Abstract
Ionotropic γ-amino butyric acid (GABA) receptors composed of heterogeneous molecular subunits are major mediators of inhibitory responses in the adult CNS. Here, we describe a novel ionotropic GABA receptor in mouse cerebellar Purkinje cells (PCs) using agents reported to have increased affinity for ρ subunit-containing GABAC over other GABA receptors. Exogenous application of the GABAC-preferring agonist cis-4-aminocrotonic acid (CACA) evoked whole-cell currents in PCs, whilst equimolar concentrations of GABA evoked larger currents. CACA-evoked currents had a greater sensitivity to the selective GABAC antagonist (1,2,5,6-tetrahydropyridin-4-yl)methylphosphinic acid (TPMPA) than GABA-evoked currents. Focal application of agonists produced a differential response profile; CACA-evoked currents displayed a much more pronounced attenuation with increasing distance from the PC soma, displayed a slower time-to-peak and exhibited less desensitization than GABA-evoked currents. However, CACA-evoked currents were also completely blocked by bicuculline, a selective agent for GABAA receptors. Thus, we describe a population of ionotropic GABA receptors with a mixed GABAA/GABAC pharmacology. TPMPA reduced inhibitory synaptic transmission at interneurone–Purkinje cell (IN–PC) synapses, causing clear reductions in miniature inhibitory postsynaptic current (mIPSC) amplitude and frequency. Combined application of NO-711 (a selective GABA transporter subtype 1 (GAT-1) antagonist) and SNAP-5114 (a GAT-(2)/3/4 antagonist) induced a tonic GABA conductance in PCs; however, TPMPA had no effect on this current. Immunohistochemical studies suggest that ρ subunits are expressed predominantly in PC soma and proximal dendritic compartments with a lower level of expression in more distal dendrites; this selective immunoreactivity contrasted with a more uniform distribution of GABAA α1 subunits in PCs. Finally, co-immunoprecipitation studies suggest that ρ subunits can form complexes with GABAA receptor α1 subunits in the cerebellar cortex. Overall, these data suggest that ρ subunits contribute to functional ionotropic receptors that mediate a component of phasic inhibitory GABAergic transmission at IN–PC synapses in the cerebellum.
The neurotransmitter GABA acts at ionotropic GABAA and GABAC receptors to mediate fast inhibition in the adult CNS. These receptor subtypes can be distinguished pharmacologically and also by their distinct molecular subunit composition (Johnston, 1996; Bormann, 2000); in regard to the latter, heteromeric GABAA receptors are encoded by α(1 − 6), β(1 − 3), γ(1 − 3), δ(1), ɛ(1), θ(1) and π(1) (Whiting et al. 1999), whilst GABAC are composed exclusively of combinations of ρ(1 − 3) subunits (Bormann, 2000). However, ρ subunits show a high degree of amino acid sequence homology with GABAA receptor subunits and have also been suggested to represent GABAA subtypes themselves (Barnard et al. 1998; Didelon et al. 2002). In expression systems, there is evidence that ρ subunits can associate with GABAA γ2 subunits (Qian & Ripps, 1999; Ekema et al. 2002; Pan & Qian, 2005). The extent to which this association occurs in vivo is largely unknown; however, it has been demonstrated that ρ subunits can coassemble with GABAA α1 and γ2 subunits in brainstem neurones (Milligan et al. 2004). The agonist CACA and the antagonist TPMPA have both been reported to have higher affinity at GABAC over other GABA receptors (Bormann, 2000). Using these agents, ionotropic GABA receptors with a mixed pharmacological profile have been reported in hippocampal (Semyanov & Kullmann, 2002; Hartmann et al. 2004) and brainstem (Milligan et al. 2004) neurones. Importantly, Milligan et al. (2004) correlated sensitivity to CACA with the expression of ρ1 subunits in individual neurones, providing the first evidence that selective pharmacology may be used to probe the expression of ρ subunits in the CNS. CACA has been shown to directly activate α6-containing GABA receptors in cerebellar granule cells (Wall, 2001); however, cerebellar PCs, the focus of the present study, do not express α6 receptor mRNA or protein (Wisden et al. 1996).
The term GABAC was originally suggested from studies in the cerebellum in which CACA, previously demonstrated to depress firing of spinal cord neurones in a bicuculline-insensitive manner (Johnston et al. 1975), had no effect on the binding of the GABAB agonist baclofen to isolated membranes (Drew et al. 1984). Studies at a molecular level have highlighted the cerebellum as being enriched in ρ subunit transcripts; ρ1 and ρ2 mRNAs were shown to be selectively expressed in rat PCs and basket-like interneurones (Rozzo et al. 2002) and surface ρ1 immunoreactivity has been reported in rat PC soma and dendrites (Boue-Grabot et al. 1998). Recently, a role for ρ subunits in the cerebellum has been further implicated with the cloning of a ρ2 subunit from bovine tissue, the first ρ subunit to be isolated outside of the retina (Lopez-Chavez et al. 2005). However, despite the cloning of ρ subunits and the demonstration of their prominent expression in PCs, the functional contribution of this subunit to ionotropic GABA responses in the cerebellum remains to be addressed. In the present study, we provide evidence that ρ subunits contribute to a novel population of GABA receptors that play a role in phasic inhibitory transmission at cerebellar IN–PC synapses, thus adding to the molecular heterogeneity of GABA receptors in the cerebellum.
Methods
Tissue preparation and solutions
Cerebellar slices were prepared according to methods previously described in detail by Southan & Robertson (1998). Three- to five-week-old male TO mice (Harlan, UK) were humanely killed by cervical dislocation and decapitated in line with UK Home Office procedures. The brain was removed and transferred into a chilled, oxygenated sucrose-based artificial cerebrospinal fluid (aCSF) solution. The cerebellum was dissected out and parasagittal cerebellar slices (300 μm thick) were cut using a Vibratome (R. & L. Slaughter, Upminster, UK). Slices were transferred into standard aCSF solution at 37°C for 1 h and then maintained at room temperature (20–24°C). The standard aCSF contained (mm): NaCl 124, KCl 3, NaHCO3 26, NaH2PO4 2.5, MgSO4 2, CaCl2 2, d-glucose 10, maintained at pH 7.3 by bubbling with 95% O2–5% CO2. The sucrose-based solution used for dissection and slicing was identical to standard aCSF, with the exception that NaCl was replaced by isosmotic sucrose (74.5 g l−1).
