Abstract
The medullary raphe (MR) and the retrotrapezoid nucleus (RTN) in the ventral medulla are two of many central chemoreceptor sites. We examine their combined function in conscious rats by focal inhibition using microdialysis. Inhibition of RTN neurons with the GABAA receptor agonist muscimol, with simultaneous dialysis of artificial cerebrospinal fluid (ACSF) in or near to the caudal MR, causes hypoventilation (decrease in the ratio of minute ventilation to oxygen consumption,  ) and reduces the ventilatory response to 7% CO2 by 24%. Inhibition of caudal MR serotonergic neurons with the 5-HT1A receptor agonist (R)-(+)-8-hydroxy-2(di-n-propylamino)tetralin (8-OH-DPAT), with simultaneous dialysis of ACSF in or near to the RTN, causes hypoventilation but has no significant effect on the CO2 response. Inhibition of both the RTN and the caudal MR simultaneously produces enhanced hypoventilation and a 51% decrease in the CO2 response. The effects of treatment on the CO2 response are similar in wakefulness and in non-rapid eye movement sleep. Comparison of the effect of 8-OH-DPAT microdialysed into a more rostral portion of the MR, where the CO2 response is reduced by 22%, demonstrates heterogeneity within the MR of the function of serotonergic neurons in breathing. We conclude that serotonergic neurons within the caudal MR provide a non-CO2-dependent tonic drive to breathe and potentiate the effects of RTN neurons that contribute to a resting chemical ‘drive to breathe’ as well as the response to added CO2. These effects of caudal MR serotonergic neurons could be at a chemoreceptor site, e.g. the RTN, or at ‘downstream’ sites involved in rhythm and pattern generation.
) and reduces the ventilatory response to 7% CO2 by 24%. Inhibition of caudal MR serotonergic neurons with the 5-HT1A receptor agonist (R)-(+)-8-hydroxy-2(di-n-propylamino)tetralin (8-OH-DPAT), with simultaneous dialysis of ACSF in or near to the RTN, causes hypoventilation but has no significant effect on the CO2 response. Inhibition of both the RTN and the caudal MR simultaneously produces enhanced hypoventilation and a 51% decrease in the CO2 response. The effects of treatment on the CO2 response are similar in wakefulness and in non-rapid eye movement sleep. Comparison of the effect of 8-OH-DPAT microdialysed into a more rostral portion of the MR, where the CO2 response is reduced by 22%, demonstrates heterogeneity within the MR of the function of serotonergic neurons in breathing. We conclude that serotonergic neurons within the caudal MR provide a non-CO2-dependent tonic drive to breathe and potentiate the effects of RTN neurons that contribute to a resting chemical ‘drive to breathe’ as well as the response to added CO2. These effects of caudal MR serotonergic neurons could be at a chemoreceptor site, e.g. the RTN, or at ‘downstream’ sites involved in rhythm and pattern generation.
There is a ‘drive to breathe’ that in part arises from central chemoreceptors and from other non-CO2-dependent sources (Nattie, 2006). Central chemoreceptors that detect increases in CO2 or H+ and stimulate breathing have been located at or near to the ventral surface of the medulla (Mitchell et al. 1963: Loeschcke, 1982) as well as in a more widely distributed set of locations in the brainstem (Coates et al. 1993; Nattie & Li, 1994, 1996, 2000, 2001, 2002a,b; Li et al. 1999; Nattie, 1999, 2001; Feldman et al. 2003; Hodges et al. 2004; Nattie et al. 2004; Li & Nattie, 2006) and cerebellum (Xu & Frazier, 2000; Xu et al. 2001; Martino et al. 2006). Widespread chemoreceptor sites have been identified in conscious animals by the presence of increased breathing in response to focal acidification as well as by reduced CO2 sensitivity after focal inhibition and specific lesions. These include the locus ceruleus (A6; Coates et al. 1993; Pineda & Aghajanian, 1997) and other catecholaminergic sites (Li & Nattie, 2006), the caudal nucleus tractus solitarius (NTS; Nattie & Li, 2002a), the rostral aspect of the ventral respiratory group (VRG; Nattie & Li, 1996), perhaps including the pre-Bötzinger complex (PBC; Feldman et al. 2003), the fastigial nucleus of the cerebellum (Xu et al. 2001; Martino et al. 2006), the retrotrapezoid nucleus (RTN; Li et al. 1999) and the medullary raphe (MR; Nattie & Li, 2001; Hodges et al. 2004). Recent work has identified serotonergic neurons in the MR (Richerson, 2004; Richerson et al. 2005) and glutamatergic neurons in the RTN (Mulkey et al. 2004; Guyenet et al. 2005) as putative chemoreceptor neurons and ATP as a putative neuromodulator in chemoreception (Gourine, 2005).
This recent work has led to discussion as to whether the serotonergic neurons of the MR or the glutamatergic neurons of the RTN are the primary central chemoreceptor (Richerson et al. 2005; Guyenet et al. 2005). Prior work in conscious rats has shown that dialysis of muscimol (1 mm) into the RTN reduces the ventilatory response to systemic inhalation of 7% CO2 (Nattie & Li, 2000) and that dialysis of the 5-HT1A receptor agonist (R)-(+)-8-hydroxy-2(di-n-propylamino)tetralin (8-OH-DPAT; 1 mm) into the MR at the level of the facial nucleus also inhibits the CO2 response (Taylor et al. 2005a). In this paper, we examine the relative importance of the RTN and the MR in central chemoreception by studying these two sites simultaneously in conscious rats during non-rapid eye movement (NREM) sleep and wakefulness. We do so by focal simultaneous inhibition using microdialysis of muscimol in the RTN and 8-OH-DPAT in the MR. Each rat has two guide tubes inserted, one in the RTN and the other in the caudal aspect of the MR (see Fig. 1A). Owing to size and anatomical constraints, we were forced to place our MR guide tube more caudally than in our previous studies (Taylor et al. 2005a,b), about 1.5 mm caudal to the facial nucleus. One group of rats was exposed on separate days to muscimol in the RTN and artificial cerebrospinal fluid (ACSF) in the MR or to ACSF in the RTN and 8-OH-DPAT in the MR. A second group of rats was exposed simultaneously to muscimol in the RTN and 8-OH-DPAT in the caudal MR. We hypothesize that inhibition of the RTN or the MR independently would have a similar effect on the ventilatory response to systemic inhalation of 7% CO2 and that when both sites were inhibited simultaneously there would be an additive inhibition. Neither site would be ‘more important’.
Figure 1. Schematic diagrams showing the experimental design (A) and the histologically determined locations of the dialysis probe tips (B–E).

These sections are drawn from the atlas of Paxinos & Watson (1998). A is a drawing of a parasaggittal view of the rat brainstem. The left-hand arrow marks the approximate location of the dialysis probes placed into the region of the retrotrapezoid nucleus (RTN) shown in cross-sections B and D, which are at −11.6, −10.6 and −13.33 mm relative to bregma. The right-hand arrow marks the approximate location of the dialysis probes placed into the region of the caudal medullary raphe (MR) shown in cross-sections C and E, which are at −13.3 mm relative to bregma. B and C show the 10 probe tip locations (•) for the 5 rats in the simultaneous treatment experiment in which each of the 5 rats was dialysed at both the RTN and the caudal MR with ACSF and then with muscimol in the RTN and 8-OH-DPAT in the caudal MR. The left-hand side of B shows 4 probe tip locations in the RTN at or near to the level −11.6 mm from bregma; the right-hand side of B shows 1 probe tip location in the RTN at a level −10.6 mm from bregma. C shows the 5 probe tip locations in the caudal MR, −13.3 mm caudal to bregma. D and E show the 10 correctly placed probe tip locations for 8 rats that were treated either with muscimol in the RTN or with 8-OH-DPAT in the caudal MR. Two rats have probe tips in both D and E (triangles). Three rats have probe tips only in D or in E (filled circles). The other probe tips for these 6 rats were not in the area of interest but were nearby; they are not shown in the figure. The scale bar represents 1 mm. Abbreviations: 7N, facial nerve; NTS, nucleus tractus solitarius; Amb, nucleus ambiguous; VII, facial nucleus; SO, superior olive; Pn, pons; RoB.
We report that inhibition of serotonergic neurons by 8-OH-DPAT dialysis in the caudal aspect of the MR: (1) causes hypoventilation with little effect on the CO2 response, an effect dramatically different from the 18% inhibition of that response with 8-OH-DPAT dialysis more rostrally (Taylor et al. 2005a); and (2) markedly potentiates the effects produced by muscimol dialysis in the RTN, causing further hypoventilation during air breathing and a substantial reduction in the CO2 response.
