Abstract
The ability to perceive and withdraw rapidly from noxious environmental stimuli is crucial for survival. When heat stimuli are applied to primate hairy skin, first pain sensation is mediated by type-II A-fibre nociceptors (II-AMHs). In contrast, the reported absence of first pain and II-AMH microneurographical responses when heat stimuli are applied to the hand palm has led to the notion that II-AMHs are lacking in this primate glabrous skin. The aim of this study was to assess the effect of hairy and glabrous skin stimulation on neural transmission of nociceptive inputs elicited by different kinds of thermal heating. We recorded psychophysical and EEG brain responses to radiant (laser-evoked potentials, LEPs) and contact heat stimuli (contact heat-evoked potentials, CHEPs) delivered to the dorsum and the palm of the hand in normal volunteers. Brain responses were analysed at a single-trial level, using an automated approach based on multiple linear regression. Laser stimulation of hairy and glabrous skin at the same energy elicited remarkably similar psychophysical ratings and LEPs. This finding provides strong evidence that first pain to heat does exist in glabrous skin, and suggests that similar nociceptive afferents, with the physiological properties of II-AMHs, mediate first pain to heat stimulation of glabrous and hairy skin in humans. In contrast, when contact heat stimuli were employed, a significantly higher nominal temperature had to be applied to glabrous skin in order to achieve psychophysical ratings similar to those obtained following hairy skin stimulation, and CHEPs following glabrous skin stimulation had significantly longer latencies (N2 wave, +25%; P2 wave, +24%) and smaller amplitudes (N2 wave, −40%; P2 wave, −44%) than CHEPs following hairy skin stimulation. Irrespective of the stimulated territory, CHEPs always had significantly longer latencies (hairy skin N2 wave, +75%; P2 wave, +56%) and smaller amplitudes (hairy skin N2 wave, −42%; P2 wave, −19%) than LEPs. These findings are consistent with the thickness-dependent delay and attenuation of the temperature waveform at nociceptor depth when conductive heating is applied, and suggest that the previously reported lack of first pain and microneurographical II-AMH responses following glabrous skin stimulation could have been the result of a search bias consequent to the use of long-wavelength radiant heating (i.e. CO2 laser) as stimulation procedure.
The ability to withdraw from noxious environmental stimuli in a timely fashion to avoid tissue damage is a crucial behaviour for survival. In primates the neural mechanism underlying the transmission of the noxious information of mechanical and heat stimuli to the spinal cord is the activation of mechano-heat A-fibre (AMHs) and C-fibre mechano heat (CMHs) skin nociceptors, which, respectively, signal first and second pain sensations in humans. Although these nociceptors are polymodal, AMHs are phylogenetically younger and have a higher degree of stimulus specificity compared to CMHs. Two populations of AMHs have been described in the primate hairy skin; they have different transduction mechanisms and differential responses to heat and mechanical stimuli (Treede et al. 1998). Type I afferents of A-fibre mechano-heat (AMHs) have low mechanical threshold (< 6 bar), a high heat threshold (median > 53°C) with a slow, wind-up response to heat (in the order of seconds), and fast conduction velocities (mean 25 m s−1). Type II afferents (II-AMHs) have high mechanical threshold (> 6 bar), low heat threshold (median 46°C) with a short latency (in the order of tens of milliseconds) and graded response to heat, and slower conduction velocities (mean 15 m s−1). These different response properties clearly indicate that I-AMHs primarily signal sharpness and pricking pain to mechanical stimuli, whereas II-AMHs signal first pain to heat stimuli.
This high degree of stimulus specificity of AMHs improves the ability of discriminating the quality of a nociceptive stimulus. This ‘population code’ for pain quality (Treede et al. 1998) is unquestionably advantageous for survival, and only the concomitant presence in the skin of AMHs with different and specific response properties provides such a discriminative ability.
Whereas I-AMHs and II-AMHs are approximately equally represented in primate hairy skin (Treede et al. 1998), there is no evidence that II-AMHs exist in primate glabrous skin. Microneurographical recordings in monkeys failed to detect II-AMH responses to heat stimuli applied to glabrous skin (Treede et al. 1995) and first pain sensation is not evoked in humans when heat stimuli are applied to the palm of the hand (Campbell & LaMotte, 1983). Taken together these findings have led to the notion that II-AMHs are lacking in primate glabrous skin (Meyer et al. 2006).
However, given that the increasing aptitude of grasping and manipulating objects has been a hallmark of primate evolution (Mountcastle, 2005), the ability to appreciate and react appropriately when the palm of the hand makes contact with hot objects would seem especially important for survival. For these reasons, from a teleological perspective the notion of the lack of nociceptors with a short-latency response to heat in glabrous skin is puzzling.
In order to clarify this issue, here we applied thermal energy to the hairy and the glabrous skin of the hand of human subjects by thermal radiation (Nd:YAP laser) or thermal conduction (contact thermode), and recorded the corresponding brain responses using scalp EEG. In particular, we took advantage of the high skin transparency to the Nd:YAP-laser radiation, to examine whether the previously observed differences between the responses elicited by stimulation of the hairy and glabrous skin may simply have resulted from a difference in skin thickness.
Methods
Subjects
Ten healthy volunteers (six men and four women) aged 25–32 years (mean 27.7 ± 2.6) participated in the study. The subjects were recruited from research staff and PhD students of the University of Oxford (UK). All participants gave their informed consent, the study conformed to the standards set by the Declaration of Helsinki, and the local ethics committee approved the procedures.
Radiant heat stimulation
Noxious radiant heat stimuli were generated by an infrared neodymium yttrium aluminium perovskite (Nd:YAP) laser with a wavelength of 1.34 μm (Electronical Engineering, Florence, Italy; http://www.elengroup.com). At this short wavelength, the skin is much more transparent to the laser radiation, in comparison with CO2 lasers (wavelength, 10.6 μm). As a consequence, Nd:YAP laser pulses activate nociceptive terminals directly (Baumgärtner et al. 2005) and do not induce the transient dyschromic spots sometimes produced by high-intensity CO2 laser pulses (Cruccu et al. 2003; Iannetti et al. 2003); however, because the exact volume of irradiated tissue is not known, the presence of less-visible lesions in the dermis cannot be excluded. In addition, the shorter wavelength of Nd:YAP lasers reduces the reproducibility of the stimulus, because of the lower skin absorption and the higher, pigmentation-dependent, skin reflectance (Plaghki & Mouraux, 2003).
In the present study, laser pulses were directed to the skin of the dorsum and of the palm of the left hand. An He–Ne pilot laser pointed to the area to be stimulated. The laser beam was transmitted through an optic fibre and its diameter was set at 6 mm (28 mm2) by focusing lenses. To avoid nociceptor fatigue or sensitization, the laser beam was moved slightly after each stimulus, and stimuli were delivered arrhythmically with 8–15 s intervals to minimize central habituation.
Contact heat stimulation
Noxious contact heat stimuli were generated by a thermode (CHEPs, Medoc Ltd, Ramat Yishai, Israel) with a square contact area of 5.7 cm2. Heating at the thermode surface was obtained using a thermofoil, and the maximal temperature rise time was 70°C s−1. The target temperature was computer controlled, and a pair of thermocouples on the surface of the thermode provided a measure of the temperature at the skin–thermode interface (sampling rate, 150 Hz). The maximal target temperature was 55°C, and the range of target temperatures was 51–55°C; at 70°C s−1 this range corresponds to a baseline-to-target time of 214–271 ms. Once the target nominal temperature was reached, a Peltier device actively cooled the thermode until the baseline temperature of 36°C was reached (cooling rate of 40°C s−1). The position of the thermode was kept constant throughout each stimulation block (see below). The thermode was fixed to the dorsum or to the palm of the left hand by means of an elastic Velcro strap provided with the equipment. Although this method does not provide accurate control of the pressure exerted by the thermode on the skin (which in turn influences the temperature at nociceptor level, Plaghki & Mouraux, 2003), maximal care in placing the thermode on the less uneven part of the hand dorsum and adjusting the Velcro strap was taken, in order to obtain a similar pressure across subjects and stimulated sites.
