:2000; Dako Cytomation, Trappes, France); for fibroblasts, rabbit anti-fibronectin (fibroblasts) (1:500; Sigma). Microglial cells were detected by Griffonia (bandeiraea) simplicifolia (GS) lectin I isolectin B4-FITC (1:100; Vector Laboratories/Alexis, Grunberg, Germany), which was applied together with secondary antibodies. GABAergic neurons were stained with a rabbit anti-glutamate decarboxylase isoform 65 (GAD65; 1:1000; Chemicon/Euromedex). Control staining ensured that the antibodies detected the respective cell type. To determine the total number of cells in a field of view, the non-selective DNA marker H33342/bis-benzimide (20 μg ml−1; Sigma) was added together with secondary antibodies. The percentage of specific cell types was determined by counting the total number of fluorescent nuclei and then the number of antibody-labelled cells. For synapse staining, neurons were labelled by mouse anti-synapsin I (clone 46.1; 1:200; Synaptic Systems, Göttingen, Germany), rabbit anti-PSD95 (1:50, Synaptic Systems) and rabbit anti-GABAA receptor (1:400; Upstate Biotechnology/Euromedex). For fluorescence labelling, Alexa 488-, Alexa 555- (Molecular Probes/Invitrogen), Cy2- and Cy3-conjugated (Jackson Immunoresearch Laboratories) goat anti-mouse or goat anti-rabbit IgG antibodies were used.
Data representation and statistical analysis
Each data set was obtained from at least three independent preparations. Statistical analysis was performed using STATISTICA 7.1 (StatSoft Inc., Maison-Alfort, France). Graphs were created by SigmaPlot 9.01 (Systat Software GmbH, Erkrath, Germany). Data samples were tested for normality (Shapiro-Wilk's test) to select appropriate statistical tests and graphical representation. Non-normally distributed values were represented by box plots (horizontal line, median; box limits, 1st and 3rd quartile; whiskers, 10th and 90th percentile) or cumulative relative frequency plots and were tested for statistically significant changes by appropriate tests (two independent samples: Mann-Whitney U test; multiple independent samples: Kruskal-Wallis ANOVA by ranks test followed by post hoc comparison of mean ranks; two dependent samples: Wilcoxon signed rank test). Changes in percentages of cells were analysed using Pearson's χ2 test. Parameters with large sample sizes (n > 500) were analysed by one-way ANOVA followed by Dunnett's post hoc test to detect changes compared with control condition. To adjust for unequal sample size, random subsamples were analysed as indicated. Levels of significance are indicated by asterisks (*P < 0.05; **P < 0.01; ***P < 0.001). Mean values are indicated together with (±) standard deviation (s.d.).
Results
Purification and culture of neurons from different regions of the postnatal mouse brain
To test whether neurons of the mouse CNS depend on glia to form synapses, we developed protocols to immunoisolate hippocampal and cerebellar neurons from postnatal mice using an antibody against the cell adhesion molecule L1 (Rathjen & Schachner, 1984). We also purified RGCs from postnatal mice using a modified version of the protocol for rat RGCs (Barres et al. 1988). The yield of cells per animal that could be harvested from the selection plates varied with the brain region (Table 1). Immunopanning of mouse RGCs yielded about a third of cells (Table 1) compared with their isolation from postnatal rats (71 000 ± 14 000; n = 75). Regardless of the brain region, the initial viability of cells, determined 3 h after plating by a microfluorometric assay, averaged at 75% (Table 1). To determine the purity of the neuronal preparations, we performed immunocytochemical staining with combinations of cell-type-specific markers. Fractions of GFAP/S100β-positive astrocytes, of O4/CNPase-positive oligodendrocytes, of fibronectin-positive fibroblasts and of GS lectin I-positive microglia were very low (Table 1) indicating that the procedures isolated > 99% pure populations of neurons from these regions. As expected from previous studies on rat (Barres et al. 1988) and chick (Butowt et al. 2000; Annies & Kroger, 2002), our preparation of mouse RGCs immunoisolated by a Thy1-specific antibody also contained very few non-neuronal cells (Table 1).
Table 1.
Characterization of immunoisolated neurons
| Fraction of non-neuronal cells (%) | ||||
|---|---|---|---|---|
| Region | Cells per animal (/1000) | Astroc./Oligodend. | Fibroblast/Microglia | Viability 3 h (%) |
| Hippocampus | 23 ± 8 (36) | 0.09 ± 0.18 (4; 2451) | 0 (3; 1363) | 74 ± 11 (n = 3) |
| Cerebellum | 1715 ± 763 (13) | 0.85 ± 0.67 (3; 1726) | 0.05 ± 0.08 (1332) | 77 ± 12 (n = 3) |
| RGCs | 27 ± 9 (27) | 1.18 ± 0.8 (8; 1691) | 75 ± 12 (n = 3) | |
Yield, fraction of non-neuronal cells and viability were determined by haemocytometer counts of tryptan blue-excluding cells, by immunocytochemical staining with cell-type-specific markers and by a microfluorometric survival assay, respectively. Values indicate mean ± s.d. with number of preparations or cells in parentheses.