Electrophysiological recording
Slices were placed in a recording chamber at room temperature and perfused at 2–4 ml min−1 with standard aCSF bubbled with 95% O2–5% CO2. Individual neurones were visualized by a ×60 water immersion lens with infrared differential interference contrast optics using an upright Olympus BX50WI microscope (Olympus, Tokyo, Japan). Whole-cell recordings were performed using an EPC-9 patch-clamp amplifier (HEKA Electronik, Lambrecht, Germany), controlled by Pulse software (HEKA) with a Macintosh G4 computer. Electrodes were made from borosilicate glass (GC150-F10, Harvard Apparatus, Kent, UK) and, when filled with an intracellular solution containing (mm): CsCl 140; MgCl2 1; CaCl2 1; EGTA 10; MgATP 4; NaGTP 0.4; Hepes 10; pH 7.3, had resistances between 3 and 7 MΩ. Series resistance was typically 5–10 MΩ and was monitored and compensated by 70–90% throughout. Data were sampled at 7 kHz and filtered at one-third of the sampling frequency. Miniature inhibitory postsynaptic currents (mIPSCs), isolated by 1 μm tetrodotoxin (TTX), were identified as rapidly activating, inward currents at a holding potential of −70 mV. Agents were applied in the presence of the non-NMDA glutamate receptor antagonist 6-nitro-7-sulfamoylbenzo(f)quinoxaline-2-3-dione (NBQX) and the GABAB antagonist CGP 55845. Data were analysed using Pulsefit (HEKA), Axograph (Axon Instruments), Mini Analysis (Synaptosoft), Igor (Wavemetrics), Origin (Microcal), Excel (Microsoft) and Prism (GraphPad) software. Cumulative frequency plots were constructed for mIPSC inter-event intervals (bin widths 5 ms) or mIPSC amplitudes (bin widths 2 pA). Data are presented as mean value ± s.e.m., where n = number of cells. Statistical significance was determined using Student's paired or unpaired t tests, one-way ANOVA followed by Tukey's HSD test or a Mann-Whitney test, as appropriate. Cumulative frequency plots were analysed by Kolmogorov-Smirnov (KS) tests. In all cases, P < 0.05 was considered significant.
Immunohistochemisty
Immunohistochemistry was performed on three- to five-week-old male TO mice (Harlan, UK) using a procedure modified from Llano et al. (2000). Briefly, mice were decapitated and the cerebellar vermis was extracted and placed in 4% paraformaldehyde in PBS (0.15 m) for 1 h. Parasagittal slices, 100 μm thick, were cut and left in the same PBS/fixative solution for a further 30 min at 22–25°C before sections were treated with a 1% bovine serum albumin (BSA)–PBS blocking buffer containing 0.4% Triton X-100 for 1 h at room temperature. Slices were washed in PBS and then incubated overnight (2–5°C) in an antibody mixture containing rabbit anti-ρ(1&2) (1:600) (a kind gift from Dr Ralf Enz, Friedrich Alexander University of Erlangen-Nurnberg) or anti-GABAA α1 (1:300) (Alomone Laboratories, USA) and mouse anti-calbindin D28K (1:2000) (Sigma, UK). Slices were then incubated with a secondary antibody mixture containing anti-rabbit FITC (1:200) and anti-mouse TRITC (1:200) (Stratech Scientific, UK), before being washed in blocking buffer and PBS and mounted on glass slides in Vectashield fluorescent mounting medium (Vector Laboratories, CA, USA). Images were acquired using a Zeiss LSM 510 Meta confocal microscope (488 nm and 540 nm excitation) and analysed off-line.
Immunoprecipitation
Cerebella dissected from three- to five-week-old male TO mice were homogenized in 50 mm Tris-HCl pH 8.0, 1 mm EDTA, 150 mm NaCl, 1.5% sodium deoxycholate, 0.1% Nonidet P-40 and Complete protease inhibitor (Roche, UK) with an Ultra-Turrax blender. Pre-cleared supernatant was incubated with rabbit anti-ρ(1&2) (1:400) overnight at 4°C. To monitor unspecific binding, the antibody was omitted from a similar sample. Following incubation, 40 μl of protein G-sepharose beads (Sigma, UK) was added and samples were incubated at 4°C for a further 4 h. The resin was collected by centrifugation and washed five times in buffer. Bound protein was eluted by boiling the resin in 40 μl of ×2 loading buffer and resolved by SDS gel electrophoresis on a 12.5% polyacrylamide gel. GABAA α1 subunits were visualized by Western blotting with a α1 antibody (1:200, Santa Cruz Biotechnology, Heidelberg, Germany) using enhanced chemiluminescence.
Pharmacology
The following agents were used: (+)bicuculline, bicuculline methiodide, CACA, GABA, picrotoxin, SNAP-5114, NO-711 (all Sigma, UK), and NBQX (disodium salt) and TPMPA (Tocris Cookson, Bristol, UK). CGP 55845 was a kind gift from Dr Wolfgang Froestl (Novartis, Basel, Switzerland). TTX was from Alomone (Jerusalem, Israel). All drugs were made up as ×1000 concentrated stock solutions in distilled water or DMSO ((+)bicuculline, CGP 55845 and SNAP-5114), aliquoted and stored at −20°C. Aliquots were thawed and dissolved in oxygenated aCSF immediately prior to use. Drugs were bath applied to achieve steady-state effects. In some experiments, drugs were applied focally by pressure ejection using low-resistance (∼1 MΩ) borosilicate glass electrodes; electrodes were placed directly above the slice and pressure ejection of drugs also served to clean the surface of cells present in the slice.
Results
Characteristics of ionotropic GABA receptor agonist-evoked currents in Purkinje cells
Ionotropic GABA receptor-mediated responses were investigated in mouse cerebellar PCs using agents with increased affinity for GABAC (which comprise ρ subunits exclusively) over other GABA receptors. We investigated (i) the effects of exogenous application of CACA, an agonist with a > 100-fold greater potency at GABAC than at GABAA receptors (Kusama et al. 1993); although CACA may activate α6-containing GABA receptors (Wall, 2001), PCs do not express α6 receptors (Wisden et al. 1996); (ii) TPMPA, a selective GABAC antagonist with a greater than 100-fold lower dissociation constant at GABAC than at GABAA receptors (Ragozzino et al. 1996); and (iii) the natural agonist GABA. Whole-cell currents were recorded in the presence of 1 μm TTX (to minimize action potential-dependent transmitter release), 5 μm NBQX and 5 μm GCP 55845 to reduce any contribution from non-NMDA and GABAB receptors, respectively.