Experimental protocol
In all subjects we recorded brain potentials evoked by radiant heat (laser-evoked potentials, LEPs). In six subjects (three men and three women, age range 26–31 years), brain potentials evoked by contact heat (contact heat-evoked potentials, CHEPs) were also recorded. LEPs and CHEPs were recorded in different sessions on different days. In each recording session one block of 30 noxious heat stimuli was directed to the skin of the dorsum of the left hand and one block of 30 noxious heat stimuli was directed to the skin of the palm of the left hand. Both the order of blocks and the order of recording sessions were balanced across subjects. At the end of each block, subjects were asked to rate verbally the perceived sensation on a numerical rating scale ranging from 0 to 10, where 0 was ‘no pain’ and 10 ‘pain as bad as it could be’ (Jensen & Karoly, 2001).
As variations in baseline temperature can lead to misinterpretations when radiant heat stimuli are applied to the skin (Tjolsen et al. 1988), by using an infrared thermometer we ensured that baseline hairy and glabrous skin temperatures were similar, both at the beginning and at the end of the recording session (difference always < 1°C).
LEP recording session
Before starting the LEP recording, we delivered noxious laser pulses to the dorsum and the palm of the left hand in a pseudo-randomised order, with the aim of (1) familiarizing the subjects with the stimuli and (2) adjusting the energy of stimulation in order to produce the same psychophysical rating after hairy and glabrous skin stimulation (target pain rating, 4/10). As in previous experiments, we found that 4 ms long, Nd:YAP laser pulses of 2 J directed to a hairy skin area of about 28 mm2 were optimal to elicit a moderately painful, pinprick sensation and evoke robust LEPs (e.g. Iannetti et al. 2005). A series of three laser pulses with these parameters was initially delivered to both hairy and glabrous skin, and average pain ratings for each site were collected. It was not necessary to change the energy of glabrous skin stimulation in any of the subjects (i.e. the same stimulus parameters gave similar psychophysical ratings after stimulation of dorsum and palm). Consequently, the same pulse energy (2J), pulse duration (4 ms) and irradiated area (∼28 mm2) were used for the stimulation of both districts in the EEG recording session.
CHEP recording session
Similarly, before the CHEP recording, contact heat stimuli were delivered to the dorsum and the palm of the left hand in a pseudo-randomised order, with the aim of (1) familiarizing the subjects with the stimuli and (2) adjusting the temperature of stimulation in order to produce the same psychophysical rating after hairy and glabrous skin stimulation (target pain rating, 4/10). A series of three contact heat stimuli was initially delivered at a nominal temperature of 46°C to both hairy and glabrous skin, and average pain ratings for each site were collected. The nominal temperature was subsequently increased (in steps of 1°C) until the target pain rating (or, if the maximal nominal temperature permitted by the device did not elicit it, the pain rating closest to target; see Results) was reached for both dorsum and palm stimulation. These temperatures were recorded and used for stimulating the corresponding districts in the CHEP recording session.
EEG recording
Participants were seated in a comfortable chair, wore protective goggles, and were asked to focus on the stimulus and relax their muscles. They were instructed to pay attention to the stimulus and keep their eyes open and gaze slightly downwards. Acoustic isolation was ensured using earplugs and headphones. Brain electrical activity was recorded with silver disc electrodes from Fz, Cz, Pz (versus linked earlobes, A1A2), T3 and T4 (versus Fz) according to the international 10–20 system, and digitized with a sampling rate of 1024 Hz and a conversion of 12 bit, giving a resolution of 0.195 μV digit−1 (System Plus; Micromed, Treviso, Italy). The electrode impedance was always kept below 5 kΩ. In order to monitor ocular movements or eye-blinks and subsequently correct contaminated trials, electro-oculographic (EOG) signals were simultaneously recorded with surface electrodes, with the active electrode over the mid lower eyelid and the reference 1 cm lateral to the lateral corner of the orbit.
EEG data analysis
EEG data were imported and all analyses carried out using EEGLAB (http://www.sccn.ucsd.edu/eeglab), an open-source toolbox running under MATLAB environment (Delorme & Makeig, 2004).
Continuous EEG data were first down sampled to 256 Hz and band-pass filtered from 0.5 to 50 Hz. EEG epochs containing the somatosensory stimuli were subsequently extracted using a window analysis time of 2 s (from 500 ms before the stimulus to 1500 ms post stimulus). For each epoch, a baseline correction for the data preceding the stimulus by 500 ms was performed. Epoched EEG recordings were visually inspected and trials contaminated with artefacts due to gross movements were removed. Trials contaminated by artefacts due to eye blinks were corrected using an independent component analysis (ICA) algorithm (Jung et al. 2001). In all datasets where this procedure was performed, individual eye movements could be seen in the independent component (IC) removed. The IC removed also had a large EOG channel contribution and a frontal scalp distribution.
To identify LEP waveforms we used two different procedures: standard averaging of single EEG sweeps and automated single-trial analysis of each EEG sweep.
Standard averaging was performed time-locked to the stimulation onset. In standard averaged data we measured the peak latency and the baseline-to-peak amplitude of the early response (N1 wave) at the temporal electrode contralateral to the stimulated side (T4 against Fz), and the peak latencies and the baseline-to-peak amplitudes of the late negative (N2) and positive (P2) waves of the late response at the vertex (Cz against A1A2). Standard averaged data were finally averaged across subjects to obtain group-level waveforms.
Single-trial analysis was performed using an original multiple linear regression approach. This method offers the important advantage of providing a simple, fast and unbiased measurement of single-trial evoked potential (EP) responses. In EP responses characterized by significant latency jitter (like pain-related EPs recorded in the present study) single-trial analysis enables disclosure of the biological information which is normally lost because of the decrease in amplitude, waveform distortion and inaccurate peak latency estimation resulting from traditional averaging (Iannetti et al. 2005; Mayhew et al. 2006). Briefly, for each subject, a basis set derived from the time-averaged data is regressed against every single-trial EP response. This provides a quantitative estimate of peak latencies and baseline-to-peak amplitudes of vertex N2 and P2 waves for each trial. For further details on this novel analytical tool, see a recent study from our group (Mayhew et al. 2006).
Trial-to-trial consistency of the main N2–P2 vertex response was qualitatively assessed by sorting single-trial responses vertically in order of occurrence, with signal amplitude colour-coded and smoothed across trials using a 10-trial rectangular moving average (Delorme & Makeig, 2004).
Statistical analysis
As both LEP and CHEP values and psychophysical ratings were distributed normally, their differences following hairy and glabrous skin stimulation were assessed by calculating paired Student's t tests. As the F test disclosed significantly different variances in latency and amplitude values of the N2 peak (P < 0.005 and P < 0.0001, respectively) and latency values of the P2 peak (P < 0.05) between the LEP and CHEP sessions, their difference was assessed by calculating a t test with Welch's correction for unequal variances.
In order to facilitate comparison of variations of responses with different latency and amplitude values, normalized percentage differences in EP values between hairy and glabrous skin stimulation were calculated, using the stimulation of hairy skin as baseline with the following formula: (EPpalm × 100/EPdorsum) − 100. Similarly, normalized percentage differences between LEP and CHEP values were calculated using the LEP values as baseline, with the following formula: (EPcontact × 100/EPlaser) − 100.
All statistical analyses were conducted using Prism 4.0 (Graphpad, Sorrento Valley, CA, USA). All values are given as arithmetic mean ± s.d.