To explore, whether our hippocampal and cerebellar cultures contained GABAergic neurons, they were stained with a GAD65-specific antibody 24 h after plating. This revealed that on average 18 ± 2% of hippocampal neurons (n = 3 preparations; 12576 cells examined) were GABAergic. About 10% of neurons (n = 154 cells total) resembled pyramidal cells (Fig. 1A, inset). Cerebellar cultures contained 7 ± 2% of GAD65-positive (n = 3 preparations.; 6678 cells) GABAergic interneurons indicating that these cultures consisted mainly of glutamatergic granule cells, as expected from the situation in vivo. This was further supported by the small somata of most cerebellar neurons, which are characteristic for granule cells. Purkinje cells, which can be recognized by their large somata, were very rare in cerebellar cultures (about 1 out of 4000). This incidence was even lower than their frequency of 0.5% in vivo. BrdU labelling showed that cerebellar cultures contained only a very small fraction of dividing cells (1.1 ± 0.8%; n = 4) indicating the absence of granule cell precursors, which are present in the cerebellum of 7-day-old mice (Swisher & Wilson, 1977).
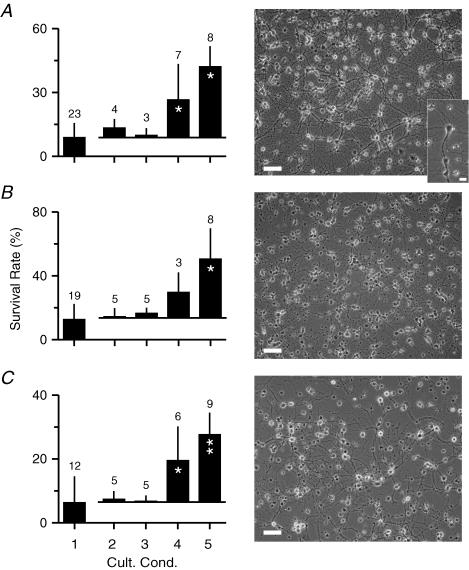
Figure 1. Serum-free culture of immunoisolated CNS neurons.
Survival rates (left; mean ± s.d.; 3 DIV) and phase-contrast micrographs (right; 7 DIV in FSM; scale bars, 80 μm) of neurons from hippocampus (A), cerebellum (B) and retina (RGCs) (C) that were immunoisolated from postnatal mice. Inset in A, phase-contrast micrograph of a hippocampal pyramidal cell. Scale bar, 20 μm. For survival assays, neurons were cultured for 3 days in MSM (1) and in MSM supplemented by forskolin (2), by BDNF–CNTF (3), by forskolin–BDNF–CNTF (4) or by forskolin–BDNF–CNTF–B27 (5). Numbers above columns indicate sample size (number of preparations). For conditions 2–5, columns indicate mean of additional rates compared with basal survival in MSM-treated control cultures induced by each respective treatment. Asterisks indicate significant changes compared with MSM (1) (paired samples; Wilcoxon signed rank test).
Next, we established chemically defined culture conditions that supported neuronal survival in the absence of glia. When immunoisolated neurons were cultured for 3 days in MSM, survival rates were very low regardless of the brain region (Fig. 1) indicating that neuronal survival required additional signals. As reported previously for RGCs (Meyer-Franke et al. 1995) and motoneurons (Hanson et al. 1998), peptide growth factors combined with an increase in the level of cyclic adenosine monophosphate enhanced survival rates of neurons from the three brain regions (Fig. 1). Addition of a commercially available medium supplement further stabilized neuronal survival (Fig. 1). After 1 week in culture with FSM, survival rates averaged 43 ± 11% (n = 4) for hippocampal neurons, 40 ± 15% for cerebellar neurons and 25 ± 6% for RGCs and all neurons showed vigorous neurite outgrowth (Fig. 1). The lower survival rate of RGCs was due to occasional preparations with very low (< 20%) viability, which were excluded from further analysis.
Synapse development in CNS neurons in the absence of glia
Next, we used our new culture preparations to test whether hippocampal and cerebellar neurons establish functional synaptic connections in the absence of glial cells. Neurons from each region were cultured for 1 week at similar densities under defined, glia-free conditions. The presence of functional synapses was determined by whole-cell patch-clamp recordings of excitatory and inhibitory synaptic activity from randomly selected cells (Figs 2 and 3). The large majority of hippocampal (Figs 2 and 7A) and of cerebellar (Figs 3 and 7B) neurons showed excitatory synaptic activity, when cultured for 1 week without glia. In both cultures, spontaneous EPSCs (sEPSCs) reached frequencies of 1000 events min−1 and higher (Figs 2B and 3B). Spontaneous inhibitory synaptic activity was observed in more than half of hippocampal neurons (Figs 2 and 9A) with spontaneous IPSCs (sIPSCs) occurring at frequencies up to 250 events min−1 (Figs 2B and 9A). The fraction of cerebellar neurons showing spontaneous IPSCs (sIPSCs) and their frequencies (Figs 3B and 9B) were lower than in hippocampal neurons (Figs 2B and 9A) reflecting the smaller percentage of GABAergic neurons in the cerebellar culture preparation.
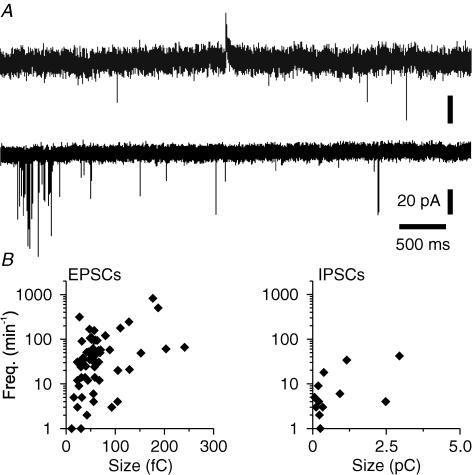
Figure 3. Presence of functional excitatory and inhibitory synapses in glia-free cultures of cerebellar neurons immunoisolated from postnatal mice.