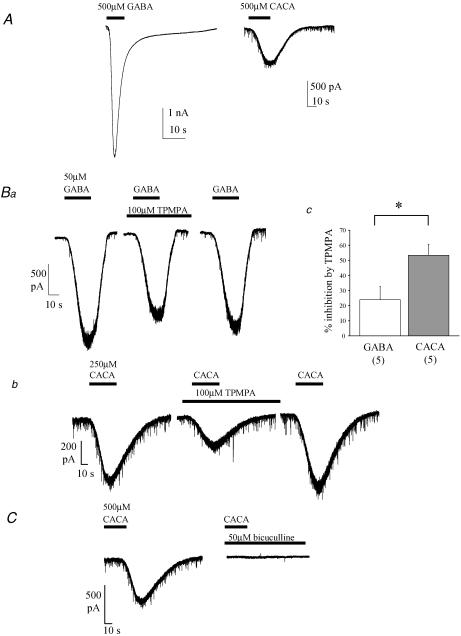
Bath application of GABA (10–500 μm) evoked concentration-dependent, inward currents at hyperpolarized holding potentials that exhibited desensitization at higher concentrations (Fig. 1A and B; see also Fig. 1 in online Supplemental material). CACA (50–500 μm) also evoked inward currents in PCs which were of smaller amplitude than those evoked by equimolar concentrations of GABA (Fig. 1A, B and C; see also online Supplemental material Fig. 1). GABA- and CACA-evoked inward currents in PCs were both significantly reduced by application of 100 μm TPMPA (Fig. 1B). The effect of TPMPA was measured at the approximated submaximal EC20 point of the GABA or CACA dose–response relationship (∼50 μm and ∼250 μm, respectively, see Supplemental material Fig. 1). Under these conditions, 100 μm TPMPA blocked a significantly larger proportion of current evoked by 250 μm CACA (53.4 ± 7.2%, n = 5; Fig. 1Bb and c) than that evoked by 50 μm GABA (23.9 ± 8.7%, n = 5, P < 0.05; Fig. 1Ba and c). These results suggest that CACA-evoked currents had an increased sensitivity to TPMPA in comparison to currents evoked by GABA. GABAC receptors are characterized by their insensitivity to bicuculline (Johnston, 1996; Bormann, 2000); however, CACA-evoked currents in PCs were abolished completely by 50 μm bicuculline (Fig. 1C; n = 6), as were GABA-evoked currents (n = 5, data not shown). In addition, CACA-evoked currents were also abolished by 10 μm picrotoxin (n = 3, data not shown). Taken together, our data suggest that PCs express a novel subtype of ionotropic GABA receptor with a mixed GABAA/GABAC pharmacology.
Figure 1. Ionotropic GABA receptors display an unusual pharmacological profile.
A, example raw data traces of GABA- and CACA-evoked inward currents. Holding potential (VH = −70 mV). Inward mIPSCs, due to spontaneous, action potential-independent release of endogenous GABA, are also evident. Note also current desensitization induced by GABA. B, example raw data traces showing reversible TPMPA-induced reduction of GABA- (a) and CACA- (b) evoked current, and summary data (c; mean ± s.e.m. with number of replicants (n) in parentheses). TPMPA produced a larger block of CACA- than GABA-evoked currents (*P < 0.01); VH = −70 mV. C, example raw data traces showing bicuculline-induced abolition of CACA-evoked current; VH = −70 mV.
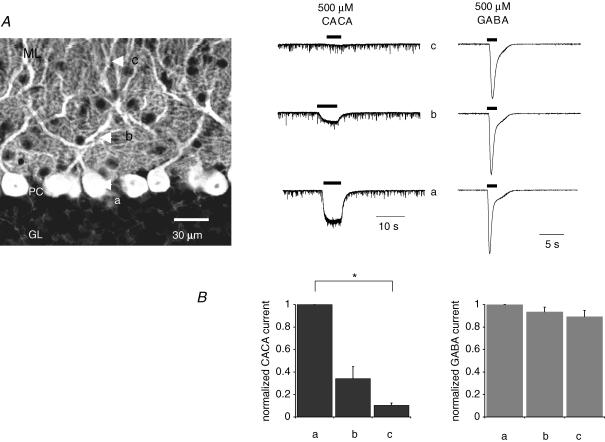
We next compared the action of CACA and GABA at distinct sites on the PC dendritic arbor. Agonists were focally applied by pressure ejection from a glass pipette (see Methods) at three distinct sites: the PC soma, proximal dendrites (∼30–60 μm away from the soma) and distal dendrites (outer edge of the molecular layer) (marked as ‘a’, ‘b’ and ‘c’, respectively, in Fig. 2). CACA-evoked currents typically displayed a sharp attenuation in amplitude with increasing distance from the soma (Fig. 2A and B); for example, when CACA was applied to the outer third of the molecular layer (position ‘c’) there was a significant 90 ± 2% (n = 5, P < 0.05) decrease in current amplitude compared to that observed when CACA was applied directly to the soma (position ‘a’) (Fig. 2B). In contrast, GABA (applied in the presence of 100 μm TPMPA to block any ρ subunit-containing GABA receptors) evoked larger, desensitizing currents that displayed far less attenuation; for example, at distal dendritic sites (c) the current attenuation was only 11 ± 5% (n = 7) (Fig. 2B). CACA-evoked currents also displayed differences in activation (quantified as time-to-peak values) than observed for GABA: for example, when agonists were applied directly to the cell soma (position ‘a’), the mean time-to-peak for CACA (2.84 ± 0.56 s, n = 5) was significantly slower than for GABA (0.45 ± 0.03 s, n = 7; P < 0.05).
Figure 2. Focal application of ionotropic GABA receptor agonists evoke membrane currents with distinct profiles.
A, agonists were focally applied to distinct sites, as approximately indicated by arrows on the left-hand schematic panel, namely directly onto the Purkinje cell (PC) soma (a), ∼30–60 μm away from the soma (b), and at the edge of the inner-third of the molecular layer (ML) (c); the granular layer (GL) is also shown for reference. Representative raw data traces for 500 μm CACA and 500 μm GABA are shown for corresponding positions (a–c); responses were normalized to peak agonist effect at the cell soma. CACA responses typically showed a sharp attenuation in amplitude with increasing distance from the soma, whilst GABA responses showed a far less pronounced attenuation. Note also that CACA-evoked currents had a slower onset and much less desensitization in comparison to those evoked by equimolar GABA. B, summary data for effects of 500 μm CACA (n = 5) and 500 μm GABA (n = 7) showing differences in degree of attenuation of current amplitude with increasing distance from the soma, *P < 0.05, unpaired t test assuming unequal variance.
Overall, the different response profiles between agonists were consistent with CACA activating a subpopulation of receptors, predominantly expressed at somatodendritic locations, whilst GABA acted non-selectively at receptor sites throughout the PC.
Effects of TPMPA on synaptic transmission
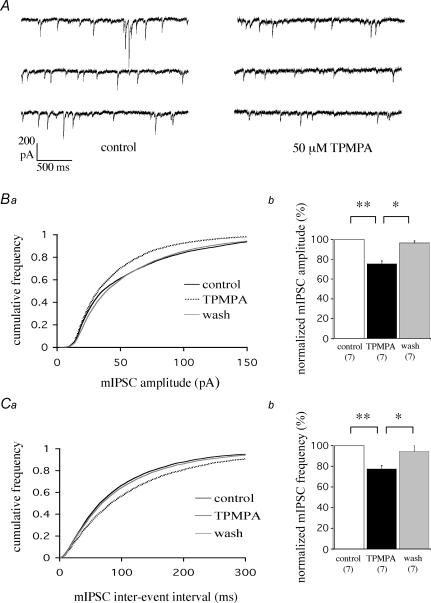
We next examined the contribution of TPMPA-sensitive receptors to inhibitory synaptic transmission at cerebellar IN–PC synapses. Bath application of 50 μm TPMPA caused a clear reduction in mean mIPSC amplitude from −58.5 ± 2.8 to −44.1 ± 2.7 pA (P < 0.001, n = 7), equivalent to a reduction of 24.6 ± 2.8% (Fig. 3A and Bb); this decrease was fully reversible (to −56.5 ± 3.1 pA, n = 7; P < 0.01). These data are reflected by a pooled cumulative frequency plot showing a reversible reduction in mean mIPSC amplitude induced by TPMPA (Fig. 3Ba; n = 7, each replicant P < 0.05, Kolmogorov-Smirnov (KS) test). Interestingly, 50 μm TPMPA also caused a decrease in mean mIPSC frequency, from 11.5 ± 1.6 to 9.0 ± 1.5 Hz (n = 7, P < 0.001), equivalent to a reduction of 22.6 ± 3.1% (Fig. 3A and Cb), with recovery to 10.7 ± 1.4 Hz (n = 7, P < 0.01). These data are reflected by a reversible increase in the mIPSC inter-event interval induced by TPMPA (Fig. 3Ca; n = 7, each replicant P < 0.05, KS test). Higher concentrations of TPMPA (100 μm) caused no further reduction in mean mIPSC amplitude (24.6 ± 1.7%) or frequency (15.2 ± 3.4%).