Results
Quality and intensity of sensation
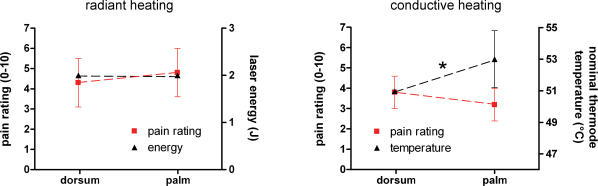
Laser stimulation of both hairy and glabrous skin at 2 J elicited a clear, pinprick sensation in all subjects. The average intensity rating to laser stimuli across all subjects was 4.3 ± 1.8 following hairy skin stimulation and 4.8 ± 1.8 following glabrous skin stimulation (Fig. 1). Ratings to hairy and glabrous skin laser stimulation were not significantly different (P > 0.2, paired t test).
Figure 1. Intensity of heat stimulation and perceived sensation.
Left graph, radiant heat (Nd:YAP laser) stimulation of hairy and glabrous skin. x axis, site of stimulation; left y axis, pain rating; right y axis, energy of laser stimulation. Right graph, contact heat (CHEP device) stimulation of hairy and glabrous skin. x axis, site of stimulation; left y axis, pain rating; right y axis, nominal temperature at the thermode surface (°C). Asterisk indicates significant difference (P < 0.05, paired t test). In order to achieve similar pain ratings, glabrous skin contact heat stimuli had to be delivered at significantly higher surface temperatures.
Contact heat stimulation of hairy skin (nominal surface temperature, 51°C) elicited a pinprick sensation in all subjects, although this sensation was consistently described as different (‘less fast’, ‘less pricking’) from the sensation elicited by laser stimuli. The average intensity rating to contact heat stimulation of hairy skin across all subjects was 3.8 ± 0.8. Because in preliminary experiments we observed that the nominal temperature used for hairy skin stimulation (51°C) was often not able to elicit a matched perceived intensity or to evoke reliable brain potentials when glabrous skin was stimulated, the temperature of glabrous skin stimulation was increased and adjusted for each subject (average nominal temperature across subjects, 53 ± 1.8°C; range, 51–55°C); this difference in temperature between hairy and glabrous skin stimulation was significant (P < 0.05, paired t test). The average intensity rating to contact heat stimulation of glabrous skin across all subjects was 3.2 ± 0.8. Ratings to hairy and glabrous skin contact heat stimulation were not significantly different (P > 0.2, paired t test, Fig. 1).
Waveforms of LEPs and CHEPs
Standard averaging
In all subjects, standard averaging analysis easily disclosed clear and reproducible LEPs time-locked to stimulus onset, following both hairy and glabrous skin stimulation. On the grand averages, the earliest identifiable scalp peak was the early latency negative wave (N1) visible in EEG data recorded from the right and left temporal leads, with a latency of 165 ms (following both hairy and glabrous skin stimulation) and an amplitude of 5.9 μV following hairy skin stimulation and 4.5 μV following glabrous skin stimulation. The N1 wave was followed by the late negative–positive complex (N2–P2) in the midline (Fz, Cz and Pz) leads; the N2 latency was approximately 210 ms following hairy skin stimulation and 215 ms following glabrous skin stimulation; the P2 latency was approximately 320 ms following hairy skin stimulation and 350 ms following glabrous skin stimulation; the N2, P2 and peak-to-peak (N2–P2) amplitudes (all maximal at Cz) were approximately 16, 18 and 34 μV following hairy skin stimulation and 14, 17 and 31 μV following glabrous skin stimulation (Figs 2 and 3).
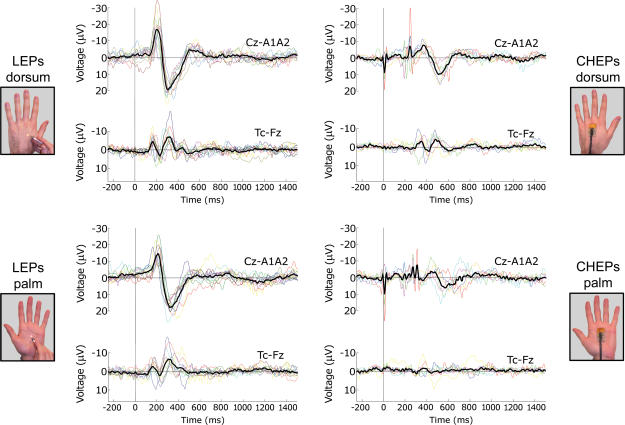
Figure 2. Single-subject waveforms and grand averages.
Brain potentials were evoked by thermal stimulation with radiant heating (laser-evoked potentials, LEPs; left column) or conductive heating (contact heat-evoked potentials, CHEPs; right column). Thermal stimuli were applied to the hairy skin (upper row) and to the glabrous skin (lower row). Brain potentials are averaged time-locked to the onset of the laser pulse or to the temperature increase of the thermode. Displayed signals are recorded from the vertex (Cz versus linked earlobes, A1A2), and from contralateral (right) temporal electrode (T4 against Fz). The coloured waveforms represent single subjects, whereas the black waveform is the grand average across subjects. Both LEPs and CHEPs show an important between-subject latency jitter, especially significant when conductive heating is applied to glabrous skin (lower right panel). This finding strongly indicates the appropriateness of a single subject-based analysis approach.
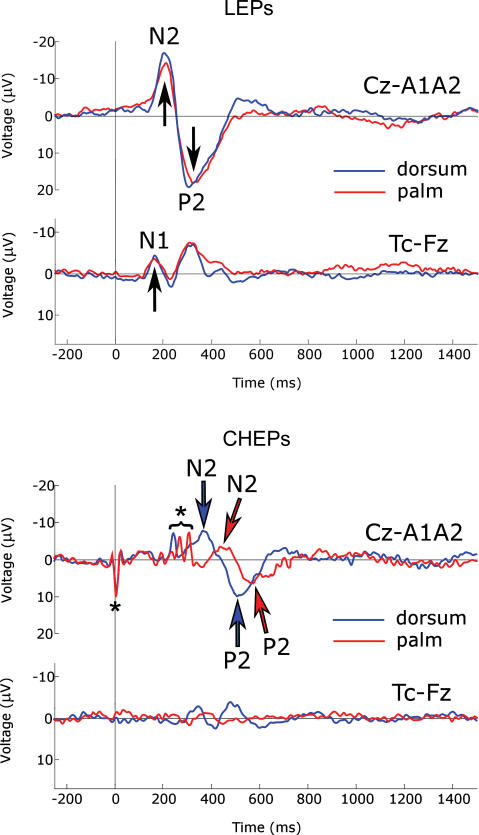
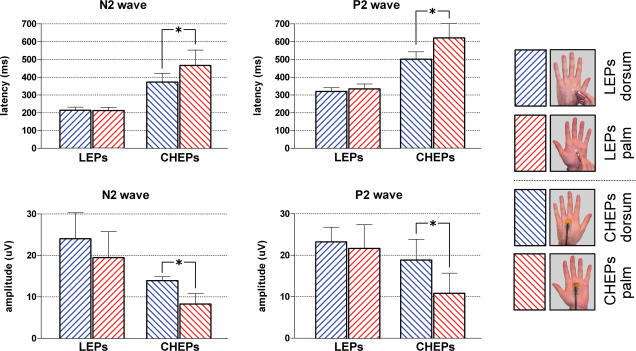
Figure 3. Comparison of grand-average waveforms.