A, recordings of spontaneous synaptic activity from cerebellar neurons at −30 mV (top trace) and at −70 mV (bottom trace). Prior to recordings, neurons were immunoisolated and cultured for 7 days in FSM. B, scatter plots of frequencies and sizes (charge transfer) of sEPSCs (left) and sIPSCs (right) in individual cerebellar neurons.
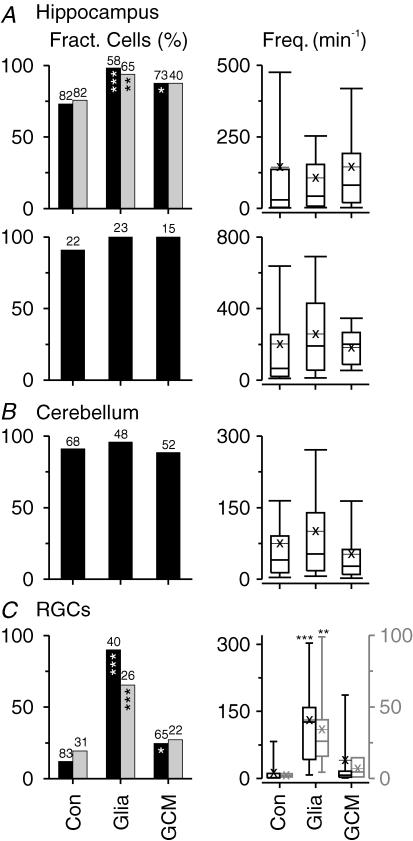
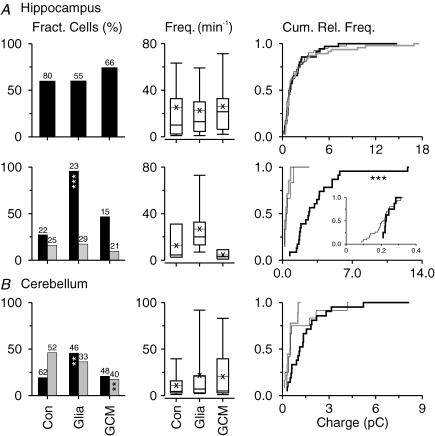
Figure 7. Effects of glial signals on incidence and frequency of spontaneous EPSCs in cultures of immunoisolated CNS neurons.
Level of spontaneous excitatory synaptic activity in hippocampal neurons (A; upper row: randomly selected cells; lower row: pyramidal cells), in cerebellar neurons (B) and in RGCs (C) cultured for 7 days in the absence of glia (Con), co-cultured with glial cells (Glia) or treated with GCM (GCM). Left, column plots showing fraction of neurons with sEPSCs (numbers indicate sample size). Right, box plots representing frequencies of sEPSCs in neurons with excitatory activity (thin lines with crosses, mean values). For parameters showing statistically significant glia-induced changes in spontaneous activity compared with control cultures, the incidence (left, grey columns) and frequency (right, grey box plots) of action potential-independent mEPSCs are indicated. Asterisks indicate significant changes compared with control cultures (left, Pearson's χ2 test; right, Kruskal-Wallis ANOVA by ranks test with post hoc comparison of mean ranks).
Figure 9. Effects of glial signals on inhibitory synaptic activity in cultures of immunoisolated CNS neurons.
Level of spontaneous inhibitory synaptic activity in hippocampal neurons (A; upper row: randomly selected cells; lower row: pyramidal cells) and in cerebellar neurons (B) cultured for 7 days in the absence of glia (Con), co-cultured with glial cells (Glia) or treated with GCM (GCM). Left, column plots showing the fraction of neurons with sIPSCs (numbers indicate sample sizes). Middle, box plots representing frequencies of sIPSCs in neurons with excitatory activity (thin lines with crosses, mean values). Right, cumulative relative frequency distribution of charge transfer of sIPSCs representing the 90th percentile in each cell in glia-free control cultures (thin black lines), in co-cultures (thick black) and in GCM-treated cultures (grey). Asterisks indicate significant changes compared with control cultures (left, Pearson's χ2 test; middle and right, Kruskal-Wallis ANOVA by ranks test with post hoc comparison of mean ranks). For parameters showing statistically significant glia-induced changes in spontaneous activity compared with control cultures, the incidence (left, grey columns) and size (A, right, inset in lower panel, pooled from all neurons tested) of action potential-independent mIPSCs are indicated.
The frequencies of spontaneous postsynaptic currents, particularly of sEPSCs, in hippocampal and cerebellar neurons ranged over nearly three orders of magnitude under glia-free conditions. To test whether this variability was cell-intrinsic or whether it reflected the presence of neuronal cell types with distinct levels of synaptic activity, we recorded spontaneous synaptic activity from hippocampal pyramidal cells, which could be identified based on their characteristic morphology (Fig. 1A, inset). In pyramidal cells cultured for 7 days, the incidence of sEPSCs and their frequency range were similar as in randomly selected hippocampal neurons (Figs 2B and 7A) indicating that the variability did not originate from cell type-specific differences. The presence of robust synaptic activity indicated that hippocampal and cerebellar neurons formed functional excitatory and inhibitory synapses in the absence of glia. This was confirmed by immunocytochemical double-staining of 7-day-old, glia-free hippocampal and cerebellar cultures with antibodies against pre- and postsynaptic proteins. As illustrated in Figs 4 and 5, neurons in both cultures showed co-localization of pre- and postsynaptic markers.