Figure 3. TPMPA reduces synaptic transmission in Purkinje cells.
A, example raw data traces showing the inhibition of mean mIPSC amplitude and frequency by TPMPA; VH = −70 mV. B, TPMPA-induced reduction in mIPSC amplitude as shown by a pooled cumulative frequency plot (n = 7) for mIPSC amplitude (a; each replicant P < 0.05, KS test), and summary of normalized data (b; *P < 0.01, **P < 0.001, unpaired t test assuming unequal variance). C, TPMPA-induced reduction in mIPSC frequency as shown by a pooled cumulative frequency plot (n = 7 cells) for mIPSC inter-event intervals (a; each replicant P < 0.05, KS test), and summary of normalized data (b; *P < 0.01; **P < 0.001, unpaired t test assuming unequal variance).
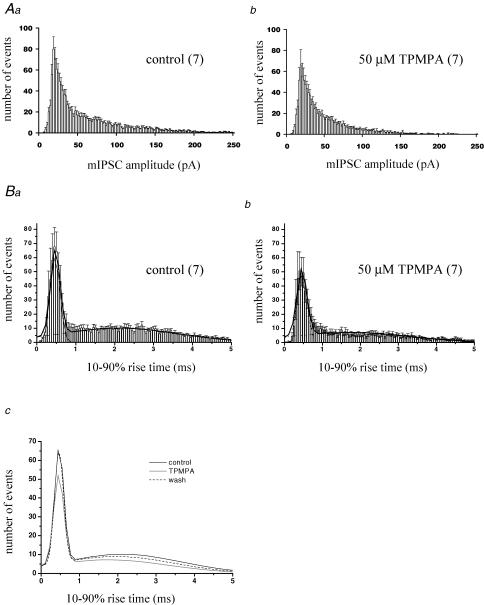
The reduction in mean mIPSC frequency by TPMPA may be due to smaller amplitude events falling below the detection threshold. However, examination of mIPSC amplitude distribution histograms (Fig. 4A) revealed that there was no clear leftward shift in the peak of the distribution (at 18 pA for both control (Fig. 4Aa) and TPMPA (Fig. 4Ab)). Interneuronal synapses which impinge on the PC soma display large amplitudes and fast rise times (see Llano et al. 2000) and are thought to arise from adjacent basket cell inputs. TPMPA caused a clear reduction in large amplitude events (for example, events > 150 pA were largely abolished in 50 μm TPMPA, Fig. 4Ab). These data suggest that postsynaptic TPMPA-sensitive receptors are present at somatodendritic IN–PC synapses, consistent with our results after exogenous CACA application (see Fig. 2A).
Figure 4. TPMPA has both pre- and postsynaptic actions.
A, mIPSC amplitude distribution histograms in control (a) and TPMPA-treated cells (b). Values are mean ± s.e.m. with number of replicants (n) in parentheses. Bin width = 2 pA. TPMPA caused a reduction in larger events, but no shift in the peak response. B, 10–90% rise time distribution histograms in control (a) and TPMPA-treated cells (b). Bin width = 0.05 ms. Data are best described by the sum of two Gaussian components fitted to grouped data, shown for clarity in c: sum of two Gaussians fitted to control, TPMPA and wash data. TPMPA reduced the number of events in both fast and slow rise-time populations.
In order to investigate the potential contribution from atypical GABA receptors expressed at presynaptic sites, we determined the effects of TPMPA on mIPSC 10–90% rise times. The distribution of rise times was best described by the sum of two Gaussian components, with a population of fast events (peak = 0.49 ± 0.03 ms, n = 7) accounting for 32 ± 4% of the total, and a population of slower events with rise times centred between 1 and 5 ms ( = slow events: peak = 2.46 ± 0.27 ms, n = 7) (Fig. 4Ba). Fast events are likely to originate from synapses on or near the PC soma, arising predominantly from inhibitory basket cell inputs; in contrast, slower events represent a more heterogeneous population, arising from proximal and more distal dendritic sites, with the slower rise time reflecting dendritic filtering of these events (King et al. 1993) and reduced fidelity of voltage clamp. Application of 50 μm TPMPA caused a reduction in the number of both fast and slow events (Fig. 4Bb and c) without any clear shift in either the fast (0.48 ± 0.04 ms, n = 7; P = 0.63) or slow (2.29 ± 0.33 ms, n = 7; P = 0.25) peak. This reduction in frequency was consistent with TPMPA acting on presynaptic receptors expressed throughout the PC dendritic arbor.
Overall, these data suggest that TPMPA reduced inhibitory synaptic transmission at IN–PC synapses by acting on a population of ionotropic GABA receptors present at both presynaptic and postsynaptic sites.