Grand averages of LEPs (upper panel) and CHEPs (lower panel) following stimulation of hairy skin (blue signal) and glabrous skin (red signal). In each panel top waveforms are recorded from the vertex (Cz versus linked earlobes, A1A2), and bottom waveforms are recorded from the contralateral (right) temporal electrode (T4 versus Fz). Negativity is plotted upwards. Arrows indicate N1, N2 and P2 peaks. N2 and P2 peaks of CHEPs have a significantly longer latency and smaller amplitude than N2 and P2 peaks of LEPs, for both hairy and glabrous skin stimulation. In addition, N2 and P2 peaks of CHEPs following glabrous skin stimulation have significantly longer latency and smaller amplitude than N2 and P2 peaks of CHEPs following hairy skin stimulation. Details of P values of statistical comparisons are given in the Results. Note the lack of early N1 component following contact heat stimulation of glabrous skin. Asterisks indicate CHEP stimulation artefacts.
Standard averaging analysis disclosed clear CHEPs time-locked to stimulus onset in all subjects following hairy skin stimulation only. On the grand average, N1 was visible in EEG data recorded from the right and left temporal leads, with a latency of 350 ms and an amplitude of 3.6 μV. The N1 wave was followed by the late negative–positive complex (N2–P2) in the midline (Fz, Cz and Pz) leads, with an N2 latency of approximately 380 ms and a P2 latency of approximately 510 ms; N2, P2 and peak-to-peak (N2–P2) amplitudes (all maximal at Cz) were 8, 10 and 18 μV (Figs 2 and 3). In contrast, grand average of CHEPs following glabrous skin stimulation was strongly affected by the important between-subjects latency jitter of the response, which was not consistently identifiable in single-subject average waveforms (Fig. 2). The N1 wave was not detectable. N2 had a latency of approximately 460 ms and P2 a latency of approximately 570 ms; N2, P2 and N2–P2 amplitudes (all maximal at Cz) were 4, 7 and 11 μV (Figs 2 and 3).
Because of the inadequacy of standard averaging for the analysis of non-stationary evoked potentials (Purves & Boyd, 1993; Iannetti et al. 2005) and the availability of a robust algorithm to extract N2 and P2 peak single-trial information from LEPs (Mayhew et al. 2006), all the results described later in this paper were obtained from single-trial N2 and P2 latency and amplitude values. The grand average waveforms and their corresponding latency and amplitude values described in the present paragraph and shown in Figs 2 and 3 are reported to facilitate comparison with previous reports using similar stimulation techniques.
Single-trial analysis
Within-subject and between-subject averages of single-trial LEP and CHEP values are shown in Figs 4 and 5. Within-subject average and standard deviation of single-trial LEP and CHEP values after hairy and glabrous skin stimulation are shown in Fig. 8, to compare within-subject variability. Across-subject averages and standard deviations are reported in Table 1 to show between-subject variabilty.
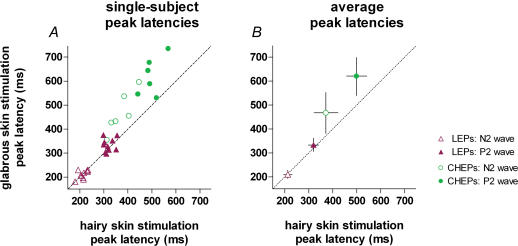
Figure 4. Peak latency of laser-evoked potentials (LEPs) and contact heat-elicited potentials (CHEPs) elicited by stimulation of hairy and glabrous skin of the hand.
x axis, peak latency (ms) after hairy skin stimulation; y axis, peak latency (ms) after glabrous skin stimulation. The left graph shows the average of N2 and P2 single-trial latencies measured from the vertex (Cz versus linked earlobes, A1A2) for each subject. The right graph shows the average (± s.d.) of peak latencies across subjects. The dashed line represents the identity line. CHEPs following glabrous skin stimulation have significantly longer latencies than CHEPs following hairy skin stimulation (N2 peak P < 0.005, P2 peak P < 0.01, paired t test).
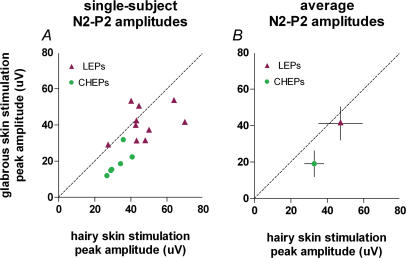
Figure 5. Amplitude of laser-evoked potentials (LEPs) and contact heat-evoked potentials (CHEPs) evoked by stimulation of hairy and glabrous skin of the hand.
x axis, N2–P2 peak-to-peak amplitude (μV) after hairy skin stimulation; y axis, N2–P2 peak-to-peak amplitude (μV) after glabrous skin stimulation. The left graph shows the average of single-trial amplitudes measured from the vertex (Cz versus linked earlobes, A1A2) for each subject. The right graph shows the average (± s.d) of amplitudes across subjects. The dashed line represents the identity line. CHEPs following glabrous skin stimulation have significantly smaller amplitude than CHEPs following hairy skin stimulation (P < 0.005, paired t test).
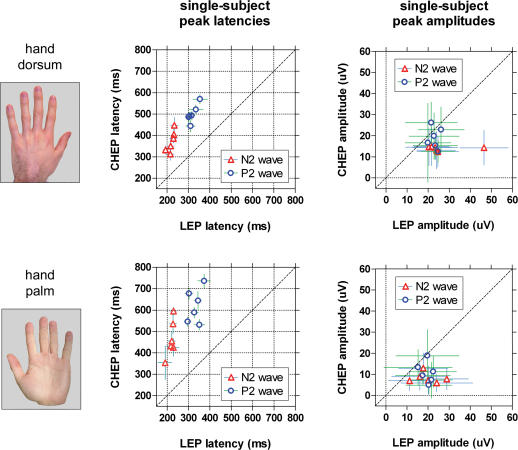
Figure 8. Single-subject averages of single-trial values of N2 and P2 peaks of laser-evoked potentials (LEPs) and contact heat-evoked potentials (CHEPs) following stimulation of hairy and glabrous skin.
Data from the six subjects in whom both LEPs and CHEPs were recorded. Single-trial values were measured from the vertex (Cz versus linked earlobes, A1A2) using a novel automated approach based on multiple linear regression (Mayhew et al. 2006). Each symbol represents a subject; error bars show within-subject variability (expressed as s.d.). Dashed line represents the identity line. Left graphs display N2 and P2 latency values: x axis, LEP latency (ms); y axis, CHEP latency (ms). Right graphs display N2 and P2 amplitude values: x axis, LEP amplitude (μV); y axis, CHEP amplitude (μV). Upper and lower rows represent responses following hairy and glabrous skin stimulation, respectively. Note the higher between-subject variability in latency and the smaller amplitude of the response when conductive heating instead of radiant heating is applied. This important latency jitter strongly indicates the appropriateness of a single-trial analysis approach.
Table 1.
Latencies and amplitudes of brain responses evoked by noxious radiant heat (LEPs) and contact heat (CHEPs) stimulation of the hairy and glabrous skin of the hand in healthy subjects
| N2 wave | P2 wave | N1 wave* | ||||
|---|---|---|---|---|---|---|
| Latency (ms) | Amplitude (μV) | Latency (ms) | Amplitude (μV) | Latency (ms) | Amplitude (μV) | |
| LEPsdorsum | 213 ± 17 | 24 ± 9.9 | 320 ± 21 | 23.2 ± 3.6 | 170 | 5.9 |
| LEPspalm | 211 ± 17 | 19.5 ± 6.3 | 334 ± 28 | 21.7 ± 5.7 | 162 | 4.3 |
| CHEPsdorsum | 372 ± 50 | 13.9 ± 0.9 | 500 ± 42 | 18.9 ± 5 | 350 | 3.6 |
| CHEPspalm | 466 ± 86 | 8.3 ± 2.5 | 620 ± 80 | 10.8 ± 4.9 | — | — |
Values are the average of single-subject, single-trial data ±s.d.; s.d. shows the variability between subjects.