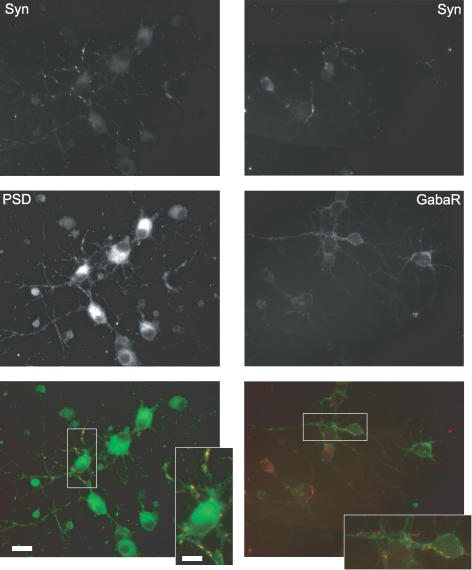
Figure 4. Formation of excitatory and inhibitory synapses by immunoisolated hippocampal neurons in the absence of glia.
Representative fluorescence micrographs of hippocampal neurons that were cultured for 7 days in FSM and then processed for immunostaining of excitatory (left) and inhibitory (right) synapses with antibodies against the presynaptic marker synapsin (top) and against postsynaptic proteins (middle left, PSD95; middle right: GABAA receptor; green bottom). Bottom, merged false colour micrographs with red and green indicating pre- and postsynaptic markers, respectively. Scale bar, 20 μm. White rectangles indicate magnified areas shown in inset. Scale bar, 10 μm.
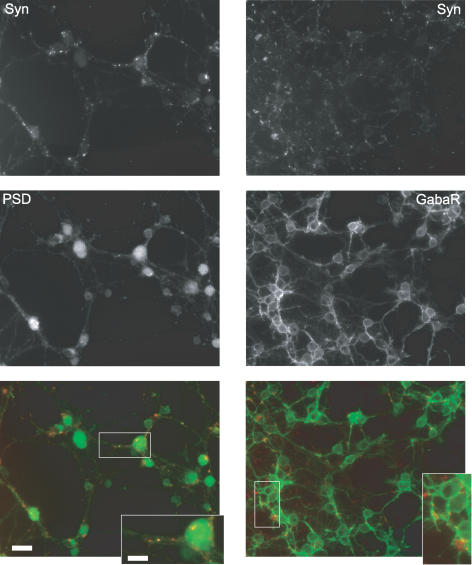
Figure 5. Formation of excitatory and inhibitory synapses by immunoisolated cerebellar neurons in the absence of glia.
Representative fluorescence micrographs of cerebellar neurons that were cultured for 7 days in FSM and then processed for immunostaining of excitatory (left) and inhibitory (right) synapses with antibodies against the presynaptic marker synapsin (top) and against postsynaptic proteins (middle left, PSD95; middle right: GABAA receptor; green bottom). Bottom, merged false colour micrographs with red and green indicating pre- and postsynaptic markers, respectively. Scale bar, 20 μm. White rectangles indicate magnified areas shown in inset. Scale bar, 10 μm.
For comparison, we analysed excitatory synaptic activity in purified mouse RGCs. Previous studies on RGCs immunoisolated from rats reported a low level of sEPSCs and a low incidence of synapses under glia-free conditions (Pfrieger & Barres, 1997; Nagler et al. 2001; Ullian et al. 2001). Our recordings confirmed these findings for mouse RGCs: only 12% of all cells tested (n = 83) showed sEPSCs when grown for 7 days in the absence of glia and their frequencies reached maximally 90 events min−1 (Figs 6 and 7C). Moreover, double immunostaining after 7 DIV confirmed the low incidence of synapses (Fig. 6) that was previously observed in RGCs isolated from rats (Nagler et al. 2001; Ullian et al. 2001). This low degree of synapse formation was in marked contrast to hippocampal and cerebellar neurons and indicated that regardless of the species, RGCs form few and inefficient synapses in the absence of glia. Taken together, CNS neurons appeared to differ in their ability to establish synaptic connections independently from glia.
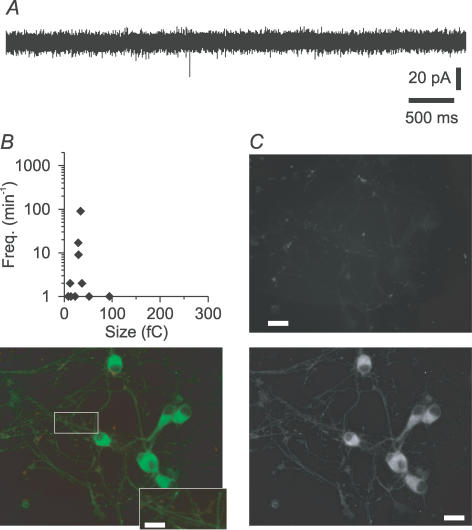
Figure 6. Low incidence of excitatory synapses in glia-free cultures of RGCs immunoisolated from postnatal mice.
A, recordings of spontaneous synaptic activity from RGCs at −70 mV. Prior to recordings, neurons were immunoisolated and cultured for 7 days in FSM. B, scatter plot of frequencies and sizes (charge transfer) of sEPSCs in individual RGCs. C, representative fluorescence micrographs of RGCs that were cultured for 7 days in FSM and then processed for immunostaining of excitatory synapses with antibodies against the presynaptic marker synapsin (top) and against the postsynaptic protein PSD95 (bottom, right). Bottom left, merged false colour micrographs with red and green indicating pre- and postsynaptic markers, respectively. Scale bar, 20 μm.