Methods
General methods
The Institutional Animal Care and Use Committee at the Dartmouth College Animal Resource Center approved all animal experimentation and surgical protocols. A total of 34 adult male Sprague–Dawley rats weighing 250–350 g were used for the experiments and they were individually housed in a light- and temperature-controlled environment with 12 h of light beginning at midnight and 12 h of darkness beginning at noon. Our normal study period (9.00–15.00 h) includes the end of the rat's sleep period and the beginning of their active period. Food and water were provided ad libitum.
Rats were surgically instrumented with a telemetric temperature probe, one microdialysis probe cannula into the caudal MR and another into the RTN, and electroencephalogram (EEG) and electromyogram (EMG) electodes. Animals were anaesthetized with injections of 100 mg kg−1 ketamine i.m. and 15 mg kg−1 xylazine i.p. The depth of anaesthesia was monitored throughout surgery by firm pinching of the hindpaw, and if necessary, additional quarter doses of ketamine and xylazine were administered. The hair on the back of the neck, the head and abdomen was thoroughly shaved, and the skin was sterilized with betadine solution and 70% ethanol. After placement of a sterilized telemetric temperature probe capsule (TA-F20, Data Sciences International, St Paul, MN, USA) within the intraperitoneal space via a mid-line abdominal incision, the animal's head was centred on a Kopf stereotaxic frame and a mid-line incision was made from the frontal bone to the base of the skull. Three EEG electrode screws were secured into the skull surface: one 2 mm rostral to bregma and 2 mm lateral to the mid-line, one 3 mm rostral to lambda and 2 mm lateral to the mid-line, and an earth electrode was placed slightly lateral between the two. Two EMG electrodes were sutured within the skeletal muscle of the neck, and electrode wires were put into a six-prong plastic pedestal. Two microdialysis probe cannulae with a prefixed distance between them were stereotaxically inserted into the brainstem, one for the caudal MR at ∼13.0–13.4 mm caudal to bregma and 0 mm from the mid-line, and 10.2–10.6 mm deep into the dorsal surface of the skull, another for the RTN at ∼10.6–11.6 mm caudal to bregma and 1.5–1.8 mm from mid-line, and 10.6–10.8 mm deep into the dorsal surface of the skull. These co-ordinates assured placement into the RTN and into the caudal MR according to the rat brain atlas of Paxinos & Watson (1998) and our own experience (Nattie & Li, 2000; Taylor et al. 2005a,b). The microdialysis cannulae and six-prong pedestal were secured to the skull with cranioplastic cement and the incision was sutured. All rats were allowed at least 7 days to recover, and any rats showing signs of illness or infection were immediately killed with an overdose of sodium pentobarbitone (intraperitoneal injection, > 75 mg kg−1). Such signs included weight loss, decreased food intake, fever, lethargy and lack of healing at the site of incision.
Microdialysis
The microdialysis probes used in this study had an 11 mm stainless-steel shaft, with a 1 mm long tip with a cuprophane membrane (0.38 mm o.d.), allowing diffusion of molecules under 6000 Da (CMA 11, CMA microdialysis, Solna, Sweden) with no injection. Solutions were dialysed through the probe at a constant rate of 4 μl min−1 maintained by a syringe pump (KD 200, KD Scientific, Holliston, MA, USA). Artificial cerebrospinal fluid (ACSF) served as the drug vehicle and for the control solution, and was comprised of (mm): 152.0 sodium, 3.0 potassium, 2.1 magnesium, 2.2 calcium, 131.0 chloride and 26.0 bicarbonate in sterile deionized water. Directly before the ventilation experiments and prior to adding the calcium, ACSF was equilibrated with 5% CO2. One millimolar muscimol hydrobromide, and 1 mm (R)-(+)-8-OH-DPAT hydrobromide (Sigma-Aldrich, St Louis, MO, USA) were prepared in ACSF. The concentration of the drugs was chosen based upon previous microdialysis studies in conscious rats (Nattie & Li, 2000; Taylor et al. 2005a,b).
Measurement of ventilation, oxygen consumption, temperature, EEG and EMG
To measure ventilation in conscious rats, we used the whole-body plethysmograph technique as previously described (Bartlett & Tenney, 1970; Nattie & Li, 2001, 2002a,b; Nattie et al. 2004). The volume of the plethysmograph was 7.6 l, with a 3.5 l top to protect the head pedestal. Analog output from the pressure transducer was sampled at 150 Hz and was converted into a digital signal by a computer using the DATAPAC 2k2 system (RUN Technologies, Laguna Hills, CA, USA). The rates of inflow and outflow to the plethysmograph were balanced to one another with a flowmeter such that flow was maintained at ≥ 1.4 l min−1 (flowmeter model 601E, Matheson Tri-gas, Montgomeryville, PA, USA), and the plethysmograph remained at atmospheric pressure. Carbon dioxide and O2 fractions were sampled from the outflow line at ∼100 ml min−1 by a combination CO2 and O2 gas analyser (Gemini Respiratory Gas Analyser, CWE Inc., Ardmore, PA, USA). To calibrate the plethysmograph before each experiment, we obtained pressure data from four 0.3 ml air injections made with a 1 ml syringe. Raw EEG and EMG outputs from the skull and skeletal muscle electrodes were sampled at 150 Hz, filtered at 0.3–50 and 0.1–100 Hz, respectively, and were recorded continuously using the DATAPAC 2k2 system.
Oxygen consumption ( ) was calculated by application of the Fick principle, using the difference in O2 content between inflow air and outflow air:
) was calculated by application of the Fick principle, using the difference in O2 content between inflow air and outflow air:
where  and
and  are inflow and outflow rates, and FIO2 and FOO2 are the fraction of inflow and outflow O2. In the plethysmograph chamber used in these experiments, any gas mixture (i.e. 7% CO2), when added to the inflow (at a flow rate of 1.4 l min−1), took approximately 15 min to reach appropriate concentrations in the outflow. Oxygen consumption was thereby calculated from outflow oxygen content data after 10 min intervals during the ventilation experiments and analysed as an average value during awake and NREM sleep conditions in room air. Core body temperature was measured every 10 min using the signal from the telemetric temperature probe within the abdomen, and animal chamber temperature was measured using a thermometer within the plethysmograph.
are inflow and outflow rates, and FIO2 and FOO2 are the fraction of inflow and outflow O2. In the plethysmograph chamber used in these experiments, any gas mixture (i.e. 7% CO2), when added to the inflow (at a flow rate of 1.4 l min−1), took approximately 15 min to reach appropriate concentrations in the outflow. Oxygen consumption was thereby calculated from outflow oxygen content data after 10 min intervals during the ventilation experiments and analysed as an average value during awake and NREM sleep conditions in room air. Core body temperature was measured every 10 min using the signal from the telemetric temperature probe within the abdomen, and animal chamber temperature was measured using a thermometer within the plethysmograph.
Experimental protocol and design
All experiments were performed at room temperature between 24.5 and 26.5°C. Ventilation experiments began following recovery from surgery. After the EEG–EMG pedestal was connected and two microdialysis probes inserted, animals were placed into the plethysmograph at ∼09.00 h and allowed to acclimate under room air breathing conditions while ACSF was perfused through both dialysis probes at 4 μl min−1. Once the animals had acclimated for at least 30 min, the experimental recording began. The ventilation was recorded under room air conditions for 30–40 min; then the inspired air was switched to 7% CO2 balanced with air. Once the gas analyser connected to the plethysmograph outflow reached 7% CO2, a further 30–40 min of ventilation data were recorded. Microdialysis of ACSF was continued throughout the entire experiment. Then the same rats were allowed to rest in the chamber for at least 1 h under room air conditions with the plethysmograph top open before drug treatment dialysis started. The identical ventilatory recordings under both room air and 7% CO2 conditions were repeated under the all three drug treatment dialysis regimes. The three drug treatment protocols are: (1) the RTN was dialysed with 1 mm muscimol while the caudal MR was dialysed with ACSF; (2) the caudal MR was dialysed with 8-OH-DPAT while the RTN was dialysed with ACSF; and (3) the RTN was dialysed with 1 mm muscimol and, simultaneously, the caudal MR was dialysed with 1 mm 8-OH-DPAT. Each brainstem site in each rat received only one drug treatment. Of a total of 34 rats that were used in the study, only 13 of them were successful in all aspects. Twelve of 34 rats were excluded from the study owing to complications of the surgery, and nine were excluded owing to inappropriate probe locations. There are total of five rats with both probes in the correct location that received simultaneous treatment at both sites. There were eight rats that received treatment at only one site. Two of these eight rats had both dialysis probes in the correct locations. These rats were treated at each site but on separate days. The other six rats had one probe in the right location either in the RTN or caudal MR with the other probe just outside but nearby the alternative region of interest. We included these rats in the study because the probes located nearby the specific regions of interest were dialysed with ACSF.