Latency and amplitude values are measured from standard-averaged data. LEPsdorsum, LEPspalm, CHEPsdorsum, CHEPspalm.
For both LEPs and CHEPs, the averages of single-trial latency values were similar to those measured in the standard averaging. In contrast, the averages of single-trial amplitude values were far higher than the corresponding ones measured in the standard averaging (e.g. LEPs following hairy skin stimulation: N2 latency, 213 versus 210 ms; N2 amplitude, 24 versus 17 μV; P2 latency, 320 versus 320 ms; P2 amplitude, 23.3 versus 18 μV, respectively). These results confirm previous reports comparing single-trial and standard-average measurements of LEPs (Iannetti et al. 2005; Mayhew et al. 2006).
N2 and P2 peak latency values of CHEPs showed a higher temporal jitter than the corresponding LEP peaks, both within and between subjects (i.e. CHEPs are less stationary than LEPs, Figs 6–8), indicating that a single-trial approach is even more valuable when analysing EEG responses evoked by contact heat stimulation.
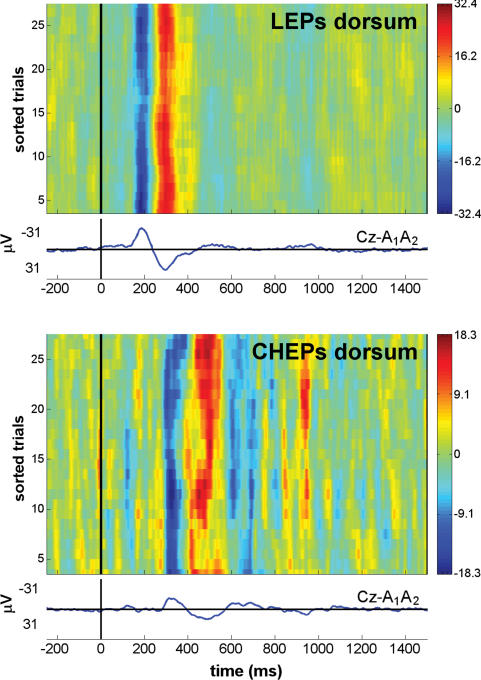
Figure 6. Reproducibility of single-trial LEP and CHEP response.
Recordings from the vertex (Cz versus linked earlobes, A1A2) in the same subject, showing the N2 and P2 waves. Upper panel, LEPs after hairy skin stimulation. Lower panel, CHEPs after hairy skin stimulation. To assess the trial-to-trial consistency, one bidimensional plot of single-trial responses is shown for each condition. Horizontal lines in the plot represent single-trial responses, with signal amplitude colour-coded at each time point. Responses are sorted vertically in order of occurrence, from bottom (first trial) to top (last trial), and between-trials smoothing with a 10-trial rectangular moving average was applied. The waveform below each plot is the average of all responses. Negativity is plotted upwards. The difference in the limits of the colour scale between upper and lower panels, due to the smaller amplitude of the CHEP response, is the reason for the apparently noisier background EEG in the CHEP recording. Note the considerably higher latency jitter of the single-trial CHEP responses, due to the slower rising time of the skin temperature after conductive heating. The higher latency jitter is partially responsible for the smaller amplitude of CHEPs than LEPs, because it causes the averaging of signals in different phase. This observation indicates that a single-trial approach is highly desirable to analyse CHEP responses.
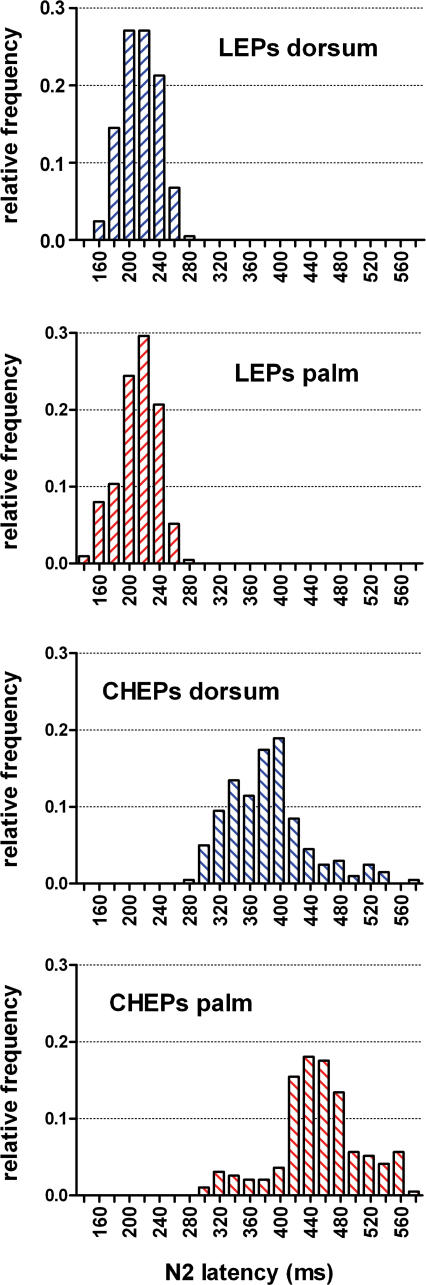
Figure 7. Frequency distribution of N2 single-trial latencies.
Single-trial latency values were measured from the vertex (Cz versus linked earlobes, A1A2) using a novel automated algorithm based on multiple linear regression (Mayhew et al. 2006). x axis, N2 latency (ms); y axis, relative frequency; bin size, 20 ms; centre of first bin, 0 ms. Note the longer latency and the higher latency jitter of single-trial CHEPs compared to single-trial LEPs.
Comparison between hairy and glabrous skin stimulation
LEP and CHEP values are shown in Figs 4, 5 and 9, and Table 1.
Figure 9. Comparison of latency (upper graphs) and amplitude values (lower graphs) of laser-evoked potentials (LEPs) and contact heat-evoked potentials (CHEPs) evoked by stimulation of hairy and glabrous skin of the hand.
Values are the average across subjects of N2 and P2 single-trial responses, measured from the vertex (Cz versus linked earlobes, A1A2) using an automated algorithm based on multiple linear regression. Error bars indicate s.d. Only CHEPs following glabrous skin stimulation have significantly longer latencies (N2, P < 0.005, P2, P < 0.01, paired t test) and smaller amplitudes (N2 and P2, P < 0.005, paired t test) than CHEPs following hairy skin stimulation.
None of the explored LEP values (N2 and P2 latencies and amplitudes) were significantly different between hairy and glabrous skin stimulation (P > 0.5, paired t test) (Fig. 9). Normalized percentage differences in LEP values between hairy and glabrous skin stimulation were calculated by using LEP responses from the stimulation of hairy skin as baseline, and were as follows: N2 latency, −0.58 ± 7.96%; P2 latency, +4.86 ± 10.44%; N2 amplitude, −13.67 ± 24.07%; P2 amplitude, 5.95 ± 22.68%.
In contrast, contact heat stimuli applied to glabrous skin yielded significantly longer N2 and P2 latencies (N2, +94 ms, P < 0.005; P2, +120 ms, P < 0.01, paired t test), and significantly smaller N2 and P2 amplitudes (N2, −5.7 μV, P < 0.005; P2, −8.1 μV, P < 0.005, paired t test) compared to contact heat stimuli applied to hairy skin (Fig. 9). Normalized percentage differences in CHEP values between hairy and glabrous skin stimulation were calculated by using CHEP responses from the stimulation of hairy skin as baseline, and were as follows: N2 latency, +24.81 ± 10.64%; P2 latency, +24.09 ± 12.74%; N2 amplitude, −39.95 ± 20.34%; P2 amplitude, −44.38 ± 15.4%.