Effects of glial signals on spontaneous synaptic activity
To study, whether glial signals affected synapse development, immunoisolated neurons were co-cultured with glial cells or treated> with GCM, which contains secreted factors. We observed different glial effects depending on the brain region and the type of synapse.
In randomly selected hippocampal neurons, co-culture with glial cells induced a slight but significant enhancement in the fraction of cells showing sEPSCs, but glial signals did not change the frequency of sEPSCs (Fig. 7A). Furthermore, co-culture with glia enhanced the size of sEPSCs in a large fraction of neurons (Fig. 8A). Inhibitory activity was not affected by the presence of glia (Fig. 9A). In pyramidal cells, glial cells also enhanced strongly the size of sEPSCs (Fig. 8A), but left their incidence and frequency unaffected (Fig. 7A). Moreover, they raised significantly the incidence of sIPSCs and their size (Fig. 9A). In cerebellar neurons, co-culture with glia increased the size of sEPSCs (Fig. 8B) and raised the incidence of inhibitory activity (Fig. 9B), but left all other parameters unaffected. Notably, changes observed in neuron-glia co-cultures were only mimicked in part by GCM.
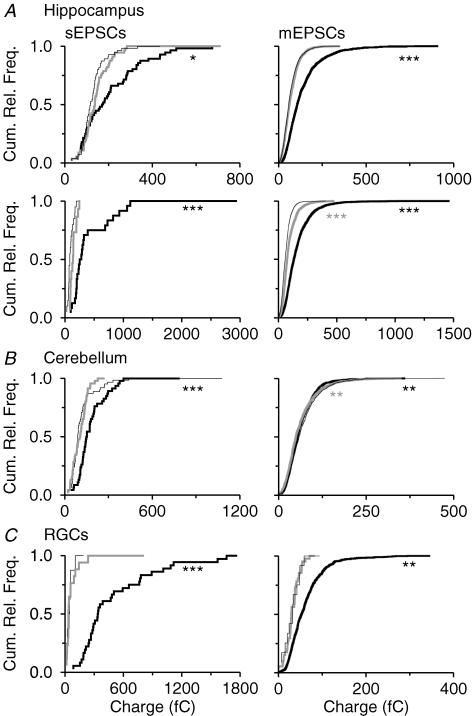
Figure 8. Glia-induced changes in the size of spontaneous and miniature EPSCs in cultures of immunoisolated neurons.
Cumulative relative frequency distributions of charge transfer of sEPSCs representing the 90th percentile in each cell (left column) and of charge transfers of individual mEPSCs pooled from all cells analysed (right column) that were recorded from hippocampal (A; upper row: randomly selected cells; lower row: pyramidal cells), cerebellar neurons (B) and from RGCs (C). Prior to recordings, neurons were cultured for 7 days in the absence of glia (thin black lines), co-cultured with glial cells (thick black lines) or treated with GCM (grey lines). For hippocampal (n ≈nbsp;2000) and cerebellar neurons (n ≈ 800), random subsamples of mEPSCs were analysed. Asterisks indicate significant changes compared with control cultures (left, Kruskal-Wallis ANOVA by ranks test with post hoc comparison of mean ranks; right, ANOVA with Dunnett's post hoc test). For RGCs, the number of individual mEPSCs (n = 12) recorded under glia-free conditions was very low compared with co-cultures (n = 582) or GCM-treated cultures (n = 41) and therefore a subsample could not be analysed. The asterisks therefore indicate a statistically significant change in random subsamples (∼40 events) from co-cultures and from GCM-treated cultures (Mann-Whitney U test).
In RGCs, co-culture with glial cells strongly enhanced synaptic activity: we observed a 7-fold increase in the incidence of sEPSCs and a nearly 10-fold increase in the frequency (Fig. 7C) and size (Fig. 8C) of sEPSCs compared with glia-free cultures. These effects confirmed previous observations on RGCs from rats (Pfrieger & Barres, 1997; Nagler et al. 2001; Ullian et al. 2001). GCM mimicked the glia-induced enhancement of synaptic activity only partially (Figs 7C and 8C). This may have been due to a low concentration of cholesterol in mouse GCM, as this component has been identified as synapse-promoting factor (Mauch et al. 2001; Goritz et al. 2005). Addition of cholesterol to the culture medium for 5 days raised the fraction of RGCs with synaptic activity nearly 3-fold (33%; n = 76 neurons tested; P < 0.01; Pearson's χ2 test) and increased the frequency of sEPSCs significantly compared with control cultures (P < 0.05; n = 25; Mann-Whitney U test). However, cholesterol did not mimick the glia-induced increase in the size of sEPSCs compared with glia-free cultures (P = 0.58; n = 25; Mann-Whitney U test).
Effects of glial cells on miniature postsynaptic currents
The observed glia-induced changes in synaptic activity in the different neuronal cell types could have been due to enhanced neuronal excitability and thus a higher level of action potential-driven activity. To address this, we tested how glial cells affected miniature postsynaptic currents, which are due to action potential-independent quantal transmitter release.
