Abstract
Background
Microvascular injury plays a key role in normal tissue radiation responses. Statins, in addition to their lipid-lowering effects, have vasculoprotective properties that may counteract some effects of radiation on normal tissues. We examined whether administration of simvastatin ameliorates intestinal radiation injury, and whether the effect depends on protein C activation.
Methods
Rats received localized, fractionated small bowel irradiation. The animals were fed either regular chow or chow containing simvastatin from 2 weeks before irradiation until termination of the experiment. Groups of rats were euthanized at 2 weeks and 26 weeks for assessment of early and delayed radiation injury by quantitative histology, morphometry, and quantitative immunohistochemistry. Dependency on protein C activation was examined in TM mutant mice with deficient ability to activate protein C.
Results
Simvastatin administration was associated with lower radiation injury scores (p<0.0001), improved mucosal preservation (p=0.0009), and reduced thickening of the intestinal wall and subserosa (p=0.008 and p=0.004), neutrophil infiltration (p=0.04), and accumulation of collagen I (p=0.0003). The effect of simvastatin was consistently more pronounced for delayed than for early injury. Surprisingly, simvastatin reduced intestinal radiation injury in TM mutant mice, indicating that the enteroprotective effect of simvastatin after localized irradiation is unrelated to protein C activation.
Conclusions
Simvastatin ameliorates the intestinal radiation response. The radioprotective effect of simvastatin after localized small bowel irradiation does not appear to be related to protein C activation. Statins should undergo clinical testing as a strategy to minimize side effects of radiation on the intestine and other normal tissues.
Keywords: Endothelium, Intestine, Radiation injuries, Hydroxymethylglutaryl-CoA reductase inhibitors
Introduction
Radiation therapy is used in 70% of all cancer patients and plays a critical role in 25% of cancer cures (1). Despite the technological advances of dose-sculpting therapies, however, normal tissue toxicity remains the single-most important obstacle to uncomplicated cancer cure.
The gastrointestinal tract is a critical dose-limiting organ system during treatment of abdominal and pelvic tumors. Symptoms of acute bowel toxicity are common and may require de-intensification of the treatment plan and/or costly supportive therapies. Delayed bowel toxicity, while less common, is highly important clinically because of its chronic and progressive nature and substantial long-term morbidity and mortality.
The notion that normal tissue radiation responses result solely from induction of apoptosis and clonogenic death of target cells has been supplanted. Hence, it is now generally recognized that the pathophysiological manifestations of normal tissue radiation injury are the result of a complex interplay among many different processes that include activation of the coagulation system, inflammation, epithelial regeneration, tissue remodeling, and collagen deposition (2).
Radiation-induced endothelial injury has been the subject of considerable interest relative to both acute and chronic radiation toxicities in normal tissues. Radiation causes endothelial cell apoptosis at high doses (3). At lower, more clinically relevant radiation doses, there is increased expression of adhesion molecules and chemokines, and loss of endothelial thromboresistance through the loss of thrombomodulin and increased expression of tissue factor and von Willebrand factor, and upregulation of the thrombin receptor, proteinase-activated receptor 1 (PAR1) (4, 5). These changes, collectively referred to as endothelial dysfunction, appear to play a central role in the development of early and delayed radiation responses and may, in fact, be the “motor” that drives the vicious cycle responsible for the progressive nature of radiation fibrosis.
The commonly used lipid-lowering compounds, statins, in addition to their effect on cholesterol, has many cholesterol-independent, vasculoprotective, so-called pleiotropic effects, many of which counteract the effects of radiation on endothelial cells. One of the most prominent among the pleiotropic effects is upregulation of thrombomodulin (TM), an endothelial cell glycoprotein that, as one of its effects, activates the natural anticoagulant protein C.
We hypothesized that statins may be able to influence the adverse effects of radiation on the intestine by protecting the vascular endothelium. The objectives of the present study were 1) to assess, in vivo, in a well established, clinically relevant rat model of localized fractionated intestinal irradiation, the extent to which statins influence early and delayed radiation responses in the intestine, and 2) to address a potential mechanism, namely the activation of protein C, by which statins may influence the intestinal radiation response.
Material and Methods
All experimental protocols were approved by the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee.
Radiation Experiments in Sprague-Dawley rats
Sixty-two male Sprague-Dawley rats, 43–49 days of age (175–200 g, Harlan, Indianapolis, IN) were housed in conventional cages with free access to tap drinking water and chow.
The surgical model for localized small bowel irradiation was prepared as described previously (6). Briefly, rats underwent bilateral orchiectomy and a loop of distal ileum was sutured to the inside of the left part of the empty scrotum. The model creates a “scrotal hernia” that contains a 4-cm loop of small intestine that can be irradiated locally without additional surgery. This model has been extensively used and validated in our laboratory.
After 3 weeks postoperative recovery, the rats were randomly assigned to receive either regular chow TD8640 (N=30) or simvastatin chow (Spectrum Laboratory Products, Gardena, CA, N=32) until termination of the experiment. Simvastatin chow was produced by Harlan Teklad by mixing in 500 mg/kg simvastatin TD8640 before pelleting. The simvastatin concentration used in this study corresponded to approximately 80 mg/kg/d and did not cause appreciable food aversion. After an additional 2-week of feeding simvastatin chow or regular chow, the rats were anesthetized with isoflurane inhalation and the transposed bowel segment within the ‘”scrotal hernia” was exposed to once-daily 5.0 Gy fractionated irradiation for 9 days. Irradiation was performed with a Seifert Isovolt 320 X-ray machine (Seifert X-Ray Corporation, Fairview Village, PA), operated at 250 kVp and 15 mA, with 3 mm Al added filtration (half-value layer 0.85 mm Cu, dose rate 4.49 Gy/min). Dosimetric considerations have been described elsewhere (7). This radiation regimen was based on data from previous experiments and intended to elicit moderate to severe radiation enteropathy.