Effects of TPMPA on tonic GABA conductances
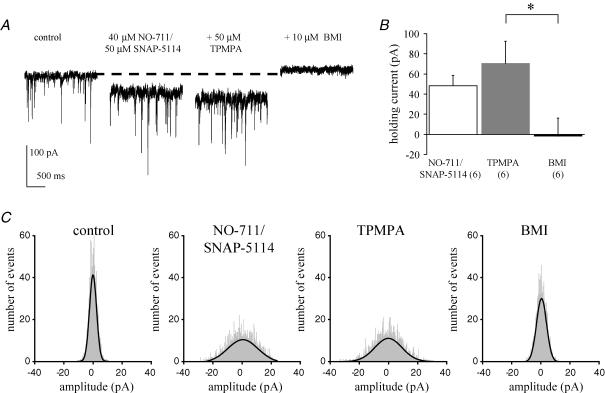
Homomeric GABAC receptors in recombinant expression systems are characterized by their lack of desensitization, and also by their high agonist affinity (reviewed by Zhang et al. 2001); as such, they represent ideal candidates to mediate tonic inhibitory currents (Semyanov et al. 2004; Kullmann et al. 2005). Tonic conductances are activated by increases in ambient GABA concentrations; in contrast, phasic currents are activated by vesicular transmitter release (see Farrant & Nusser, 2005). Therefore, we investigated the potential contribution of atypical ionotropic GABA receptors to inhibitory tonic currents. Under basal conditions, application of TPMPA or bicuculline had no clear effect on the holding current required to voltage clamp PCs (data not shown). A tonic conductance could be induced in PCs by the combined application of NO-711 (a selective GABA transporter subtype 1 (GAT-1) antagonist) and SNAP-5114 (a GAT-2/3/4 antagonist). Application of 40 μm NO-711 and 50 μm SNAP-5114 (in the continued presence of the GABAB antagonist CGP 55845) induced a significant increase in the PC holding current (+48 ± 10 pA, n = 6 cells) (Fig. 5A and B). Baseline root mean square (RMS) noise (4.4 ± 0.2 pA, n = 6) was also significantly increased by NO-711/SNAP-5114 application (7.9 ± 0.6 pA, n = 6; P < 0.001), as also illustrated in Fig. 5A. All-point histograms, constructed from 500 ms of event-free recordings, revealed a broader distribution of baseline noise in the presence of NO-711/SNAP-5114 than seen in control (Fig. 5C). When NO-711 or SNAP-5114 (both up to 100 μm) was applied individually they were without effect (data not shown). The increase in holding current and baseline noise are characteristic of a tonic conductance and these results suggest that, under conditions of high ambient GABA concentrations, different GAT protein isoforms synergistically modulate extrasynaptic levels of GABA at PC synapses. Application of 50 μm TPMPA had no clear effect on the amplitude of the NO-711/SNAP-5114-induced current (+71 ± 22 pA, n = 6, P = 0.17; Fig. 5A and B) or baseline RMS noise levels (9.2 ± 0.9 pA, n = 6), as reflected in all-point histograms (Fig. 5C). In contrast, subsequent application of 10 μm bicuculline methiodide (BMI) to the same cell reduced the NO-711/SNAP-5114-induced current (−2 ± 17 pA in comparison to control levels, n = 6, P < 0.01; Fig. 5A and B), reduced RMS noise (4.4 ± 0.5 pA, n = 6, P < 0.01; see also Fig. 5A and C), and also blocked all mIPSCs.
Figure 5. TPMPA has no effect on a NO-711/SNAP-5114-induced tonic GABA current in Purkinje cells.
A, example raw data trace of NO-711/SNAP-5114-induced increase in membrane holding current, lack of effect of TPMPA and subsequent block of NO-711/SNAP-5114-induced current by bicuculline methiodide (BMI). Note also the increase in baseline noise induced by NO-711/SNAP-5114 and subsequent reduction in noise by BMI (but not TPMPA); VH = −70 mV. B, summary data for effects on holding current. VH = −70 mV, *P < 0.01. C, representative all-point histograms of 500 ms event-free recording under different conditions. NO-711/SNAP-5114 caused a broadening in the distribution of baseline noise (shaded region) compared to control cells; whilst TPMPA was without effect, BMI narrowed the noise distribution. Mean values of single Gaussian fits to distributions from 6 cells were 4.2 ± 0.3 pA (control); 7.8 ± 0.6 pA (NO-711/SNAP-5114; P < 0.01 versus control); 8.7 ± 0.7 pA (TPMPA; P = 0.10 versus NO-711/SNAP-5114) and 4.5 ± 0.5 pA (BMI; P < 0.01 versus TPMPA).
Taken together, our data suggest that atypical GABA receptors do not significantly contribute to tonic GABA conductances in PCs. A further finding here is that the novel receptor identified in PCs can be distinguished from the tonic GABAA conductance on the basis of sensitivity to TPMPA (but not to bicuculline). Thus, whilst TPMPA caused a clear reduction in inhibitory synaptic transmission mediated by phasic GABA release (Figs 3 and 4), we saw no clear effect of TPMPA on the GAT antagonist-induced increase in holding current or membrane noise.
Immunohistochemical localization and co-immunoprecipitation of GABA ρ and α1 subunits
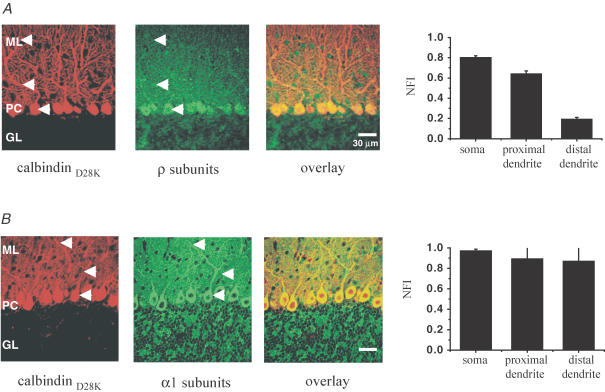
The electrophysiological actions of CACA and TPMPA may be consistent with the presence of ρ subunit-containing ionotropic GABA receptors in PCs; therefore, we investigated the expression of ρ subunits in mouse cerebellum. Double immunolabelling for ρ subunits and calbindin D28K, a specific marker for PCs, revealed an abundant expression of ρ subunits in the somatodendritic and proximal dendritic compartments of PCs and low-level expression in the distal dendrites (Fig. 6A). Moreover, punctate staining was observed throughout the molecular layer and pinceau regions of PCs, indicating the possible expression of ρ subunits on axon terminals of cerebellar basket and stellate cells. The ρ subunit distribution in PCs was consistent with the profile of CACA/TPMPA responses in electrophysiological experiments, supporting the hypothesis that ρ subunit-containing GABA receptors mediate the atypical pharmacological responses reported in PCs. We also compared ρ subunit immunoreactivity with that of the GABAA α1 subunit, a prominent subunit in PCs (Fritschy & Mohler, 1995; Wisden et al. 1996). As illustrated in Fig. 6B, in contrast to the more restricted distribution of ρ subunits, α1 subunits were expressed uniformly throughout the PC soma, proximal and distal dendrites. The α1 staining also correlated well with electrophysiological responses to GABA (Fig. 2), suggesting that α1-containing receptors mediate a major component of GABA action (Fritschy et al. 2006).
Figure 6. ρ subunit expression in mouse cerebellum, and comparison with α1 subunit expression.
Confocal images showing ρ(1&2) subunit (green; A) and α1 subunit (green) together with calbindin D28K (red) immunoreactivity (B) in the mouse cerebellar cortex (> 3 weeks). A, strong ρ(1&2) subunit immunostaining can be observed in the somatic and somatodendritic regions of Purkinje cells (PC) while moderate staining is observed in the molecular layer (ML). B, strong, uniform α1 subunit immunostaining can be observed in the somatic, proximal and distal dendritic regions of PCs and in the ML. The mean normalized fluorescence intensities (NFI) of ρ(1&2) and α1 subunit immunoreactivity in somatic, proximal dendritic and distal dendritic locations (see arrows) of cerebellar PCs are also shown (n = 4 separate slices each).