In hippocampal neurons including pyramidal cells, glial cells affected mEPSCs in a similar manner as spontaneous excitatory events: compared with glia-free cultures, co-culture with glia increased the fraction of neurons showing mEPSCs (Fig. 7A) and their size (Fig. 8A). The glia-induced changes in the incidence and size of sIPSCs of pyramidal cells were not observed in mIPSCs (Fig. 9A). This indicated that glial cells affected the development or function of excitatory, but not of inhibitory synaptic connections in hippocampal neurons. Recent studies showed that glia-derived tumour necrosis factor alpha enhances the size of mEPSCs in hippocampal cultures (Beattie et al. 2002; Stellwagen & Malenka, 2006). However, this component did not mimick the glia-induced increase in mEPSC size in hippocampal neurons (n = 15 cells, 3 preparations). In cerebellar cultures, glial signals decreased the size of mEPSCs (Glia: n = 38; GCM: n = 39) compared with glia-free cultures (n = 66; Fig. 8B) indicating that the enhanced size of sEPSCs in these neurons was due to a change in action potential-dependent synaptic activity. In RGCs, the glia-induced increase in the incidence, frequency and size of sEPSCs was also observed for mEPSCs (Figs 7C and 8C), thus confirming previous observations that glial cells promote the development of synapses in these neurons.
Discussion
Our study on new primary cultures of immunoisolated CNS neurons from postnatal mice revealed that hippocampal and cerebellar neurons formed functional synapses in the absence of glia, whereas RGCs did not. Co-culture with glia strongly promoted the frequency of spontaneous synaptic activity only in RGCs, but not in hippocampal and cerebellar neurons and this effect was mimicked in part by cholesterol, thus confirming previous results (Mauch et al. 2001; Goritz et al. 2005). On the other hand, glial signals enhanced the size of spontaneous excitatory synaptic events in all three regions, and in hippocampal neurons and RGCs this effect was due to an increase in the size of action potential-independent miniature synaptic events. Finally, no effect of glia on inhibitory synapses was detected.
Hippocampal and cerebellar neurons, but not RGCs show robust synapse formation in the absence of glia
A main finding of our study is that CNS neurons differ in their ability to form synapses without glia. Within 1 week in culture, most hippocampal and cerebellar neurons formed functional excitatory and inhibitory synaptic contacts in the absence of glia as indicated by robust spontaneous synaptic activity and by the presence of immunocytochemically stained synapses. The frequencies of EPSCs and IPSCs in glia-free cultures were similar or even higher than those reported previously in conventional hippocampal (Mennerick et al. 1995; Bouron & Reuter, 1997; Boehm, 1999) and cerebellar (Virginio et al. 1995; Losi et al. 2002; Fiszman et al. 2005) cultures derived from postnatal rats or mice. This indicated that under the present culture conditions, purified neurons reach the same level of connectivity as those growing in glia-containing primary cultures. This was in contrast to cultured RGCs, which formed only very few connections and showed a very low level of activity in the absence of glia thus confirming previous studies (Pfrieger & Barres, 1997; Nagler et al. 2001; Ullian et al. 2001).
Our isolation procedure and culture conditions may have selected for those neurons that form synapses autonomously. However, hippocampal and cerebellar cultures contained inhibitory neurons as well as principal neurons like pyramidal and Purkinje cells at ratios that can be expected from the situation in vivo. Thus, our preparation preserved the diversity of neuronal cell types rather than selecting for synaptogenesis-competent cells. It may also be argued that synaptic connections in our cultures were ‘non-natural’ and that their formation proceeds independently from glia. This argument does not apply for hippocampal cultures, where pyramidal cells, which represent principal synaptic targets, received synaptic input from excitatory and inhibitory neurons. In cerebellar cultures, which consisted mainly of granule cells, excitatory synaptic activity originated from somewhat non-natural synapses among granule cells, as these are the only cerebellar cell type that can form excitatory glutamatergic connections. However, the same argument applies for cultures of immunoisolated RGCs (Nagler et al. 2001; Ullian et al. 2001) and motoneurons (Ullian et al. 2004b), which lack natural partner neurons. In contrast to cerebellar granule cells, however, these neurons required glia to form non-natural synaptic connections. This corroborates our statement that neurons differ in their ability to form synapses autonomously. Due to the rare occurrence (< 0.5%) of Purkinje cells in our cultures it was not possible to determine whether they receive natural input from granule cells.
Glial signals promote formation of synapses by RGCs, but not by hippocampal or cerebellar neurons
Glial signals did not enhance the frequency of excitatory or inhibitory synaptic events in hippocampal and cerebellar cultures indicating that they did not promote the formation of new functional connections by these neurons. This is different from previous reports that glial signals promote synaptic activity and synapse formation in cultures of hippocampal neurons (Yang et al. 2003; Hama et al. 2004; Elmariah et al. 2005). The divergence may be explained by differences in culture conditions and in the age of animals from which neurons were obtained. The cited studies prepared neurons from embryonic animals. At this stage, only few glial cells have been generated and so the culture preparations can be regarded as glia-free. It is possible that at the embryonic stage, neurons require glial signals for synaptogenesis, whereas postnatally they become autonomous. We note that the glia-induced increase in a small fraction of hippocampal neurons (but not pyramidal cells) that showed excitatory synaptic events may reflect an induction of synaptic input and thus synapse formation in a subset of hippocampal neurons.
In contrast to hippocampal and cerebellar neurons, glial signals strongly promoted synapse formation in RGCs as indicated by the large increase in the incidence of synaptic activity and in the frequency of spontaneous and miniature synaptic events. This confirmed previous reports that the glia-induced enhancement of synaptic activity in these neurons is due to enhanced synaptogenesis (Nagler et al. 2001; Ullian et al. 2001). The lack of effect by mouse GCM, which differs from the situation in the rat (Pfrieger & Barres, 1997; Nagler et al. 2001), was possibly due to a lower cholesterol concentration in mouse GCM, since addition of cholesterol mimicked the effects of co-culture on the frequency of sEPSCs at least in part.