Data analysis
For sleep–wake state analysis, a fast Fourier transform was performed on the EEG–EMG signal at 3.6 s-long pochs, in addition to studying the raw EEG–EMG signals. We designated frequency bands delta, theta, and sigma as 0.3–5, 6–9, and 10–17 Hz, respectively, based upon algorithms for rat sleep scoring (Bennington et al. 1994). Experiments were performed between 09.00 and 15.00 h, and both wakefulness and NREM sleep states were observed consistently through this period. Sleep states were categorized in the following way as previously described (Li et al. 1999; Nattie & Li, 2001, 2002a,b; Nattie et al. 2004): (1) wakefulness, raw EEG low, EMG present, delta power low; (2) non-rapid eye movement sleep, EMG low, delta power high, the product of sigma and theta power high; (3) REM sleep, raw EEG low, EMG absent, delta power low, theta to delta power ratio high. Periods of REM sleep were short and were not present in every experiment. As a result of this, ventilation events that occurred during REM or when sleep state was indeterminate were excluded from our analysis.
Using the DATAPAC 2k2 software, breath events were individually selected. Breaths occurring during rat movement (sniffing, sighs, grooming etc.) were excluded. Breath events were grouped into bins of 500–2000 events, depending upon arousal state. The minute ventilation ( ) and tidal volume (VT) of each breath were calculated using rat body temperature, plethysmograph chamber temperature, and the barometric pressure of the day on which the experiment was performed, and normalized against rat body weight (Bartlett & Tenney, 1970). Ventilatory values are expressed as means for quiet wakefulness and NREM sleep during room air or 7% CO2 conditions. For statistical analysis, we used pre- and post-treatment
) and tidal volume (VT) of each breath were calculated using rat body temperature, plethysmograph chamber temperature, and the barometric pressure of the day on which the experiment was performed, and normalized against rat body weight (Bartlett & Tenney, 1970). Ventilatory values are expressed as means for quiet wakefulness and NREM sleep during room air or 7% CO2 conditions. For statistical analysis, we used pre- and post-treatment  , VT, and frequency (f) data as repeated measures with state (awake versus NREM sleep) and gas (room air versus hypercapnia) as categorical variables. Post hoc evaluation was via modified (Bonferroni) t test (SYSTAT Software Inc., Point Richmond, CA, USA). To determine whether treatments affected the level of ventilation relative to metabolism, we calculated the ratio of
, VT, and frequency (f) data as repeated measures with state (awake versus NREM sleep) and gas (room air versus hypercapnia) as categorical variables. Post hoc evaluation was via modified (Bonferroni) t test (SYSTAT Software Inc., Point Richmond, CA, USA). To determine whether treatments affected the level of ventilation relative to metabolism, we calculated the ratio of  to
to  during air breathing. Since the plethysmograph wash-out time was slow relative to the awake to NREM sleep cycle time, we averaged these
during air breathing. Since the plethysmograph wash-out time was slow relative to the awake to NREM sleep cycle time, we averaged these  data over both awake and NREM sleep period. We compared the
data over both awake and NREM sleep period. We compared the  values using a one-way repeated measures ANOVA with
values using a one-way repeated measures ANOVA with  pre- and post-treatment as the repeated measure and treatment group as a categorical variable.
pre- and post-treatment as the repeated measure and treatment group as a categorical variable.
Anatomy
At the conclusion of the experimental protocol, rats were killed with an overdose of sodium pentobarbitone injected intraperitoneally (> 75 mg kg−1). The brainstem of the animals was quickly dissected and frozen rapidly on dry ice, and stored at −15°C until 50 μm thick coronal sections were made using a leica cryostat. After being mounted upon gelatin-coated slides, sections were fixed in 4% paraformaldehyde and stained with Cresyl Violet. The distance between bregma and the probe tip (rostral–caudal level) was calculated by measuring the distance between the centre of the probe and the most caudal section showing the entire facial nerve (−10.04 mm relative to bregma in the rat brain atlas of Paxinos & Watson, 1998). Animals that had probes falling well outside of the MRR or RTN were not included in the data analysis.
Results
The guide tubes were surgically implanted in the locations outlined schematically in Fig. 1A, with the actual locations for rats reported in this paper shown in Fig. 1B–E. In the five rats used for the simultaneous dialysis of muscimol into the RTN and 8-OH-DPAT into the caudal MR, the probe tips were perfectly placed, as shown in Fig. 1B and C. Each of these rats was dialysed with ACSF at both sites and then with muscimol and 8-OH-DPAT at both sites. They received no further treatment.
For the two single site dialysis protocols, Fig. 1D shows the five locations of the probes for rats that received muscimol in the RTN, and Fig. 1E the five locations for rats that received 8-OH-DPAT in the MR. A total of eight rats contributed to the two single site experiments. Two rats had both the RTN and the MR probes correctly placed and they contributed results obtained, on separate days, to both the RTN muscimol and the MR 8-OH-DPAT experiments. Three rats had only the RTN probe correctly placed and they contributed only RTN muscimol results; three other rats had only the MR probe correctly placed and they contributed only MR 8-OH-DPAT results. Figure 1D and E shows the 10 correct probe placements from these eight rats; incorrect probe placements are not shown. In the three rats with RTN probes near to the RTN (not shown), the probe tips were at −10.3 mm caudal to bregma and were dorsomedial to the rostral aspect of the facial nucleus. In the three rats with caudal MR probes near to the caudal MR (not shown), the probe tips were at −13.3 mm caudal to bregma and were ∼1 mm lateral to the mid-line.
Rat body temperature did decrease a small but significant amount during the time course of the experiment (P < 0.001; repeated measures ANOVA with rat temperature as the repeated measure and treatment [RTN, caudal MR, RTN plus caudal MR] as the categorical variable). The mean initial and final body temperatures in the three experiments did not differ among the experimental groups, however. The initial and final body temperatures (in °C) were for the RTN experiment, 37.8 ± 0.1 and 37.2 ± 0.2; for the MR experiment, 38.1 ± 0.2 and 37.5 ± 0.1; and for the simultaneous caudal MR and RTN experiment, 37.9 ± 0.1 and 37.4 ± 0.1. Oxygen consumption similarly varied a small but significant amount during the time course of the experiment but without any significant difference among protocols (P < 0.001; repeated measures ANOVA with rat oxygen consumption as the RM and treatment [RTN, caudal MR, RTN plus caudal MR] as the categorical variable). The initial and final oxygen consumption values were (in ml (100 g)−1 min−1) for the RTN experiment, 2.5 ± 0.2 and 2.2 ± 0.2; for the caudal MR experiment, 2.3 ± 0.2 and 2.2 ± 0.2; and for the simultaneous caudal MR and RTN experiment, 2.3 ± 0.2 and 2.3 ± 0.2.
All three treatment groups developed hypoventilation during air breathing as estimated by the ratio of  to
to  averaged over both wakefulness and NREM sleep (Fig. 2). The ratio of
averaged over both wakefulness and NREM sleep (Fig. 2). The ratio of  to
to  was significantly lower during dialysis with muscimol in the RTN (−17%), 8-OH-DPAT in the MR (−20%) and with simultaneous dialysis of muscimol in the RTN and 8-OH-DPAT in the MR (−30%; P < 0.001, one-way RM ANOVA; P < 0.05 post hoc t test with Bonferroni correction).
was significantly lower during dialysis with muscimol in the RTN (−17%), 8-OH-DPAT in the MR (−20%) and with simultaneous dialysis of muscimol in the RTN and 8-OH-DPAT in the MR (−30%; P < 0.001, one-way RM ANOVA; P < 0.05 post hoc t test with Bonferroni correction).
Figure 2. Each treatment caused hypoventilation.
Mean (s.e.m.)  values for animals in each group before (filled bars) and after dialysis treatment (shaded bars) in the RTN with muscimol, the MR with 8-OH-DPAT or at both sites with both agents. There was a significant decrease in
values for animals in each group before (filled bars) and after dialysis treatment (shaded bars) in the RTN with muscimol, the MR with 8-OH-DPAT or at both sites with both agents. There was a significant decrease in  after each treatment (*P < 0.05 post hoc with Bonferroni correction; one-way repeated measures ANOVA, P < 0.001). These
after each treatment (*P < 0.05 post hoc with Bonferroni correction; one-way repeated measures ANOVA, P < 0.001). These  values were averaged over the awake and NREM sleep periods (see text for explanation).
values were averaged over the awake and NREM sleep periods (see text for explanation).