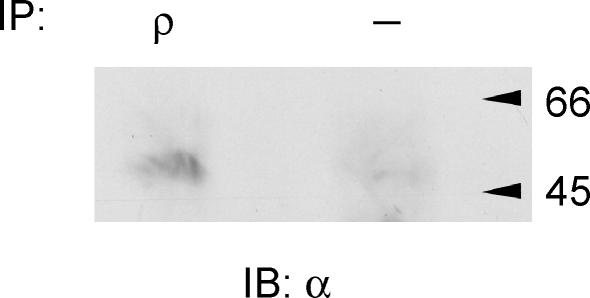
An attractive possibility, suggested by the mixed GABAA/GABAC pharmacological properties and the subunit distribution, is that the atypical receptors represent novel heteromers comprising conventional GABAA subunits together with ρ subunits. Therefore, we performed co-immunoprecipitation studies to investigate if ρ subunits could coassemble in the cerebellum. Protein complexes were precipitated from cerebellum lysates using ρ antibody and these complexes were found also to contain α1 subunits (Fig. 7); thus, it appears that ρ subunits are expressed in the same neuronal compartment as α1 subunits. Taken together our data suggest that ρ subunits may coassemble with α1 subunits to form functional GABA receptor heteromultimers which contribute to inhibitory synaptic transmission at IN–PC synapses in the cerebellum.
Figure 7. ρ subunits co-immunoprecipitate with α1 subunits in cerebellum lysates.
Protein complexes precipitated from cerebellum lysates using anti-ρ(1&2) and probed with the α1 antibody resulted in a band at the molecular weight appropriate to the α1 subunit. No bands were detected when the ρ(1&2) antibody was omitted. The data are representative of n = 3 separate experiments. IP, immunoprecipitation antibody; IB, immunoblot antibody.
Discussion
Identification of a novel ionotropic GABA receptor in Purkinje cells
The present study identifies an ionotropic GABA receptor subtype in mouse cerebellar PCs with a number of features that distinguish it from other receptors previously described in the cerebellum (Wisden et al. 1996). We show that the GABAC-preferring agonist CACA activates a current with sensitivity both to the selective GABAC antagonist TPMPA and the GABAA antagonist bicuculline. Focal application of CACA produced effects that differed from those seen with the natural agonist GABA; thus, CACA-evoked currents exhibited a far sharper attenuation with increasing distance from the PC soma, had a slower time-to-peak and displayed little or no desensitization. We also demonstrate that TPMPA-sensitive receptors mediate a component of GABAergic transmission at IN–PC synapses. TPMPA-induced reductions in mean mIPSC amplitude suggest a postsynaptic localization of receptors, consistent with responses to exogenous CACA. TPMPA also caused a reduction in frequency of events with both ‘fast’ and ‘slow’ rise times, consistent with these receptors also being expressed at presynaptic sites.
Ionotropic GABA receptors with a pharmacological profile similar to that reported here have also been described in other neurones in the CNS; thus, CACA-activated currents with sensitivity to both bicuculline and TPMPA have been reported in neurones from the hippocampus (Semyanov & Kullmann, 2002; Hartmann et al. 2004) and brainstem (Milligan et al. 2004). In this regard, could it be that CACA and TPMPA are less selective for GABAC over GABAA receptors than suggested in the literature? CACA has been reported to activate α6-containing GABA receptors (Wall, 2001); however, α6 receptor mRNA or protein is not expressed in PCs (Wisden et al. 1996). Moreover, currents mediated by α6 receptors are not blocked by TPMPA (Wall, 2001) and also have a somewhat low sensitivity to bicuculline (Thompson et al. 1996). In the present study, CACA-evoked currents had a greater sensitivity to TPMPA than GABA-evoked currents, similar to that observed in hippocampal neurones (Hartmann et al. 2004). In addition, the clear differences in response profile between CACA and the natural agonist GABA (see above) are consistent with CACA activating a subpopulation of receptors. We demonstrate that 50 μm TPMPA reduced synaptic transmission at IN–PC synapses, with no further effect at higher (100 μm) concentrations; these levels are well below the reported dissociation constant (∼320 μm) for TPMPA at GABAA receptors in Xenopus oocytes expressing cerebral cortex poly(A) RNA (Ragozzino et al. 1996). At 100 μm, TPMPA has no action on GABA-evoked currents in recombinant α1β2γ2 receptors (P. Thomas, UCL, personal communication) and is reported to be without effect on GABAA receptors in native neurones (Wall, 2001; Fischer et al. 2005).
In summary, the pharmacological responses to GABAC-preferring agents in cerebellar PCs are consistent with a role for a novel ionotropic GABA receptor subtype. Overall, these atypical receptors display some properties of GABAC receptors, but their sensitivity to bicuculline places them formally in the GABAA receptor family.
Potential contribution of ρ subunits to atypical GABA receptors in Purkinje cells
Having identified a novel ionotropic GABA receptor subtype in PCs with a mixed GABAC/GABAA pharmacology, we can speculate on its potential molecular composition. An attractive possibility is that responses to GABAC-preferring agents are mediated by ionotropic GABA receptors containing ρ subunits (see Zhang et al. 2001). There is extensive evidence for the presence of transcripts for ρ1 and ρ2 subunits within the mammalian cerebellum (Boue-Grabot et al. 1998; Rozzo et al. 2002; Lopez-Chavez et al. 2005). More specifically, ρ1 and ρ2 mRNAs were shown to be selectively expressed in rat PCs and basket-like interneurones (Rozzo et al. 2002). Although, there is not necessarily a linear relationship between mRNA levels and protein expression, Boue-Grabot et al. (1998) reported ρ1 immunoreactivity in the soma and dendrites of PCs. Consistent with the latter study, we demonstrate prominent ρ subunit staining in somatodendritic regions, but with reduced expression at more distal sites. Importantly, the ρ subunit distribution in PCs exhibited a clear correlation with the profile of CACA responses (Fig. 2) and TPMPA action on inhibitory synaptic transmission at IN–PC synapses (Figs 3 and 4), thus indicating that ρ subunit-containing receptors mediate the atypical pharmacological responses in PCs.
Recently, a ρ2 subunit has been cloned from bovine cerebellum and functionally expressed in Xenopus oocytes (Lopez-Chavez et al. 2005); homomeric ρ2 receptors supported bicuculline-insensitive, TPMPA-sensitive currents, as reported for other ρ2 subunits (Pan et al. 2005; Alakuijala et al. 2005). The present study suggests that similar homomeric ρ subunit receptors do not contribute to functional responses in cerebellar PCs. In particular, the sensitivity of CACA-evoked currents to bicuculline means that the GABA receptor subtype identified here cannot be ascribed exclusively to the ρ subunit-containing GABAC class of receptors (as discussed above). Overall, a consensus view of our data may be that atypical ionotropic GABA receptors represent novel heteromers comprising conventional GABAA subunits together with ρ subunits. In support of this hypothesis, we show that ρ subunits can coassemble with GABAA receptor α1 subunits in the mouse cerebellum, similar to findings in rat brainstem (Milligan et al. 2004). The demonstration that ρ and α1 subunits are present in the same neuronal compartment suggests that these subunits may coassemble in the cerebellum and that heteromer formation is likely to be obligatory for the functionality of ρ subunit-containing receptors in PCs. Moreover, the immunohistochemistry data argue that the presence of ρ subunits would restrict expression of such a complex principally to somatic and proximal dendritic regions of PCs.