Glial cells enhance the size of miniature synaptic currents in hippocampal neurons and RGCs
Glial cells enhanced the size of spontaneous excitatory postsynaptic currents in all three culture preparations. In hippocampal neurons and RGCs, this was due to an increase in the size of action potential-independent miniature synaptic currents. In cerebellar cultures, which consist mainly of granule cells, the effect was action potential-dependent and may have been caused by an increased excitability or by an increased efficacy of evoked transmitter release. Spontaneous inhibitory synaptic currents were only affected by glia in pyramidal cells, where co-culture led to a higher incidence and larger size of sIPSCs. This effect, which did not occur in miniature inhibitory synaptic currents and was therefore action potential-dependent, may have been indirect via elevated excitatory synaptic activity. This may have excited GABAergic interneurons providing synaptic input to pyramidal cells. Alternatively, glial cells, namely astrocytes, may have directly activated interneurons as reported recently (Liu et al. 2004). Apart from this effect in pyramidal cells, glial cells increased only the size of excitatory postsynaptic currents. Such a selective effect on glutamatergic rather than GABAergic synapses is in line with the fact that inhibitory synapses develop before glutamatergic synapses (Ben-Ari, 2002), and thus may not require glial signals.
To increase the size of mEPSCs in hippocampal neurons and RGCs, glial signals may have enhanced postsynaptically the efficacy of glutamate receptors or presynaptically the glutamate concentration in synaptic vesicles. These changes may have been due to enhanced maturation of excitatory synaptic connections, for example by increased clustering of postsynaptic glutamate receptors, or due to enhanced function of normally developed synapses, for example by glial support of the glutamate–glutamine cycle (Hertz & Zielke, 2004). Except for hippocampal pyramidal cells, the glia-induced increase in miniature size was only seen in neuron–glia co-cultures and was not mimicked by soluble glial factors contained in GCM. This is in line with our previous observation on immunoisolated rat RGCs that co-culture with glia enhanced the size of mEPSCs more strongly than GCM (Nagler et al. 2001). Astrocyte-derived tumour necrosis factor alpha, which has been shown to enhance the surface expression of glutamate receptors (Beattie et al. 2002; Stellwagen & Malenka, 2006), did not mimick the glia-induced increase in miniature size in our cultures.
Taken together, our results indicate that in the mammalian CNS the way that glial signals promote the formation and efficacy of excitatory synapses varies with the neuronal cell type. This underlines the importance of comparative studies on different neurons. Our new culture preparations may serve as first experimental models to study the relevance of glial signals on postnatal neuronal development.
Acknowledgments
The authors thank D. Dalencon for administrative assistance and gratefully acknowledge support by the Ara Parseghian Medical Research Foundation (I.B., F.W.P.), CNRS (K.N., F.W.P.), Fondation NRJ – Institut de France (F.W.P.), Fondation pour la Recherche Medicale (T.C., C.S.), Max-Planck Society (F.W.P.), Ministere de l'Education et de la Recherche (C.S.) and Retina France (I.B., T.C., F.W.P.).
References
- Allen NJ, Barres BA. Signaling between glia and neurons: focus on synaptic plasticity. Curr Opin Neurobiol. 2005;15:542–548. doi: 10.1016/j.conb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Annies M, Kroger S. Isoform pattern and AChR aggregation activity of agrin expressed by embryonic chick retinal ganglion neurons. Mol Cell Neurosci. 2002;20:525–535. doi: 10.1006/mcne.2002.1125. [DOI] [PubMed] [Google Scholar]
- Barres BA, Silverstein BE, Corey DP, Chun LLY. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. doi: 10.1016/0896-6273(88)90127-4. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Boehm S. Presynaptic α2-adrenoceptors control excitatory, but not inhibitory, transmission at rat hippocampal synapses. J Physiol. 1999;519:439–449. doi: 10.1111/j.1469-7793.1999.0439m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouron A, Reuter H. Muscarinic stimulation of synaptic activity by protein kinase C is inhibited by adenosine in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 1997;94:12224–12229. doi: 10.1073/pnas.94.22.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butowt R, Jeffrey PL, von Bartheld CS. Purification of chick retinal ganglion cells for molecular analysis: combining retrograde labeling and immunopanning yields 100% purity. J Neurosci Meth. 2000;95:29–38. doi: 10.1016/s0165-0270(99)00149-1. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Colomar A, Robitaille R. Glial modulation of synaptic transmission at the neuromuscular junction. Glia. 2004;47:284–289. doi: 10.1002/glia.20086. [DOI] [PubMed] [Google Scholar]
- Elmariah SB, Oh EJ, Hughes EG, Balice-Gordon RJ. Astrocytes regulate inhibitory synapse formation via Trk-mediated modulation of postsynaptic GABAA receptors. J Neurosci. 2005;25:3638–3650. doi: 10.1523/JNEUROSCI.3980-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Carmignoto G. Neurone-to-astrocyte signalling in the brain represents a distinct multifunctional unit. J Physiol. 2004;559:3–15. doi: 10.1113/jphysiol.2004.063214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Koirala S, Ko CP. Synapse–glia interactions at the vertebrate neuromuscular junction. Neuroscientist. 2005;11:503–513. doi: 10.1177/1073858405277409. [DOI] [PubMed] [Google Scholar]