Dialysis of 1 mm muscimol into the RTN with ACSF being dialysed in or near to the caudal MR decreased significantly during 7% CO2 breathing in both wakefulness and NREM sleep without any significant effect on  during air breathing (Fig. 3;
during air breathing (Fig. 3;  as repeated measures comparing pre- to post-treatment, P < 0.001; interaction between treatment and gas breathed, P < 0.001; P < 0.05, post hoc comparison at 7% CO2 with Bonferroni correction). This treatment also decreased VT significantly during 7% CO2 breathing in both wakefulness and NREM sleep and during air breathing in wakefulness but not in NREM sleep (Fig. 3; VT as repeated measures comparing pre- to post-treatment, P < 0.001; interaction between treatment and gas breathed, P < 0.03; P < 0.05, post hoc comparison at 7% CO2 and air breathing with Bonferroni correction). Breathing frequency (f) was decreased significantly during 7% CO2 breathing only in NREM sleep (Fig. 3; interaction between treatment and gas breathed, P < 0.04; P < 0.05, post hoc comparison at 7% CO2 and with Bonferroni correction). The CO2 response,
as repeated measures comparing pre- to post-treatment, P < 0.001; interaction between treatment and gas breathed, P < 0.001; P < 0.05, post hoc comparison at 7% CO2 with Bonferroni correction). This treatment also decreased VT significantly during 7% CO2 breathing in both wakefulness and NREM sleep and during air breathing in wakefulness but not in NREM sleep (Fig. 3; VT as repeated measures comparing pre- to post-treatment, P < 0.001; interaction between treatment and gas breathed, P < 0.03; P < 0.05, post hoc comparison at 7% CO2 and air breathing with Bonferroni correction). Breathing frequency (f) was decreased significantly during 7% CO2 breathing only in NREM sleep (Fig. 3; interaction between treatment and gas breathed, P < 0.04; P < 0.05, post hoc comparison at 7% CO2 and with Bonferroni correction). The CO2 response,  , was reduced by 24.4 ± 5.6% in wakefulness (P < 0.02, students paired t test;
, was reduced by 24.4 ± 5.6% in wakefulness (P < 0.02, students paired t test;  pre- versus post-treatment) and 27.7 ± 3.6% in NREM sleep (P < 0.01, students paired t test;
pre- versus post-treatment) and 27.7 ± 3.6% in NREM sleep (P < 0.01, students paired t test;  pre- versus post-treatment). This effect was on the Δf response to CO2, which was reduced by 25.1 ± 14% in wakefulness and by 25.8 ± 13% in NREM sleep. TheseΔf values were not significantly different in wakefulness or NREM sleep (students paired t test,Δf pre- versus post-treatment), but if the awake and NREM data were combined for the two states, there was a significant treatment effect (P < 0.03, students paired t test,Δf pre- versus post-treatment). Note that
pre- versus post-treatment). This effect was on the Δf response to CO2, which was reduced by 25.1 ± 14% in wakefulness and by 25.8 ± 13% in NREM sleep. TheseΔf values were not significantly different in wakefulness or NREM sleep (students paired t test,Δf pre- versus post-treatment), but if the awake and NREM data were combined for the two states, there was a significant treatment effect (P < 0.03, students paired t test,Δf pre- versus post-treatment). Note that  itself did not change significantly, while the
itself did not change significantly, while the  did indicate hypoventilation.
did indicate hypoventilation.
Figure 3. Effects of muscimol dialysis in the RTN (with dialysis of ACSF into the caudal medullary raphe) on  , VT and f.
, VT and f.
Pretreatment (•) and post-treatment (○) mean ± s.e.m. ventilatory values are shown for wakefulness (left-hand panels) and NREM sleep (right-hand panels). Asterisk indicates values that are significantly different comparing pre- to post-treatment (P < 0.05 for Bonferroni post hoc comparison;  , VT and f as repeated measures pre- and post-treatment with gas breathed as a categorical variable).
, VT and f as repeated measures pre- and post-treatment with gas breathed as a categorical variable).
Dialysis of 1 mm 8-OH-DPAT into the caudal MR with ACSF being dialysed into the RTN had no significant effect on  in air or 7% CO2 in wakefulness or NREM sleep, although the values after treatment were lower than before treatment (Fig. 4). Tidal volume was decreased significantly during air breathing only in both wakefulness and NREM sleep (Fig. 4; VT as repeated measures comparing pre- to post-treatment, P < 0.001; interaction between treatment and gas breathed, P < 0.05; P < 0.05, post hoc comparison during air breathing with Bonferroni correction). Frequency was unaffected. There was no significant treatment effect on
in air or 7% CO2 in wakefulness or NREM sleep, although the values after treatment were lower than before treatment (Fig. 4). Tidal volume was decreased significantly during air breathing only in both wakefulness and NREM sleep (Fig. 4; VT as repeated measures comparing pre- to post-treatment, P < 0.001; interaction between treatment and gas breathed, P < 0.05; P < 0.05, post hoc comparison during air breathing with Bonferroni correction). Frequency was unaffected. There was no significant treatment effect on  . Note that
. Note that  itself did not change significantly, while the
itself did not change significantly, while the  ratio did indicate hypoventilation.
ratio did indicate hypoventilation.
Figure 4. Effects of 8-OH-DPAT dialysis in the caudal MR (with dialysis of ACSF into the RTN) on  , VT and f.
, VT and f.
Pretreatment (•) and post-treatment (○) mean ± s.e.m. ventilatory values are shown for wakefulness (left-hand panels) and NREM sleep (right-hand panels). Asterisk indicates values that are significantly different comparing pre- to post-treatment (P < 0.05 for Bonferroni post hoc comparison;  , VT and f as repeated measures pre- and post-treatment with gas breathed as a categorical variable).
, VT and f as repeated measures pre- and post-treatment with gas breathed as a categorical variable).
Simultaneous dialysis of 1 mm muscimol into the RTN and 1 mm 8-OH-DPAT into the MR decreased VT significantly during both 7% CO2 and air breathing in both wakefulness and NREM sleep (Fig. 5). The effect was statistically the same in air and in 7% CO2. (VT as repeated measures comparing pre- to post-treatment, P < 0.001; no significant interaction between treatment and gas breathed; P < 0.05, post hoc comparison of 7% CO2 and air breathing with Bonferroni correction). The frequency was decreased significantly during 7% CO2 but not during air breathing in wakefulness and in NREM sleep (Fig. 5; f as repeated measures comparing pre- to post-treatment, P < 0.001; interaction between treatment and gas breathed, P < 0.001; P < 0.05, post hoc comparison at 7% CO2 with Bonferroni correction). As a result,  was decreased significantly during both air and 7% CO2 breathing in both wakefulness and NREM sleep (Fig. 5;
was decreased significantly during both air and 7% CO2 breathing in both wakefulness and NREM sleep (Fig. 5;  as repeated measures comparing pre- to post-treatment, P < 0.001; interaction between treatment and gas breathed, P < 0.001; P < 0.05, post hoc comparison of 7% CO2 and air breathing with Bonferroni correction). The CO2 response,
as repeated measures comparing pre- to post-treatment, P < 0.001; interaction between treatment and gas breathed, P < 0.001; P < 0.05, post hoc comparison of 7% CO2 and air breathing with Bonferroni correction). The CO2 response,  , was reduced by 50.7 ± 7.0% in wakefulness (P < 0.002, paired t test;
, was reduced by 50.7 ± 7.0% in wakefulness (P < 0.002, paired t test;  pre- versus post-treatment) and 52.1 ± 5.1% in NREM sleep (P < 0.001, paired t test; Δf pre- versus post-treatment). This effect resulted from theΔf response to CO2, which was reduced by 50 ± 11% in wakefulness and by 48.6 ± 9% in NREM sleep. These Δf values were significantly different in wakefulness (P < 0.01, paired t test, Δf pre- versus post-treatment) and in NREM sleep (P < 0.01, paired t test, Δf pre- versus post-treatment). Note that in this case, both
pre- versus post-treatment) and 52.1 ± 5.1% in NREM sleep (P < 0.001, paired t test; Δf pre- versus post-treatment). This effect resulted from theΔf response to CO2, which was reduced by 50 ± 11% in wakefulness and by 48.6 ± 9% in NREM sleep. These Δf values were significantly different in wakefulness (P < 0.01, paired t test, Δf pre- versus post-treatment) and in NREM sleep (P < 0.01, paired t test, Δf pre- versus post-treatment). Note that in this case, both  itself in either wakefulness or NREM sleep and the
itself in either wakefulness or NREM sleep and the  ratio averaged over both states decreased, indicating hypoventilation.
ratio averaged over both states decreased, indicating hypoventilation.
Figure 5. Effects of simultaneous muscimol dialysis into the RTN and 8-OH-DPAT dialysis into the caudal MR on  , VT and f.
, VT and f.