Comparison between LEPs and CHEPs
LEP and CHEP values are shown in Figs 4 and 5, and Table 1. N2 and P2 latency values of LEPs were significantly shorter than the corresponding values of CHEPs, for both hairy and glabrous skin stimulation (N2dorsum and N2palm, P < 0.001; P2dorsum and P2palm, P < 0.0005, unpaired t test with Welch's correction for unequal variances). N2 and P2 amplitude values of LEPs were significantly larger than the corresponding values of CHEPs, for both hairy and glabrous skin stimulation (N2dorsum, P < 0.05, unpaired t test with Welch's correction; N2palm, < 0.0005, unpaired t test with Welch's correction; P2dorsum, P < 0.05, unpaired t test; P2palm, P < 0.005, unpaired t test).
Normalized percentage differences between LEP and CHEP average values were calculated by using LEP values as baseline, and were as follows: N2dorsum latency, +75.47%; N2palm latency, +120.85%; N2dorsum amplitude, −41.67%; N2palm amplitude, −57.95%; P2dorsum latency, +56.25%; P2palm latency, +85.33%; P2dorsum amplitude, −18.7%; P2palm amplitude, −50.91%.
Discussion
Our results show that relatively short-wavelength (1.34 μm) radiant laser pulses of identical energy directed to the dorsum and the palm of the hand elicit qualitatively and quantitatively similar pain sensations, and evoke brain potentials of similar latency and amplitude. This finding indicates that first pain to heat does exist in glabrous skin, and suggests that similar nociceptive afferents mediate psychophysical and electrophysiological responses to thermal stimulation of glabrous and hairy skin in humans.
In addition, contact heat stimuli applied to the dorsum and the palm of the hand elicit pain sensations and evoke brain potentials as well, although with significantly lower signal-to-noise ratio compared to laser-evoked potentials. However, the palm has to be stimulated with a significantly higher thermode temperature than the dorsum in order to obtain similar pain sensations, and despite this higher temperature of stimulation brain responses have significantly longer latencies and smaller amplitudes. These findings are consistent with the thickness-dependent delay and attenuation of the temperature waveform at nociceptor level when conductive heating is applied. We suggest that the previously reported lack of first pain and microneurographical II-AMH responses following glabrous skin stimulation has been the result of a search bias consequent to the use of conductive heating and long-wavelength radiant heating (i.e. CO2 laser) as stimulation procedures.
Contact versus radiant heat nociceptive stimulation of hairy skin
Noxious heat stimuli are frequently used to study the nociceptive system, because they activate a nociceptive-specific transduction mechanism (Julius & Basbaum, 2001). Nociceptive nerve endings can be heated by either thermal conduction or thermal radiation. The advent of devices able to raise quickly the skin temperature by either infrared thermal radiation (laser stimulators) or thermal conduction (contact thermodes) allowed the recording of stimulus-evoked brain potentials (LEPs and CHEPs) in the electroencephalogram (Carmon et al. 1978; Chen et al. 2001). Whereas the possibility of recording CHEPs has been reported only recently (Chen et al. 2001), LEPs have been extensively used in basic and clinical research to study the temporal dynamics of nociceptive processing, and to date are the best available tool for assessing the function of nociceptive pathways in patients (Cruccu et al. 2004).
Our results show that, matching intensity of perception and stimulated territory, LEPs have a significantly higher signal-to-noise ratio than CHEPs (Figs 1–3 and 6). This finding is consistent with the different biophysical properties of radiant versus conductive heat–skin interactions. Compared to conductive heating, the infrared radiation produced by modern solid-state lasers (such as thulium-YAG or neodymium-YAP) has the following advantages, which are relevant for the interpretation of the results reported in the present study.
(1) Radiant heat activates the nociceptive afferents in a selective fashion (Plaghki & Mouraux, 2003). In contrast, contact thermodes unavoidably produce concomitant stimulation of low-threshold mechanoreceptors, which modulate the spinal transmission of both nociceptive and heat information (Nathan et al. 1986).
(2) Because the laser energy is confined to a narrow beam of nearly parallel monochromatic electromagnetic waves (i.e. the energy fluence is extremely high), the rise of surface skin temperature is particularly fast (≫ 1000°C s−1, compared to 70°C s−1 of fastest contact thermodes, Chen et al. 2001).
(3) Infrared radiation reaches directly nociceptive terminals, which terminate between 20 and 570 μm below the skin surface (Tillman et al. 1995). Given that thulium-YAG and neodymium-YAP radiations have a respective extinction length of 350 and > 500 μm (Baumgartner et al. 2005), nociceptive terminals are thus activated in a direct and rapid fashion (Spiegel et al. 2000; Iannetti et al. 2004).
(4) As a consequence of (3), the temperature increase induced at the specific receptor depth by infrared radiation is reproducible between trials (although not necessarily reproducible between subjects when relatively short wavelengths are used, because of differences in skin reflectance). In contrast, because contact heat stimulators receive a feedback temperature signal from a thermocouple embedded in the surface of the stimulator, the temperature waveform at the nociceptor level is both delayed and attenuated by thermal conduction between the skin surface and nociceptive nerve terminals (Magerl & Treede, 1996). Notably, these time and intensity differences between temperature profiles at surface and nociceptor level become more pronounced as the heating rate increases (Tillman et al. 1995). Thus, the faster the rate of conducting heating applied, the more difficult the prediction of temperature at nociceptor level.
(5) When lasers with sufficiently high extinction lengths are applied, the temperature waveform at nociceptor level is relatively independent of thickness of the epidermis; in other words, the epidermis is transparent to this kind of radiant heat.
Following hairy skin stimulation, we observed that CHEPs had significantly longer latencies and significantly smaller amplitudes than LEPs (Figs 2–5 and 8, and Table 1).
The average latency of the first negative CHEP peak at the vertex (N2) was 159 ms longer than the corresponding LEP peak (Figs 4B and 9 and Table 1). The heat ramp of the thermode lasted 214 ms (i.e. the time spent in order to reach the target temperature (51°C) from the baseline temperature (36°C) at a rate of 70°C s−1). Because II-AMHs have a median thermal threshold of ∼46°C (Treede et al. 1995; 1998), their activation is expected to happen not earlier than the second half of this 214 ms- long ramp. In addition, because of the reasons outlined above, the temperature rise at nociceptor depth is expected to be delayed by thermal conduction from the skin surface. Besides the direct effect of rise time, a further contribution to the longer latency of the CHEP response may be ascribed to the weaker spatial and temporal summation of the nociceptive input (synchronization effect). Because of the physiological variance in nociceptor thresholds, the delayed temperature profile induced by contact heat stimuli excites nociceptors with slightly higher thresholds with a longer delay, making the afferent volley less synchronized and exerting a less effective spatial summation at central synapses.
The average amplitude of the first negative CHEP peak at the vertex (N2) was 10.1 μV smaller than the corresponding LEP peak (Fig. 9 and Table 1). This finding is certainly the result of at least two phenomenona. First, a smaller synchronization effect (i.e. weaker spatial and temporal summation of the afferent volley at the central synapses) when conductive heating is applied; this is consistent with the previously reported reduction of the amplitude of the brain and psychophysical responses when heat stimuli of the same energy but longer duration are applied (Pertovaara et al. 1988; Treede et al. 1994; Iannetti et al. 2004). Second, a more important nociceptor fatigue and habituation during contact heat stimulation; this is consistent with a recent report of significantly smaller subjective ratings and CHEP amplitudes when contact heat stimuli are applied to a fixed surface (i.e. as in the present study) as compared to when their position was varied after each trial (Greffrath et al. 2006).