Functional relevance of atypical GABA receptors in Purkinje cells
The present study characterizes a population of novel ionotropic GABA receptors which functionally mediate a component of inhibitory synaptic transmission at IN–PC synapses. Moreover, our findings are consistent with a contribution of ρ subunits to the molecular composition of such receptors. The actions of exogenous CACA, together with clear reduction in mIPSC amplitude by TPMPA, suggest a postsynaptic localization. These data are supported by ρ subunit immunoreactivity and identify a new substrate for GABAergic inhibition in the cerebellum.
The potential role of presynaptic ionotropic receptors in transmitter release has been highlighted (Engelman & MacDermott, 2004). As discussed above, the TPMPA-induced reduction in mIPSC frequency suggest that atypical receptors may also have a presynaptic localization; these data are supported by punctate ρ subunit staining consistent with expression on axon terminals of basket and stellate cells. However, the corresponding TPMPA-induced reduction in mIPSC amplitude makes it difficult to assess accurately the relative contribution of any pre- versus postsynaptic actions (see also Hartmann et al. 2004). The antagonist data suggest that receptors expressed at IN–PC synapses would normally act to increase transmitter release. Anionic presynaptic receptors have been reported to mediate depolarizing responses leading to increased transmitter release (Turecek & Trussell, 2001, 2002), and such actions are likely to be dependent on the reversal potential of permeant Cl− ions (see Engelman & MacDermott, 2004). Interestingly, although the intracellular Cl− concentrations in presynaptic terminals are unknown here, the effects of presynaptic GABAA receptor activation may be either facilitatory or inhibitory amongst individual cerebellar interneurone synapses (Chavas & Marty, 2003), making the overall impact on transmitter release difficult to assess. However, such effects are likely to impinge on presynaptic inhibition mediated by other classes of GABA receptors at these synapses (Harvey & Stephens, 2004).
Homomeric GABAC receptors have high agonist affinity and are associated with persistently open channels, suggesting a possible contribution of ρ subunit-containing receptors to tonic GABA conductances. We demonstrate that combined application of NO-711 (GAT-1 antagonist) and SNAP-5114 (GAT-2/3/4 antagonist) induced a tonic current in PCs. These data suggest for the first time that different GAT protein isoforms, in addition to GAT-1 (Chiu et al. 2005), are involved in modulating extrasynaptic levels of GABA at PC synapses. In this regard, we show that GAT-1 acts synergistically with GAT-2/3/4 isoforms to regulate GABA levels, as also reported for tonic conductances in neocortical neurones (Keros & Hablitz, 2005). The NO-711/SNAP-5114-induced tonic current in PCs was not affected by TPMPA, but was blocked by bicuculline. These data suggest that atypical GABA receptors do not contribute to tonic GABA conductances in PCs, and that distinct GABA receptor subtypes, composed of different combinations of subunits, may underlie tonic and phasic signalling (Farrant & Nusser, 2005). This study also provides further evidence that TPMPA is not acting as a non-selective GABA antagonist. Taken together, our data suggest that atypical ionotropic GABA receptors act selectively to mediate a component of phasic inhibitory synaptic transmission at cerebellar IN–PC synapses.
In summary, we describe a novel population of GABA receptors with a mixed GABAA/GABAC pharmacology that contributes to functional responses in PCs. We present evidence that GABA ρ subunits contribute to these atypical receptors and may form heteromeric complexes with GABAA α1 subunits. This study increases our understanding of the diversity of ionotropic GABA receptors within the cerebellum and suggests distinct substrates for inhibition in the CNS.
Acknowledgments
We wish to thank Dr Ralf Enz, Friedrich Alexander University of Erlangen-Nurnberg for the kind gift of rabbit anti-ρ(1&2) antibody. We would also like to thank Professor Trevor Smart (University College London (UCL)) for critical comment on the manuscript and Dr Philip Thomas (UCL) for useful input. This work was supported by The Wellcome Trust.
Supplementary material
The online version of this paper can be accessed at:
DOI:10.1113/jphysiol.2006.112482
http://jp.physoc.org/cgi/content/full/jphysiol.2006.112482/DC1 and contains supplemental material consisting of a figure and legend entitled:
Ionotropic GABA receptor agonists evoke membrane currents in Purkinje cells
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Alakuijala A, Talvioja K, Pasternack A, Pasternack M. Functional characterization of rat ρ2 subunits expressed in HEK 293 cells. Eur J Neurosci. 2005;21:692–700. doi: 10.1111/j.1460-9568.2005.03880.x. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Bormann J. The ‘ABC’ of GABA receptors. Trends Pharmacol Sci. 2000;21:16–19. doi: 10.1016/s0165-6147(99)01413-3. [DOI] [PubMed] [Google Scholar]
- Boue-Grabot E, Roudbaraki M, Bascles L, Tramu G, Bloch B, Garret M. Expression of GABA receptor ρ subunits in rat brain. J Neurochem. 1998;70:899–907. doi: 10.1046/j.1471-4159.1998.70030899.x. [DOI] [PubMed] [Google Scholar]
- Chavas J, Marty A. Coexistence of excitatory and inhibitory GABA synapses in the cerebellar interneuron network. J Neurosci. 2003;23:2019–2031. doi: 10.1523/JNEUROSCI.23-06-02019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CS, Brickley S, Jensen K, Southwell A, Mckinney S, Cull-Candy S, Mody I, Lester HA. GABA transporter deficiency causes tremor, ataxia, nervousness, and increased GABA-induced tonic conductance in cerebellum. J Neurosci. 2005;25:3234–3245. doi: 10.1523/JNEUROSCI.3364-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didelon F, Sciancalepore M, Savic N, Mladinic M, Bradbury A, Cherubini E. γ-Aminobutyric acidAρ receptor subunits in the developing rat hippocampus. J Neurosci Res. 2002;67:739–744. doi: 10.1002/jnr.10178. [DOI] [PubMed] [Google Scholar]
- Drew CA, Johnston GA, Weatherby RP. Bicuculline-insensitive GABA receptors: studies on the binding of (–)-baclofen to rat cerebellar membranes. Neurosci Lett. 1984;52:317–321. doi: 10.1016/0304-3940(84)90181-2. [DOI] [PubMed] [Google Scholar]
- Ekema GM, Zheng W, Lu L. Interaction of GABA receptor/channel ρ1 and γ2 subunit. Invest Ophthalmol Vis Sci. 2002;43:2326–2333. [PubMed] [Google Scholar]