Pretreatment (•) and post-treatment (○) mean ± s.e.m. ventilatory values are shown for wakefulness (left-hand panels) and NREM sleep (right-hand panels). Asterisk indicates values that are significantly different comparing pre- to post-treatment (P < 0.05 for Bonferroni post hoc comparison;  , VT and f as repeated measures pre- and post-treatment with gas breathed as a categorical variable).
, VT and f as repeated measures pre- and post-treatment with gas breathed as a categorical variable).
Discussion
It was difficult to place both probes at the correct locations in individual rats. This difficulty resulted in the use of 34 rats to produce the successful experiments reported in this paper. For the simultaneous dialysis of muscimol into the RTN and 8-OH-DPAT into the caudal MR, we used five rats in which the probe tips were perfectly placed (Fig. 1B and C). For the single site treatment experiments, two rats had both probes perfectly placed (Fig. 1D and E). Three rats had one probe placed within the RTN (shown in Fig. 2) and the other probe placed near to the caudal MR (not shown). Three different rats had one probe placed within the caudal MR (shown in Fig. 1) and the other probe placed near to the RTN (not shown). We included data from these rats because probes in the non-treated site that were dialysed with ACSF were quite close to the specific regions of interest.
The RTN probe tip location provides access to the neurons within the RTN, which is a thin sheet of neurons lying beneath the facial nucleus and extending from the rostral aspect of the facial nucleus to a few hundred micrometres caudal to the facial nucleus, medially to the pyramids and laterally just past the lateral margin of the facial nucleus (Cream et al. 2002; Mulkey et al. 2004; Guyenet et al. 2005). These neurons were originally described by retrograde tracing experiments following injections into the dorsal or ventral respiratory groups (Smith et al. 1989). Subsequent experiments demonstrated the importance of this small region, since focal inhibition or lesions in anaesthetized animals caused apnoea and severe reductions in CO2 sensitivity (Nattie et al. 1991; Takakura et al. 2006) and diminished breathing and CO2 sensitivity in conscious animals (Akilesh et al. 1997; Nattie & Li, 2002b), while focal acidification stimulated breathing (Nattie & Li, 2001) via neurons subsequently identified by juxtacellular labelling as glutamatergic (Mulkey et al. 2004; Guyenet et al. 2005).
The caudal MR probe location provides access to neurons within the raphe pallidus and the raphe obscurus. Prior work on the role of the MR in the control of breathing in conscious rats focused on the more rostral aspect of the MR, that lying at the level of the facial nucleus and including raphe pallidus and magnus (Nattie & Li, 2001; Taylor et al. 2005a,b).
Reverse microdialysis is a useful approach to reliably stimulate or inhibit neurons within the region of drug diffusion surrounding the tip of the dialysis probe (Cream et al. 1999; Nattie & Li, 2000). The semipermeable tip of the probe that we use is 1 mm in length and 240 μm in diameter. As a crude index of spread of the dialysed muscimol or 8-OH-DPAT, we have previously analysed the spread of dialysed fluorescein, which has a relative molecular mass of 332, compared to 195 for muscimol and 328 for 8-OH-DPAT. The average spread of fluorescein in the rat RTN region after dialysis at 1 mm in the dialysate (n = 5 rats) was over a total volume of 590 nl, which was calculated using, for each rat, the measured average radius of spread in the cross-section with the largest amount of fluorescein (Cream et al. 1999). This average radius was 555 μm, with similar results found in two rats in a separate study (Nattie & Li, 2000). The shape of the diffused fluorescein is like that of a football with the longer axis in the rostral to caudal direction. Studies from other laboratories have shown in cortex that there is a 35% tissue delivery of dialysed glutamate at 10–1000 mm concentration in the dialysate (Alessandri et al. 1996). Applying these chemical data to our studies would suggest that our muscimol or 8-OH-DPAT concentration just outside the probe would be ∼350 μm. This approach allows specific drug delivery to the RTN and/or to the caudal MR. Within the RTN region, the neurons affected could include the parafacial nucleus paragigantocellularis and the parapyramidal region. Within the caudal MR, the neurons affected could be in both raphe pallidus and raphe obscurus as well as adjacent mid-line neurons.
In this study, we limited the exposure to increased CO2 to 30–40 min, a period that minimized time-dependent brain pH regulatory processes but still allowed study of responses in wakefulness and NREM sleep. However, this protocol did not allow us to sample sufficient REM periods for formal analysis.
Microdialysis of muscimol alone in RTN
In a previous study from this laboratory, we dialysed muscimol at 1 and 10 mm concentrations into the RTN region of the conscious rat (Nattie & Li, 2000) with results similar to those of the present study. At the end of the 30 min muscimol dialysis period, we observed a decrease in  during room air breathing without change in
during room air breathing without change in  such that
such that  decreased, i.e. there was hypoventilation. In the present study, we observed no change in
decreased, i.e. there was hypoventilation. In the present study, we observed no change in  with dialysis of muscimol into the RTN; however, with a small increase in
with dialysis of muscimol into the RTN; however, with a small increase in  ,
, again decreased, indicating hypoventilation. In our previous study, we observed a significant 19% decrease in the
again decreased, indicating hypoventilation. In our previous study, we observed a significant 19% decrease in the  during 7% CO2 stimulation, a value similar to the 24% decrease observed in this study. Both studies demonstrated a significant decrease in VT during both air and 7% CO2 breathing. Dialysis with ACSF had no significant effect. These experiments are unilateral, while the RTN is a bilateral structure. It is possible that the effects observed would be greater were we to inhibit the RTN bilaterally. This is technically difficult.
during 7% CO2 stimulation, a value similar to the 24% decrease observed in this study. Both studies demonstrated a significant decrease in VT during both air and 7% CO2 breathing. Dialysis with ACSF had no significant effect. These experiments are unilateral, while the RTN is a bilateral structure. It is possible that the effects observed would be greater were we to inhibit the RTN bilaterally. This is technically difficult.
The RTN contains glutamatergic neurons that respond to CO2 and stimulate breathing (Mulkey et al. 2004; Guyenet et al. 2005), some of which are also activated by carotid body stimulation (Takakura et al. 2006). The effects on breathing during 7% CO2 produced by RTN inhibition by muscimol could reflect inhibition of either the CO2 chemosensitivity of these neurons or their effect on carotid body afferent integration or both. Inhibition of the RTN does affect the hypoxic ventilatory response in decerebrate cats, indicating that RTN neurons do affect the carotid body reflex control of breathing (Nattie et al. 1991). The presence of hypoventilation during muscimol inhibition of the RTN indicates the presence of a tonic drive to breathe that arises from the RTN (see Nattie, 2006). The mechanistic explanation for this is likely to involve tonic firing of RTN chemosensitive neurons that is dependent on local CO2 stimulation and/or tonic afferent activity from the carotid body. In turn, RTN neurons connect to neurons in the PBC and VRG (Connelly et al. 1989; Cream et al. 2002) that determine respiratory rhythm and pattern (Feldman et al. 2003). Inhibition of these tonically firing neurons by muscimol decreases resting ventilation and diminishes their responsiveness to added CO2.
Microdialysis of 8-OH-DPAT alone in MR
In a previous study from this laboratory (Taylor et al. 2005a), we dialysed 8-OH-DPAT at 1, 10 and 30 mm concentrations into the rostral portion of the MR at the level of the facial nucleus, i.e. in the raphe pallidus and magnus. Figure 6 shows that the location of the dialysis probe tip in the present study is well caudal to that for the previous study. This more caudal probe placement was by necessity not design, since it is technically impossible to place one dialysis guide tube into the RTN and another into the adjacent rostral MR at the same time owing to anatomical and size constraints. At the more rostral site, the dialysis of 8-OH-DPAT at the 1 mm dose significantly decreased  during air breathing without significant effect on
during air breathing without significant effect on  such that
such that  decreased (Taylor et al. 2005a). We also observed a significant decrease in
decreased (Taylor et al. 2005a). We also observed a significant decrease in  during dialysis of 1 mm 8-OH-DPAT into the more caudal aspect of the MR.
during dialysis of 1 mm 8-OH-DPAT into the more caudal aspect of the MR.
Figure 6. Comparison of the effects of dialysis of 8-OH-DPAT into the rostral medullary raphe (data taken from Taylor et al. 2005a) and the caudal medullary raphe (present study).
The schematic drawing at the top shows a parasaggittal view of the medulla with the arrows at the top to demonstrate the location of the dialysis probes in the two studies, one in the rostral MR the other in the caudal MR. The circles represent the approximate areas of drug diffusion, with the rostral arrow pointing to the overall effects on the  response to 7% CO2 (shown as
response to 7% CO2 (shown as  ) and the caudal arrow pointing to the same for the present study. Abbreviations: DR, dorsal raphe; mR, median raphe; pR, pontine raphe; RMg, raphe magnus; RPa, raphe pallidus; and ROb, raphe obscurus.