- Fiszman ML, Barberis A, Lu C, Fu Z, Erdelyi F, Szabo G, Vicini S. NMDA receptors increase the size of GABAergic terminals and enhance GABA release. J Neurosci. 2005;25:2024–2031. doi: 10.1523/JNEUROSCI.4980-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goritz C, Mauch DH, Pfrieger FW. Multiple mechanisms mediate cholesterol-induced synaptogenesis in a CNS neuron. Mol Cell Neurosci. 2005;29:190–201. doi: 10.1016/j.mcn.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Hama H, Hara C, Yamaguchi K, Miyawaki A. PKC signaling mediates global enhancement of excitatory synaptogenesis in neurons triggered by local contact with astrocytes. Neuron. 2004;41:405–415. doi: 10.1016/s0896-6273(04)00007-8. [DOI] [PubMed] [Google Scholar]
- Hanson MG, Jr, Shen S, Wiemelt AP, McMorris FA, Barres BA. Cyclic AMP elevation is sufficient to promote the survival of spinal motor neurons in vitro. J Neurosci. 1998;18:7361–7371. doi: 10.1523/JNEUROSCI.18-18-07361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Hertz L, Zielke HR. Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends Neurosci. 2004;27:735–743. doi: 10.1016/j.tins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Liu QS, Xu Q, Arcuino G, Kang J, Nedergaard M. Astrocyte-mediated activation of neuronal kainate receptors. Proc Natl Acad Sci U S A. 2004;101:3172–3177. doi: 10.1073/pnas.0306731101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losi G, Prybylowski K, Fu Z, Luo JH, Vicini S. Silent synapses in developing cerebellar granule neurons. J Neurophysiol. 2002;87:1263–1270. doi: 10.1152/jn.00633.2001. [DOI] [PubMed] [Google Scholar]
- Mauch DH, Nägler K, Schumacher S, Göritz C, Müller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- Mazzanti M, Haydon PG. Astrocytes selectively enhance N-type calcium current in hippocampal neurons. Glia. 2003;41:128–136. doi: 10.1002/glia.10135. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Que J, Benz A, Zorumski CF. Passive and synaptic properties of hippocampal neurons grown in microcultures and in mass cultures. J Neurophysiol. 1995;73:320–332. doi: 10.1152/jn.1995.73.1.320. [DOI] [PubMed] [Google Scholar]
- Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- Nagler K, Mauch DH, Pfrieger FW. Glia-derived signals induce synapse formation in neurones of the rat central nervous system. J Physiol. 2001;533:665–679. doi: 10.1111/j.1469-7793.2001.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Glial modulation of synaptic transmission in the retina. Glia. 2004;47:268–274. doi: 10.1002/glia.20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliet SH, Piet R, Poulain DA, Theodosis DT. Glial modulation of synaptic transmission: Insights from the supraoptic nucleus of the hypothalamus. Glia. 2004;47:258–267. doi: 10.1002/glia.20032. [DOI] [PubMed] [Google Scholar]
- Peng HB, Yang JF, Dai Z, Lee CW, Hung HW, Feng ZH, Ko CP. Differential effects of neurotrophins and Schwann cell-derived signals on neuronal survival/growth and synaptogenesis. J Neurosci. 2003;23:5050–5060. doi: 10.1523/JNEUROSCI.23-12-05050.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Araque A. Glial calcium signaling and neuron-glia communication. Cell Calcium. 2005;38:375–382. doi: 10.1016/j.ceca.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells in vitro. Science. 1997;277:1684–1687. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- Rathjen FG, Schachner M. Immunocytological and biochemical characterization of a new neuronal cell surface component (L1 antigen) which is involved in cell adhesion. EMBO J. 1984;3:1–10. doi: 10.1002/j.1460-2075.1984.tb01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy LV, Koirala S, Sugiura Y, Herrera AA, Ko CP. Glial cells maintain synaptic structure and function and promote development of the neuromuscular junction in vivo. Neuron. 2003;40:563–580. doi: 10.1016/s0896-6273(03)00682-2. [DOI] [PubMed] [Google Scholar]
- Slezak M, Pfrieger FW. New roles for astrocytes: regulation of CNS synaptogenesis. Trends Neurosci. 2003;26:531–535. doi: 10.1016/j.tins.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-α. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Swisher DA, Wilson DB. Cerebellar histogenesis in the lurcher (Lc) mutant mouse. J Comp Neurol. 1977;173:205–218. doi: 10.1002/cne.901730112. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Christopherson KS, Barres BA. Role for glia in synaptogenesis. Glia. 2004a;47:209–216. doi: 10.1002/glia.20082. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Harris BT, Wu A, Chan JR, Barres BA. Schwann cells and astrocytes induce synapse formation by spinal motor neurons in culture. Mol Cell Neurosci. 2004b;25:241–251. doi: 10.1016/j.mcn.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Virginio C, Martina M, Cherubini E. Spontaneous GABA-mediated synaptic currents in cerebellar granule cells in culture. Neuroreport. 1995;6:1285–1289. doi: 10.1097/00001756-199506090-00014. [DOI] [PubMed] [Google Scholar]
- Volterra A, Steinhauser C. Glial modulation of synaptic transmission in the hippocampus. Glia. 2004;47:249–257. doi: 10.1002/glia.20080. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M, Duan S. Contribution of astrocytes to hippocampal long-term potentiation through release of d-serine. Proc Natl Acad Sci U S A. 2003;100:15194–15199. doi: 10.1073/pnas.2431073100. [DOI] [PMC free article] [PubMed] [Google Scholar]