It is worth highlighting that all the results described here are obtained from an automatic and unbiased analysis of single-trial N2 and P2 latency and amplitude values (Mayhew et al. 2006). This novel approach allowed us to compare fairly the amplitude values of LEP and CHEP peaks of single trials, without incurring the bias that is normally introduced by the latency jitter when EP amplitude values are measured in standard, time-locked averages. As the latency jitter of nociceptive-related response is significant because of the relatively slow conduction velocity of nociceptive fibres (Purves & Boyd, 1993), the signal averaged across trials is blurred, and its amplitude is lower than the average of amplitudes of single-trial responses (mostly because the latency jitter between trials causes the averaging of signals which are out of phase, Iannetti et al. 2005). Accordingly, in the present study, the average amplitude of single-trial EPs was bigger than the amplitude of standard averages (e.g. after hairy skin stimulation: LEPs: N2standard, 16 μV; N2single-trial, 24 μV; CHEPs: N2standard, 8 μV; N2single-trial, 14 μV). Given that CHEPs showed a higher latency jitter than LEPs both within and between subjects (Figs 4 and 6–8), if standard averaged data was used, overestimation of the reduction of amplitude observed in CHEPs (e.g. N2 after hairy skin stimulation: standard amplitudes, 16 versus 8 μV; single-trial amplitudes, 24 versus 14 μV) would occur. Independently of the modality of heating, these findings clearly indicate the need for a single-trial approach in the analysis of heat-evoked potentials, especially when meaningful and unbiased comparisons between conditions with different latencies and signal-to-noise ratios are required (e.g. experimental modulations of response amplitudes, and lesions of the nociceptive pathways in clinical practice).
Evidence for II-AMHs in glabrous skin
There is little doubt that II-AMHs are the peripheral afferents responsible for first pain sensation to heat in hairy skin, and that they conduct the volley that elicits LEPs in the central nervous system. II-AMHs are the only heat nociceptors with short response latency and graded response to heat (Treede et al. 1998), and their threshold distribution matches the threshold distribution of pricking pain and late LEPs (Treede et al. 1994).
Microneurographical recordings in monkeys failed to detect II-AMH responses to CO2 laser heat stimuli applied to glabrous skin (Treede et al. 1995) and first pain sensation is not evoked when CO2 laser heat stimuli are applied to the palm of the hand in humans (Campbell & LaMotte, 1983). Taken together these findings have led to the notion that first pain and II-AMHs are lacking in primate glabrous skin (Meyer et al. 2006).
Our results challenge this notion, because they show that Nd:YAP laser stimuli of identical energy applied to the dorsum and the palm of the hand elicit qualitatively and quantitatively indistinguishable sensations (Fig. 1), and elicit brain potentials with extremely similar latency and amplitude values (Fig. 3 and Table 1). It is worth noting that variations in baseline temperature can lead to misinterpretations when radiant heat stimuli are applied to the skin (Tjolsen et al. 1988), because the differential temperature increase induced by radiant heating is independent of the baseline skin temperature (i.e. the stimulus-induced temperature increase adds to the baseline, see Fig. 2 in Iannetti et al. 2004). We excluded this potential confounding factor from our results, by ensuring that baseline hairy and glabrous skin temperatures were similar at the beginning and at the end of the recording session (difference always < 1°C).
These findings provide strong evidence for the existence, in human glabrous skin, of a population of heat nociceptors with physiological features (activation threshold, response latency, spatial distribution and conduction velocity) very similar to II-AMHs, and indicate that these afferents mediate first pain to heat.
The notion of the lack of first pain to heat in glabrous skin is puzzling, when examined from a finalistic perspective. The ability to appreciate and react appropriately to the contact between the palm of the hand and hot objects seems especially important for survival, particularly in animals with an extreme ability for manipulation like primates. Indeed, a well-timed withdrawal reflex when the palm of the hand gets in contact with a hot saucepan when cooking is a common experience of everyday life. Our results provide experimental evidence for the neural circuitry subserving this common behaviour, and indicate that first pain to heat in glabrous skin does exist.
We believe that previous negative results could have been due to a search bias explained by the biophysics of heat skin interactions. Both microneurographical and psychophysical experiments that have failed to reveal fast responses to heat when glabrous skin was stimulated have been conducted using a CO2 laser controlled by radiometric feedback, with a temperature rise time of approximately 100 ms (Meyer et al. 1976). CO2 laser has a wavelength in the far infrared (10.6 μm). Because of the characteristics of reflectance, transmissions and absorption of the human epidermis, at this wavelength the extinction length is in the order of single micrometers (Hardy & Muschenheim, 1934; Plaghki & Mouraux, 2003) (i.e. radiation is nearly extinct well above the depth where nociceptive free nerve endings terminate; 20–570 μm below skin surface in hairy skin (Tillman et al. 1995)). For these reasons, the heating induced by a CO2 infrared radiation behaves rather similarly to the heating induced by a very fast contact heat stimulus (although the heat ramp of the CO2 stimulus can be several orders of magnitude greater than that of a contact thermode): once the CO2 radiation is absorbed in the very first micrometers of the epidermis, the heating is subsequently transmitted by thermal conduction to the deeper epidermal layers where the activation of nociceptors occurs. During this transmission, the temperature waveform is progressively delayed and attenuated in a depth-dependent process, as has been shown in simulation and modelling studies (Bromm & Treede, 1984; Spiegel et al. 2000; Plaghki & Mouraux, 2003). As a consequence, when CO2 radiant heat stimuli of an intensity effectively eliciting first pain in hairy skin are applied to skin territories where a thicker epidermal layer is interposed between nociceptors and the skin surface (such as the glabrous skin of the palm, where the thickness of the stratum corneum is at least twice that in hairy skin, Whitton & Everall, 1973; Nouveau-Richard et al. 2004; Mountcastle, 2005), they can easily fail to reach the nociceptor activation threshold. Alternatively, the threshold is reached but without an optimal heat ramp profile, especially when pulses with relatively long rise times are applied (e.g. 100 ms in Treede et al. 1995; between 200 and 250 ms in Campbell & LaMotte, 1983). We believe that these mechanisms could have constituted a bias in the search for the neural afferents mediating first pain sensations to heat in glabrous skin. This hypothesis is strengthened by several observations. First, contact heat stimuli of identical intensity elicit lower pain ratings when applied to glabrous skin, and only when the nominal thermode temperature is significantly increased similar pain ratings (although qualitatively described as ‘less pricking’ than hairy skin stimulation) are obtained (Fig. 1). Second, when glabrous skin is stimulated, CHEPs show a slower latency with a bigger jitter, and a smaller amplitude than CHEPs following hairy skin stimulation (Figs 3–5 and 9). Reasons for these findings are the longer delay and the higher variability in nociceptor activation time when the temperature profile is delayed and attenuated by the transmission through the thicker stratum corneum of the hand palm. Third, short-lasting CO2 laser stimuli at the intensity sufficient to elicit LEPs following hand dorsum stimulation are not able to elicit LEPs following palm stimulation (G. Cruccu, personal communication), and only when the intensity of stimulation is significantly increased, LEPs to glabrous skin stimulation appear (Towell et al. 1996).
Although only dedicated microneurographical recordings using short-wavelength laser pulses as test stimuli will permit a full characterization of the response properties of nociceptors mediating first pain and late LEPs following glabrous skin stimulation, our results provide strong evidence that a population of nociceptors with properties very similar to II-AMHs is responsible for the sensation of first pain to heat in glabrous skin in humans.
Acknowledgments
The authors wish to thank Mike Lee and Richard Wise for their invaluable feedback on this manuscript. This study was partly supported by Pfizer UK Ltd (G.D.I.) and Higher Education Funding Council for England (I.T.).