) and the caudal arrow pointing to the same for the present study. Abbreviations: DR, dorsal raphe; mR, median raphe; pR, pontine raphe; RMg, raphe magnus; RPa, raphe pallidus; and ROb, raphe obscurus.
In contrast, dialysis of 1 mm 8-OH-DPAT into the more rostral MR produced a significant 22% decrease in  during 7% CO2 breathing (Taylor et al. 2005a; Fig. 6), an effect that is substantially greater than that observed in the present study, 2.9% (Fig. 6). Inhibition of the caudal portion of the MR did not have the expected effect on chemoreception. With 1 mm 8-OH-DPAT dialysed into the caudal MR, there was essentially no effect on the CO2 response. This 8-OH-DPAT treatment in the caudal MR also decreased VT during air breathing in wakefulness and in NREM sleep, an effect that was not observed at this dose with the more rostral MR experiments. These data indicate the presence of a heterogeneity in MR function with respect to chemoreception and breathing, an important new finding. Our previous study with focal CO2 dialysis into the MR, which demonstrated a stimulatory effect in sleep, was also limited to the rostral aspect of the MR at the level of the facial nucleus (Nattie & Li, 2001). Our data in conscious adult rats support the presence of chemoreception at the rostral MR but do not support the presence of chemoreception at the more caudal MR. Veasey et al. (1995) did report in conscious cats that hypercapnia stimulated the firing of six neurons in the raphe obscurus, which would be in the vicinity of our more caudal dialysis sites. These workers interpreted this finding to reflect an association of serotonergic activity with the motor activity of breathing and not with chemosensitivity. In transverse slices from neonatal rats, focal CO2 in raphe obscurus does stimulate hypoglossal nerve activity (Peever et al. 2001), an effect that may not be measurable as a change in breathing in the intact conscious adult rat. Serotonergic neurons in MR can also modulate the hypoxic ventilatory response. Serotonergic neurons in MR express c-fos with hypoxic or carotid body stimulation (Erickson & Millhorn, 1994), and focal inhibition of the rostral MR in conscious rats affects the hypoxic ventilatory response in a temperature-dependent manner (Taylor et al. 2005b). We do not as yet know whether the more caudal MR modulates the ventilatory response to hypoxia.
during 7% CO2 breathing (Taylor et al. 2005a; Fig. 6), an effect that is substantially greater than that observed in the present study, 2.9% (Fig. 6). Inhibition of the caudal portion of the MR did not have the expected effect on chemoreception. With 1 mm 8-OH-DPAT dialysed into the caudal MR, there was essentially no effect on the CO2 response. This 8-OH-DPAT treatment in the caudal MR also decreased VT during air breathing in wakefulness and in NREM sleep, an effect that was not observed at this dose with the more rostral MR experiments. These data indicate the presence of a heterogeneity in MR function with respect to chemoreception and breathing, an important new finding. Our previous study with focal CO2 dialysis into the MR, which demonstrated a stimulatory effect in sleep, was also limited to the rostral aspect of the MR at the level of the facial nucleus (Nattie & Li, 2001). Our data in conscious adult rats support the presence of chemoreception at the rostral MR but do not support the presence of chemoreception at the more caudal MR. Veasey et al. (1995) did report in conscious cats that hypercapnia stimulated the firing of six neurons in the raphe obscurus, which would be in the vicinity of our more caudal dialysis sites. These workers interpreted this finding to reflect an association of serotonergic activity with the motor activity of breathing and not with chemosensitivity. In transverse slices from neonatal rats, focal CO2 in raphe obscurus does stimulate hypoglossal nerve activity (Peever et al. 2001), an effect that may not be measurable as a change in breathing in the intact conscious adult rat. Serotonergic neurons in MR can also modulate the hypoxic ventilatory response. Serotonergic neurons in MR express c-fos with hypoxic or carotid body stimulation (Erickson & Millhorn, 1994), and focal inhibition of the rostral MR in conscious rats affects the hypoxic ventilatory response in a temperature-dependent manner (Taylor et al. 2005b). We do not as yet know whether the more caudal MR modulates the ventilatory response to hypoxia.
While we did not observe an effect on the CO2 response of 8-OH-DPAT dialysis into the caudal MR, we did observe hypoventilation, i.e. a decrease in  . We interpret this to reflect the loss of a drive to breathe that arises from within the caudal MR that is independent of chemoreception. The source of this drive is not clear from our data, but serotonergic neurons recorded in vivo are active and especially so in wakefulness (Veasey et al. 1995). This activity in non-CO2-excited serotonergic neurons may arise from other afferent inputs to these neurons. Carbon dioxide is not the sole stimulus that drives breathing at rest, and this input from caudal MR serotonergic neurons is likely to reflect one of many other sources of a drive to breathe. There is a possible alternative explanation for the hypoventilation caused by dialysis of 8-OH-DPAT into the caudal MR, namely that 5-HT1A receptors on neurons within the ventral respiratory group were affected. There are known serotonergic projections from the MR to neurons within the ventral respiratory group (Connelly et al. 1989; Holtman et al. 1990; Sun et al. 2002), but receptor ligand autoradiography (Thor et al. 1992; Niblock et al. 2005) and immunohistochemistry (Helke et al. 1997) demonstrate the presence of few, if any, 5-HT1A receptors there. Lalley et al. (1994) have shown effects of ionophoresis of 8-OH-DPAT directly on expiratory neurons within the ventral respiratory group, but these were located well caudal to our dialysis locations. Present evidence does not support the interpretation that our 8-OH-DPAT could affect ventral respiratory group neurons directly, although this remains a possibility.
. We interpret this to reflect the loss of a drive to breathe that arises from within the caudal MR that is independent of chemoreception. The source of this drive is not clear from our data, but serotonergic neurons recorded in vivo are active and especially so in wakefulness (Veasey et al. 1995). This activity in non-CO2-excited serotonergic neurons may arise from other afferent inputs to these neurons. Carbon dioxide is not the sole stimulus that drives breathing at rest, and this input from caudal MR serotonergic neurons is likely to reflect one of many other sources of a drive to breathe. There is a possible alternative explanation for the hypoventilation caused by dialysis of 8-OH-DPAT into the caudal MR, namely that 5-HT1A receptors on neurons within the ventral respiratory group were affected. There are known serotonergic projections from the MR to neurons within the ventral respiratory group (Connelly et al. 1989; Holtman et al. 1990; Sun et al. 2002), but receptor ligand autoradiography (Thor et al. 1992; Niblock et al. 2005) and immunohistochemistry (Helke et al. 1997) demonstrate the presence of few, if any, 5-HT1A receptors there. Lalley et al. (1994) have shown effects of ionophoresis of 8-OH-DPAT directly on expiratory neurons within the ventral respiratory group, but these were located well caudal to our dialysis locations. Present evidence does not support the interpretation that our 8-OH-DPAT could affect ventral respiratory group neurons directly, although this remains a possibility.
We use 8-OH-DPAT as a selective serotonergic 5-HT1A receptor agonist (Arvidsson et al. 1981) in order to inhibit serotonergic neurons within the MR. It binds to 5-HT1A receptors on serotonergic neurons, causes hyperpolarization and inhibits serotonin release (Hjorth & Magnusson, 1988). Our interpretation of the inhibition of the CO2 response produced by 8-OH-DPAT dialysis into the rostral MR is that the activity of chemosensitive serotonergic neurons is being reduced (Messier et al. 2004; Taylor et al. 2005a). We interpret the effects of 8-OH-DPAT dialysis into the caudal MR as resulting from inhibition of serotonergic neurons at that site, which then affects either other chemoreceptor sites or the PBC–VRG rhythm- and pattern-generating sites. There are 5-HT1A receptors at postsynaptic sites within our sphere of influence (Helke et al. 1997), which could respond to the 8-OH-DPAT dialysis as well (Kirby et al. 2003).
Simultaneous inhibition of RTN and MR
Based on previous results, we had two expectations when we designed this study. First, we expected that dialysis of muscimol into the RTN would inhibit the CO2 response, as it did, and that dialysis of 8-OH-DPAT into the MR would also inhibit the CO2 response, which it did not. Second, we expected that simultaneous inhibition of two chemoreceptor sites, the RTN and the MR, would produce an additive inhibition; the  during 7% CO2 breathing would be decreased by the sum of the two independent inhibitory effects. This expectation also was not met. Inhibition of the caudal MR, which we now believe does not by itself contribute to chemoreception directly, at the same time as inhibition of the RTN potentiated the RTN effects.
during 7% CO2 breathing would be decreased by the sum of the two independent inhibitory effects. This expectation also was not met. Inhibition of the caudal MR, which we now believe does not by itself contribute to chemoreception directly, at the same time as inhibition of the RTN potentiated the RTN effects.