- Engelman HS, MacDermott AB. Presynaptic ionotropic receptors and control of transmitter release. Nat Rev Neurosci. 2004;5:135–145. doi: 10.1038/nrn1297. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fischer H, Harper AA, Anderson CR, Adams DJ. Developmental changes in expression of GABAA receptor-channels in rat intrinsic cardiac ganglion neurones. J Physiol. 2005;564:465–474. doi: 10.1113/jphysiol.2005.084012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Panzanelli P, Kralic JE, Vogt KE, Sassoe-Pognetto M. Differential dependence of axo-dendritic and axo-somatic GABAergic synapses on GABAA receptors containing the α1 subunit in Purkinje cells. J Neurosci. 2006;26:3245–3255. doi: 10.1523/JNEUROSCI.5118-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann K, Stief F, Draguhn A, Frahm C. Ionotropic GABA receptors with mixed pharmacological properties of GABAA and GABAC receptors. Eur J Pharmacol. 2004;497:139–146. doi: 10.1016/j.ejphar.2004.06.044. [DOI] [PubMed] [Google Scholar]
- Harvey VL, Stephens GJ. Mechanism of GABA receptor-mediated inhibition of spontaneous GABA release onto cerebellar Purkinje cells. Eur J Neurosci. 2004;20:684–700. doi: 10.1111/j.1460-9568.2004.03505.x. [DOI] [PubMed] [Google Scholar]
- Johnston GA. GABAC receptors: relatively simple transmitter-gated ion channels? Trends Pharmacol Sci. 1996;17:319–323. [PubMed] [Google Scholar]
- Johnston GA, Curtis DR, Beart PM, Game CJ, McCulloch RM, Twitchin B. Cis- and trans-4-aminocrotonic acid as GABA analogues of restricted conformation. J Neurochem. 1975;24:157–160. doi: 10.1111/j.1471-4159.1975.tb07642.x. [DOI] [PubMed] [Google Scholar]
- Keros S, Hablitz JJ. Subtype-specific GABA transporter antagonists synergistically modulate phasic and tonic GABAA conductances in rat neocortex. J Neurophysiol. 2005;94:2073–2085. doi: 10.1152/jn.00520.2005. [DOI] [PubMed] [Google Scholar]
- King JS, Chen YF, Bishop GA. An analysis of HRP-filled basket cell axons in the cat's cerebellum. II. Axonal distribution. Anat Embryol (Berl) 1993;188:299–305. doi: 10.1007/BF00188220. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Ruiz A, Rusakov DM, Scott R, Semyanov A, Walker MC. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusama T, Spivak CE, Whiting P, Dawson VL, Schaeffer JC, Uhl G. Pharmacology of GABA ρ1 and GABA α/β receptors expressed in Xenopus oocytes and COS cells. Br J Pharmacol. 1993;109:200–206. doi: 10.1111/j.1476-5381.1993.tb13554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I, Gonzalez J, Caputo C, Lai FA, Blayney LM, Tan YP, Marty A. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- Lopez-Chavez A, Miledi R, Martinez-Torres A. Cloning and functional expression of the bovine GABACρ2 subunit. Molecular evidence of a widespread distribution in the CNS. Neurosci Res. 2005;53:421–427. doi: 10.1016/j.neures.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Milligan CJ, Buckley NJ, Garret M, Deuchars J, Deuchars SA. Evidence for inhibition mediated by coassembly of GABAA and GABAC receptor subunits in native central neurons. J Neurosci. 2004;24:7241–7250. doi: 10.1523/JNEUROSCI.1979-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Khalili P, Ripps H, Qian H. Pharmacology of GABAC receptors: responses to agonists and antagonists distinguish A- and B-subtypes of homomeric ρ receptors expressed in Xenopus oocytes. Neurosci Lett. 2005;376:60–65. doi: 10.1016/j.neulet.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Pan Y, Qian H. Interactions between ρ and γ2 subunits of the GABA receptor. J Neurochem. 2005;94:482–490. doi: 10.1111/j.1471-4159.2005.03225.x. [DOI] [PubMed] [Google Scholar]
- Qian H, Ripps H. Response kinetics and pharmacological properties of heteromeric receptors formed by coassembly of GABA ρ and γ2-subunits. Proc R Soc Lond B Biol Sci. 1999;266:2419–2425. doi: 10.1098/rspb.1999.0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino D, Woodward RM, Murata Y, Eusebi F, Overman LE, Miledi R. Design and in vitro pharmacology of a selective γ-aminobutyric acidC receptor antagonist. Mol Pharmacol. 1996;50:1024–1030. [PubMed] [Google Scholar]
- Rozzo A, Armellin M, Franzot J, Chiaruttini C, Nistri A, Tongiorgi E. Expression and dendritic RNA localization of GABAC receptor ρ1 and ρ2 subunits in developing rat brain and spinal cord. Eur J Neurosci. 2002;15:1747–1758. doi: 10.1046/j.1460-9568.2002.02013.x. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Kullmann DM. Relative picrotoxin insensitivity distinguishes ionotropic GABA receptor-mediated IPSCs in hippocampal interneurons. Neuropharmacol. 2002;43:726–736. doi: 10.1016/s0028-3908(02)00123-5. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Southan AP, Robertson B. Patch-clamp recordings from cerebellar basket cell bodies and their presynaptic terminals reveal an asymmetric distribution of voltage-gated potassium channels. J Neurosci. 1998;18:948–955. doi: 10.1523/JNEUROSCI.18-03-00948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SA, Whiting PJ, Wafford KA. Barbiturate interactions at the human GABAA receptor: dependence on receptor subunit combination. Br J Pharmacol. 1996;117:521–527. doi: 10.1111/j.1476-5381.1996.tb15221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turecek R, Trussell LO. Presynaptic glycine receptors enhance transmitter release at a mammalian central synapse. Nature. 2001;411:587–590. doi: 10.1038/35079084. [DOI] [PubMed] [Google Scholar]
- Turecek R, Trussell LO. Reciprocal developmental regulation of presynaptic ionotropic receptors. Proc Natl Acad Sci U S A. 2002;99:13884–13889. doi: 10.1073/pnas.212419699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MJ. Cis-4-amino-crotonic acid activates α6 subunit-containing GABAA but not GABAC receptors in granule cells of adult rat cerebellar slices. Neurosci Lett. 2001;316:37–40. doi: 10.1016/s0304-3940(01)02363-1. [DOI] [PubMed] [Google Scholar]
- Whiting PJ, Bonnert TP, McKernan RM, Farrar S, le Bourdelles B, Heavens RP, et al. Molecular and functional diversity of the expanding GABAA receptor gene family. Ann N Y Acad Sci. 1999;868:645–653. doi: 10.1111/j.1749-6632.1999.tb11341.x. [DOI] [PubMed] [Google Scholar]
- Wisden W, Korpi ER, Bahn S. The cerebellum: a model system for studying GABAA receptor diversity. Neuropharmacol. 1996;35:1139–1160. doi: 10.1016/s0028-3908(96)00076-7. [DOI] [PubMed] [Google Scholar]
- Zhang D, Pan ZH, Awobuluyi M, Lipton SA. Structure and function of GABAC receptors: a comparison of native versus recombinant receptors. Trends Pharmacol Sci. 2001;22:121–132. doi: 10.1016/s0165-6147(00)01625-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ionotropic GABA receptor agonists evoke membrane currents in Purkinje cells