References
- Baumgärtner U, Cruccu G, Iannetti GD, Treede RD. Laser guns and hot plates. Pain. 2005;116:1–3. doi: 10.1016/j.pain.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Bromm B, Treede RD. Nerve fibre discharges, cerebral potentials and sensations induced by CO2 laser stimulation. Human Neurobiol. 1984;3:33–40. [PubMed] [Google Scholar]
- Campbell JN, LaMotte RH. Latency to detection of first pain. Brain Res. 1983;266:203–208. doi: 10.1016/0006-8993(83)90650-9. [DOI] [PubMed] [Google Scholar]
- Carmon A, Dotan Y, Sarne Y. Correlation of subjective pain experience with cerebral evoked responses to noxious thermal stimulations. Exp Brain Res. 1978;33:445–453. doi: 10.1007/BF00235566. [DOI] [PubMed] [Google Scholar]
- Chen AC, Niddam DM, Arendt-Nielsen L. Contact heat evoked potentials as a valid means to study nociceptive pathways in human subjects. Neurosci Lett. 2001;316:79–82. doi: 10.1016/s0304-3940(01)02374-6. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Anand P, Attal N, Garcia-Larrea L, Haanpaa M, Jorum E, Serra J, Jensen TS. EFNS guidelines on neuropathic pain assessment. Eur J Neurol. 2004;11:153–162. doi: 10.1111/j.1468-1331.2004.00791.x. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Pennisi E, Truini A, Iannetti GD, Romaniello A, Le Pera D, De Armas L, Leandri M, Manfredi M, Valeriani M. Unmyelinated trigeminal pathways as assessed by laser stimuli in humans. Brain. 2003;126:2246–2256. doi: 10.1093/brain/awg227. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Greffrath W, Baumgärtner U, Treede RD. Habituation of human heat pain and evoked potentials reflects fatigue of peripheral nociceptors. Acta Physiol (Oxf) 2006;186(suppl. 1):121. [Google Scholar]
- Hardy JD, Muschenheim C. The Radiation of heat from the human body. IV. The emission, reflection, and transmission of infra-red radiation by the human skin. J Clin Invest. 1934;13:817–831. doi: 10.1172/JCI100624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetti GD, Leandri M, Truini A, Zambreanu L, Cruccu G, Tracey I. Adelta nociceptor response to laser stimuli: selective effect of stimulus duration on skin temperature, brain potentials and pain perception. Clin Neurophysiol. 2004;115:2629–2637. doi: 10.1016/j.clinph.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Truini A, Romaniello A, Galeotti F, Rizzo C, Manfredi M, Cruccu G. Evidence of a specific spinal pathway for the sense of warmth in humans. J Neurophysiol. 2003;89:562–570. doi: 10.1152/jn.00393.2002. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Zambreanu L, Cruccu G, Tracey I. Operculoinsular cortex encodes pain intensity at the earliest stages of cortical processing as indicated by amplitude of laser-evoked potentials in humans. Neuroscience. 2005;131:199–208. doi: 10.1016/j.neuroscience.2004.10.035. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk D, Melzack R, editors. Handbook of Pain Assessment. New York: The Guilford Press; 2001. pp. 15–34. [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ. Analysis and visualization of single-trial event-related potentials. Hum Brain Mapp. 2001;14:166–185. doi: 10.1002/hbm.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magerl W, Treede RD. Heat-evoked vasodilatation in human hairy skin: axon reflexes due to low-level activity of nociceptive afferents. J Physiol. 1996;497:837–848. doi: 10.1113/jphysiol.1996.sp021814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew SD, Iannetti GD, Woolrich MW, Wise RG. Automated single-trial measurement of amplitude and latency of laser-evoked potentials (LEPs) using multiple linear regression. Clin Neurophysiol. 2006;117:1331–1344. doi: 10.1016/j.clinph.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Ringkamp M, Campbell JN, Raja SN. Peripheral mechanisms of cutaneous nociception. In: McMahon SB, Koltzenburg M, editors. Wall and Melzalck's Textbook of Pain. Elsevier: Churchill Livingstone; 2006. pp. 3–34. [Google Scholar]
- Meyer RA, Walker RE, Mountcastle VB., Jr A laser stimulator for the study of cutaneous thermal and pain sensations. IEEE Trans Biomed Eng. 1976;23:54–60. doi: 10.1109/tbme.1976.324616. [DOI] [PubMed] [Google Scholar]
- Mountcastle V. The Sensory Hand. Neural Mechanisms of Somatic Sensation. Harvard University Press; 2005. [Google Scholar]
- Nathan PW, Smith MC, Cook AW. Sensory effects in man of lesions of the posterior columns and of some other afferent pathways. Brain. 1986;109:1003–1041. doi: 10.1093/brain/109.5.1003. [DOI] [PubMed] [Google Scholar]
- Nouveau-Richard S, Monot M, Bastien P, De Lacharriere O. In vivo epidermal thickness measurement: ultrasound vs. confocal imaging. Skin Res Technol. 2004;10:136–140. doi: 10.1111/j.1600-0846.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- Pertovaara A, Morrow TJ, Casey KL. Cutaneous pain and detection thresholds to short CO2 laser pulses in humans: evidence on afferent mechanisms and the influence of varying stimulus conditions. Pain. 1988;34:261–269. doi: 10.1016/0304-3959(88)90121-2. [DOI] [PubMed] [Google Scholar]
- Plaghki L, Mouraux A. How do we selectively activate skin nociceptors with a high power infrared laser? Physiology and biophysics of laser stimulation. Neurophysiol Clin. 2003;33:269–277. doi: 10.1016/j.neucli.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Purves AM, Boyd SG. Time-shifted averaging for laser evoked potentials. Electroencephalogr Clin Neurophysiol. 1993;88:118–122. doi: 10.1016/0168-5597(93)90062-t. [DOI] [PubMed] [Google Scholar]
- Spiegel J, Hansen C, Treede RD. Clinical evaluation criteria for the assessment of impaired pain sensitivity by thulium-laser evoked potentials. Clin Neurophysiol. 2000;111:725–735. doi: 10.1016/s1388-2457(99)00297-7. [DOI] [PubMed] [Google Scholar]
- Tillman DB, Treede RD, Meyer RA, Campbell JN. Response of C fibre nociceptors in the anaesthetized monkey to heat stimuli: estimates of receptor depth and threshold. J Physiol. 1995;485:753–765. doi: 10.1113/jphysiol.1995.sp020766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjolsen A, Berge OG, Eide PK, Broch OJ, Hole K. Apparent hyperalgesia after lesions of the descending serotonergic pathways is due to increased tail skin temperature. Pain. 1988;33:225–231. doi: 10.1016/0304-3959(88)90094-2. [DOI] [PubMed] [Google Scholar]
- Towell AD, Purves AM, Boyd SG. CO2 laser activation of nociceptive and non-nociceptive thermal afferents from hairy and glabrous skin. Pain. 1996;66:79–86. doi: 10.1016/0304-3959(96)03016-3. [DOI] [PubMed] [Google Scholar]
- Treede R, Meyer RL, Lesser RP. Similarity of threshold temperatures for first pain sensation, laser-evoked potentials and nociceptor activation. In: Gebhart GF, Hammond DC, Jensen TS, editors. Proceedings of the 7th World Congress on Pain. Progress in Pain Research and Management. Vol. 2. Seattle: IASP Press; 1994. pp. 857–865. [Google Scholar]
- Treede RD, Meyer RA, Campbell JN. Myelinated mechanically insensitive afferents from monkey hairy skin: heat-response properties. J Neurophysiol. 1998;80:1082–1093. doi: 10.1152/jn.1998.80.3.1082. [DOI] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Raja SN, Campbell JN. Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. J Physiol. 1995;483:747–758. doi: 10.1113/jphysiol.1995.sp020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton JT, Everall JD. The thickness of the epidermis. Br J Dermatol. 1973;89:467–476. doi: 10.1111/j.1365-2133.1973.tb03007.x. [DOI] [PubMed] [Google Scholar]