Muscimol inhibition of the RTN decreased VT in air and in 7% CO2 and decreased the f response to CO2. The  /
/ was decreased by 17%, indicating hypoventilation, and the CO2 response was reduced by 24%. Simultaneous dialysis of 8-OH-DPAT into the caudal MR potentiated these effects. The decrease in VT during both air and 7% CO2 breathing was enhanced, as was the inhibition of the f response to CO2. The result was a 28 and 30% reduction in
was decreased by 17%, indicating hypoventilation, and the CO2 response was reduced by 24%. Simultaneous dialysis of 8-OH-DPAT into the caudal MR potentiated these effects. The decrease in VT during both air and 7% CO2 breathing was enhanced, as was the inhibition of the f response to CO2. The result was a 28 and 30% reduction in  in air breathing during wakefulness and NREM sleep, respectively, a 30% reduction in averaged over both wakefulness and NREM sleep, and a 50.7 and 52% reduction in the
in air breathing during wakefulness and NREM sleep, respectively, a 30% reduction in averaged over both wakefulness and NREM sleep, and a 50.7 and 52% reduction in the  CO2 response in wakefulness and NREM sleep, respectively. We interpret these results as a reduction in the ‘drive to breathe’ that arises from both central and peripheral chemoreceptors (Nattie, 2006; Smith et al. 2006) as well as from non-CO2-dependent caudal MR neurons and a reduction in chemosensitivity. Mechanistically, caudal MR serotonergic neurons may modulate breathing in three general ways: (1) directly at central chemoreceptor sites, e.g. at the RTN or the NTS (Nattie & Li, 2000); (2) at downstream sites within the VRG, including the PBC; and (3) at sites that affect the afferent chemosensitive input from the carotid body, e.g. the RTN and NTS (Nattie et al. 1991; Takakura et al. 2006). Wherever it occurs, the effects of muscimol inhibition at the RTN are then potentiated by the effects of inhibition at the caudal MR. This remarkable potentiation of the hypoventilation and the inhibition of the CO2 response produced by muscimol in the RTN with simultaneous inhibition of the caudal MR is summarized in Fig. 7.
CO2 response in wakefulness and NREM sleep, respectively. We interpret these results as a reduction in the ‘drive to breathe’ that arises from both central and peripheral chemoreceptors (Nattie, 2006; Smith et al. 2006) as well as from non-CO2-dependent caudal MR neurons and a reduction in chemosensitivity. Mechanistically, caudal MR serotonergic neurons may modulate breathing in three general ways: (1) directly at central chemoreceptor sites, e.g. at the RTN or the NTS (Nattie & Li, 2000); (2) at downstream sites within the VRG, including the PBC; and (3) at sites that affect the afferent chemosensitive input from the carotid body, e.g. the RTN and NTS (Nattie et al. 1991; Takakura et al. 2006). Wherever it occurs, the effects of muscimol inhibition at the RTN are then potentiated by the effects of inhibition at the caudal MR. This remarkable potentiation of the hypoventilation and the inhibition of the CO2 response produced by muscimol in the RTN with simultaneous inhibition of the caudal MR is summarized in Fig. 7.
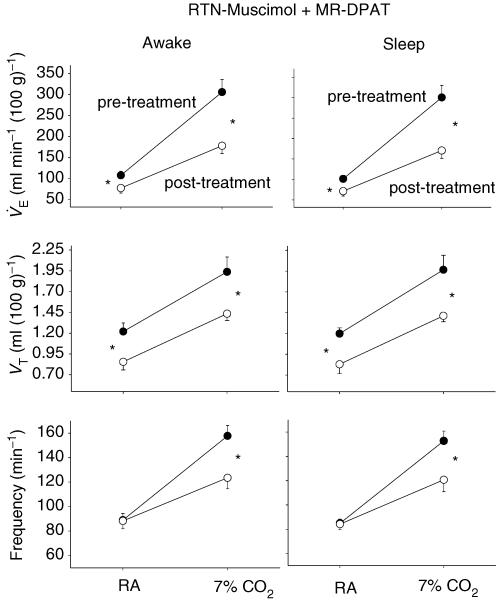
Figure 7. Simultaneous dialysis of muscimol into the RTN and 8-OH-DPAT into the caudal MR (bottom panel) potentiates the inhibitory effect of either treatment alone (top panels).
Pre- and post-treatment values of  (means ± s.e.m.) are shown in each panel. Note that dialysis of muscimol into the RTN reduces the
(means ± s.e.m.) are shown in each panel. Note that dialysis of muscimol into the RTN reduces the  value at 7% CO2 by 24%, dialysis of 8-OH-DPAT into the caudal MR reduces it by 2.5%, while simultaneous dialysis of muscimol into the RTN and 8-OH-DPAT into the caudal medullary raphe reduces it by 50.7%. These data are for wakefulness; NREM sleep data show the same pattern of response.
value at 7% CO2 by 24%, dialysis of 8-OH-DPAT into the caudal MR reduces it by 2.5%, while simultaneous dialysis of muscimol into the RTN and 8-OH-DPAT into the caudal medullary raphe reduces it by 50.7%. These data are for wakefulness; NREM sleep data show the same pattern of response.
There are anatomical and physiological data that support all three ways by which caudal MR serotonergic neurons can modulate breathing. The MR has known projections to the RTN (Connelly et al. 1989; Cream et al. 2002), but we know of no data describing serotonergic receptors on RTN neurons. There are serotonergic receptors on neurons in the PBC and VRG (Tryba et al. 2006) and in the NTS (Raul, 2003), and serotonergic agents affect neuronal function at these sites.
Conclusions
First, in conscious rats, focal manipulation by dialysis of the rostral and caudal MR demonstrates a heterogeneity of function with respect to breathing. Second, inhibition of the caudal MR with 8-OH-DPAT causes hypoventilation without an effect on the CO2 response and markedly potentiates the effects of simultaneous RTN inhibition with muscimol, causing further hypoventilation and a substantially reduced CO2 response. Third, serotonergic neurons in the caudal MR modulate breathing via effects either at chemoreceptor sites, e.g. the RTN, or at respiratory neurons that determine rhythm and pattern in the VRG and PBC, and provide a tonic drive to breathe that is independent of chemosensitivity.
Acknowledgments
This research was supported by the National Institutes of Health research grant HL 28066.
References
- Akilesh MR, Kamper M, Li A, Nattie EE. Effects of unilateral lesions of retrotrapezoid nucleus on breathing in awake rats. J Appl Physiol. 1997;82:469–479. doi: 10.1152/jappl.1997.82.2.469. [DOI] [PubMed] [Google Scholar]
- Alessandri B, Landolt H, Langemann H, Gregorin J, Hall J, Gratzl O. Application of glutamate in the cortex of rats: a microdialysis study. Acta Neurochir. 1996;67(Suppl):6–12. doi: 10.1007/978-3-7091-6894-3_2. [DOI] [PubMed] [Google Scholar]
- Arvidsson LE, Hacksell U, Nilsson JL, Hjorth S, Carlsson A, Lindberg P, et al. 8-Hydroxy-2-(di-n-propylamino) tetralin, a new centrally acting 5-hydroxytryptamine. J Med Chem. 1981;24:921–923. doi: 10.1021/jm00140a002. [DOI] [PubMed] [Google Scholar]
- Bartlett D, Jr, Tenney SM. Control of breathing in experimental anemia. Resp Physiol. 1970;10:384–395. doi: 10.1016/0034-5687(70)90056-3. [DOI] [PubMed] [Google Scholar]
- Bennington JH, Kodali SK, Heller HC. Scoring transitions to REM in rats based on the EEG phenomena of pre-REM sleep: an improved analysis of sleep structure. Sleep. 1994;17:28–36. doi: 10.1093/sleep/17.1.28. [DOI] [PubMed] [Google Scholar]
- Coates EL, Li A, Nattie EE. Widespread sites of brain stem ventilatory chemoreceptors. J Appl Physiol. 1993;75:5–14. doi: 10.1152/jappl.1993.75.1.5. [DOI] [PubMed] [Google Scholar]
- Connelly CA, Ellenberger HH, Feldman JL. Are there serotonergic projections from raphe and retrotrapezoid nucleus to the central respiratory group in the rat? Neurosci Lett. 1989;105:34–40. doi: 10.1016/0304-3940(89)90007-4. [DOI] [PubMed] [Google Scholar]
- Cream C, Li A, Nattie E. The retrotrapzoid nucleus (RTN): local cytoarchitecture and afferent connections. Respir Physiol Neurobiol. 2002;130:121–137. doi: 10.1016/s0034-5687(01)00338-3. [DOI] [PubMed] [Google Scholar]
- Cream C, Nattie E, Li A. TRH microdialysis into the RTN of the conscious rat increases breathing, metabolism, and temperature. J Appl Physiol. 1999;87:673–682. doi: 10.1152/jappl.1999.87.2.673. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotonergic neurons of the rat brainstem. J Comp Neurol. 1994;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- Feldman JR, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV. On the peripheral and central chemoreception and control of breathing: an emerging role of ATP. J Physiol. 2005;568:715–724. doi: 10.1113/jphysiol.2005.095968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK. Retrotrapezoid nucleus: a litmus test for the identification of central chemoreceptors. Exp Physiol. 2005;90:247–253. doi: 10.1113/expphysiol.2004.029637. [DOI] [PubMed] [Google Scholar]
- Helke CJ, Capuano S, Tran N, Zhou H. Immunocytochemical studies of the 5-HT1A receptor in ventral medullary neurons that project to the intermediolateral column and contain serotonin or tyrosine hydroxylase immunoreactivity. J Comp Neurol. 1997;379:261–270. [PubMed] [Google Scholar]
- Hjorth S, Magnusson T. The 5-HT 1A receptor agonist, 8-OH-DPAT, preferentially activates cell body 5-HT autoreceptors in rat brain in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1988;338:463–471. doi: 10.1007/BF00179315. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Martino P, Davis S, Opansky C, Pan LG, Forster HV. Effects on breathing of focal acidosis at multiple medullary raphe sites in awake goats. J Appl Physiol. 2004;97:2303–2309. doi: 10.1152/japplphysiol.00645.2004. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr, Marion LJ, Speck DF. Origin of serotonin-containing projections to the ventral respiratory group in the rat. Neuroscience. 1990;37:541–552. doi: 10.1016/0306-4522(90)90422-z. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Pernar L, Valentino RJ, Beck SG. Distinguishing characteristics of serotonin and non-serotonin-containing cells in the dorsal raphe nucleus: electrophysiological and immunohistochemical studies. Neuroscience. 2003;116:669–683. doi: 10.1016/s0306-4522(02)00584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM, Bischoff AM, Richter DW. 5-HT-1A receptor-mediated modulation of medullary expiratory neurons in the cat. J Physiol. 1994;476:117–130. [PMC free article] [PubMed] [Google Scholar]
- Li A, Nattie EE. Catecholamine neurons in rats modulate sleep, breathing, central chemoreception, and breathing variability. J Physiol. 2006;570:385–396. doi: 10.1113/jphysiol.2005.099325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Randall M, Nattie EE. CO2 microdialysis in retrotrapezoid nucleus of the rat increases breathing in wakefulness but not in sleep. J Appl Physiol. 1999;87:910–919. doi: 10.1152/jappl.1999.87.3.910. [DOI] [PubMed] [Google Scholar]
- Loeschcke HH. Central chemosensitivity and the reaction theory. J Physiol. 1982;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino PF, Hodges MR, Davis S, Opansky C, Pan LG, Krause K, Qian B, Foster HV. CO2/H+ Chemoreceptors in the cerebellar fastigial nucleus do not uniformly affect breathing of awake goats. J Appl Physiol. 2006;101:241–248. doi: 10.1152/japplphysiol.00968.2005. [DOI] [PubMed] [Google Scholar]
- Messier ML, Li A, Nattie EE. Inhibition of medullary raphe serotonergic neurons has age-dependent effects on the CO2 response in newborn piglets. J Appl Physiol. 2004;96:1909–1919. doi: 10.1152/japplphysiol.00805.2003. [DOI] [PubMed] [Google Scholar]
- Mitchell RA, Loeschcke HH, Massion WH, Severinghaus JW. Respiratory responses mediated through superficial chemosensitive areas on the medulla. J Appl Physiol. 1963;18:523–533. doi: 10.1152/jappl.1963.18.3.523. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Nattie E. CO2, brainstem chemoreceptors and breathing. Prog Neurobiol. 1999;59:299–331. doi: 10.1016/s0301-0082(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Nattie EE. Central chemosensitivity, sleep, and wakefulness. Respir Physiol. 2001;129:257–268. doi: 10.1016/s0034-5687(01)00295-x. [DOI] [PubMed] [Google Scholar]
- Nattie EE. The retrotrapezoid nucleus and the ‘drive’ to breathe. J Physiol. 2006;572:311. doi: 10.1113/jphysiol.2006.107284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li A. Retrotrapezoid nucleus lesions decrease phrenic activity and CO2 sensitivity in rats. Respir Physiol. 1994;97:63–77. doi: 10.1016/0034-5687(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Central chemoreception in the region of the ventral respiratory group in the rat. J Appl Physiol. 1996;81:1987–1995. doi: 10.1152/jappl.1996.81.5.1987. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Muscimol dialysis in the retrotrapezoid nucleus region inhibits breathing in the awake rat. J Appl Physiol. 2000;89:153–162. doi: 10.1152/jappl.2000.89.1.153. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol. 2001;90:1247–1257. doi: 10.1152/jappl.2001.90.4.1247. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in nucleus tractus solitarius region of rat increases ventilation in sleep and wakefulness. J Appl Physiol. 2002a;92:2119–2130. doi: 10.1152/japplphysiol.01128.2001. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Substance P–saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J Physiol. 2002b;544:603–616. doi: 10.1113/jphysiol.2002.020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li A, Richerson GB, Lappi DA. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol. 2004;556:235–253. doi: 10.1113/jphysiol.2003.059766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li A, St John WM. Lesions in retrotrapezoid nucleus decrease ventilatory output in anesthetized or decerebrate cats. J Appl Physiol. 1991;71:1364–1375. doi: 10.1152/jappl.1991.71.4.1364. [DOI] [PubMed] [Google Scholar]
- Niblock MM, Luce CJ, Belliveau RA, Paterson DS, Kelly ML, Sleeper LA, Filiano JJ, Kinney HC. Comparative anatomical assessment of the piglet as a model for the developing human medullary serotonergic system. Brain Res Brain Res Rev. 2005;50:169–183. doi: 10.1016/j.brainresrev.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1998. [Google Scholar]
- Peever JH, Necakov A, Duffin J. Nucleus raphe obscurus modulates hypoglossal output of neonatal rat in vitro transverse brain stem slices. J Appl Physiol. 2001;90:269–279. doi: 10.1152/jappl.2001.90.1.269. [DOI] [PubMed] [Google Scholar]
- Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus ceruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neurosci. 1997;77:723–743. doi: 10.1016/s0306-4522(96)00485-x. [DOI] [PubMed] [Google Scholar]
- Raul L. Serotonin2 receptors in the nucleus tractus solitarius: characterization and role in the baroreceptor reflex arc. Cell Mol Neurobiol. 2003;23:709–727. doi: 10.1023/A:1025096718559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Hodges MR, Dohle CI, Diez-Samprado A. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp Physiol. 2005;90:259–266. doi: 10.1113/expphysiol.2005.029843. [DOI] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Smith CA, Rodman JR, Chenuel BJ, Henderson KS, Dempsey JA. Response time and sensitivity of the ventilatory response to CO2 in unanesthetized intact dogs: central vs. peripheral chemoreceptors. J Appl Physiol. 2006;100:13–19. doi: 10.1152/japplphysiol.00926.2005. [DOI] [PubMed] [Google Scholar]
- Sun QJ, Berkowitz RG, Goodchild A, Pilowsky PM. Serotonin inputs to inspiratory laryngeal motoneurons in the rat. J Comp Neurol. 2002;451:91–98. doi: 10.1002/cne.10329. [DOI] [PubMed] [Google Scholar]
- Takakura ACT, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol. 2005a;566:543–557. doi: 10.1113/jphysiol.2005.083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Ventilatory effects of muscimol microdialysis into the rostral medullary raphe region of conscious rats. Respir Physiol Neurobiol. 2005b doi: 10.1016/j.resp.2005.11.005. December 7; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Thor KB, Blitz-Siebert A, Helke CJ. Autoradiographic localization of 5HT1 binding sites in the medulla oblongata of the rat. Synapse. 1992;10:185–205. doi: 10.1002/syn.890100303. [DOI] [PubMed] [Google Scholar]
- Tryba AK, Peña F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Frazier DT. Modulation of respiratory motor output by cerebellar deep nuclei in the rat. J Appl Physiol. 2000;89:996–1004. doi: 10.1152/jappl.2000.89.3.996. [DOI] [PubMed] [Google Scholar]
- Xu F, Zhang Z, Frazier DT. Microinjection of acetazolamide into the fastigial nucleus augments respiratory output in the rat. J Appl Physiol. 2001;91:2342–2350. doi: 10.1152/jappl.2001.91.5.2342. [DOI] [PubMed] [Google Scholar]