Generation of TM Mutant Mice
The thrombin-thrombomodulin-protein C system appears to play a role in the development of acute and chronic radiation responses and evidence from total body irradiation experiments suggest that activation of protein C is particularly important (unpublished observations, 2006–2007). Therefore, we investigated the role of protein C activation in the intestinal radiation response after localized irradiation.
Homozygous TM-deficiency in mice is embryonically lethal at about embryonic day 8.5, before the assembly of a functional cardiovascular system. Heterozygous TM-deficient (TM+/−) mice, on the other hand, are phenotypically normal. Compared to wild-type (TM+/+) littermates, TM+/− mice have approximately 50% reduction TM mRNA and TM protein levels (8).
TMPro/Pro and TM+/− breeders for producing TM+/+ and TMPro/− mice for the present study were on a mixed C57BL/6J and 129 background and were provided by Dr. Hartmut Weiler (Blood Research Institute, BloodCenter of Wisconsin, Milwaukee, WI). The TMPro/Pro mutants had been generated by a single amino acid substitution (Glu404→Pro) in the TM gene. Homozygous TMPro/Pro mice exhibit approximately 1000-fold reduction in protein C activation and approximately 100-fold reduction in the binding of thrombin (9). Heterozygous TM-deficient (TM+/−) mice had been generated by insertion of the β-galactosidase reporter gene into one of the TM alleles, leading to complete elimination of TM expression at that allele (10). The Glu404→Pro mutation is specific for the activation of protein C, i.e., it does not affect binding of high mobility group box 1 (HMGB1) or activation of thrombin-activated fibrinolysis inhibitor (TAFI). In the knock-out allele, these functions are absent, presumably resulting in an overall 50% reduction in HMGB1 binding and TAFI activation in TMPro/− mice compared to TM+/+.
TMPro/Pro and TM+/− mice were cross-bred to produce TMPro/− offspring (9, 11). Compared to TM+/+ mice, TMPro/− exhibit a 2000-fold reduction in activated protein C activation and 200-fold reduction in thrombin binding (9, 11). Wild type control mice (TM+/+) were obtained from homozygous TM+/+ breeders or from TM+/− breeders. The use of TMPro/− mice, rather than TMPro/Pro mice has the following important advantages: 1) because one allele of the TM gene is completely knocked out, TMPro/− mice have a more profound deficiency in the ability to activate protein C; and 2) because both TMPro/− mice and TM+/+ mice are generated from the same breeding colony (of TMPro/Pro and TM+/− mice) it is not necessary to rely on control mice from a commercial vendor, which invariably exhibit other differences than those related just to the knocked out or mutated gene.
Radiation Experiments in TM Mutant (TMPro/− and TM+/+) mice
A total of 119 TM mutant mice, 2–4 months of age, were used for these experiments: 65 TM+/+ mice (32 female and 33 male) and 54 TMPro/− mice (31 female and 23 male). Genotypes of the animals were identified by lacZ reporter gene analysis and by PCR before the experiment and at euthanasia.
The mice were fed the same simvastatin chow and control chow as the rats. After 2 weeks, the mice were anesthetized with 60 mg/kg Nembutal (Abbott Laboratories, Chicago, IL). A 5-cm segment of ileum, located 10 cm from the ileocecum, was exteriorized through an abdominal incision and marked for identification with a tantalum clip on the mesentery. The mice were placed on a heating pad (maintained at 38°C). The exteriorized ileum was covered with saline-moistened gauze and exposed to 20 Gy localized single dose x-irradiation using the radiation machine and parameters mentioned above. After irradiation, the ileum was replaced into the abdomen and the incision was closed with 5-0 polypropylene (12).
Assessment of the Intestinal Radiation Response
Groups of rats and mice were euthanized 2 weeks (34 rats [17 controls, 17 simvastatin treated], 22 TMPro/− mice [12 controls, 10 simvastatin treated] and 34 TM+/+ mice [16 controls, 18 simvastatin treated]) and 26 weeks (28 rats [13 controls, 15 simvastatin treated], 16 TMPro/− mice [10 controls, 6 simvastatin treated] and 22 TM+/+ mice [13 controls, 9 simvastatin treated]) after the end of the radiation course. These time points are representative of early (subacute) and delayed (chronic) radiation enteropathy in our model system (6, 13). Specimens of irradiated and unirradiated intestine were procured and fixed in methanol-Carnoy’s solution for histological, morphometric, and immunohistochemical analysis. There was no significant difference in radiation response between male and female TM+/+ and TMPro/− mice or in the distribution of male versus female mice in the treatment groups.
Quantitative histopathology and morphometry
Radiation injury score
The overall severity of structural radiation injury was assessed using the radiation injury score (RIS) scoring system. The RIS is a composite histopathological scoring system that provides a global measure of structural radiation injury (13, 14). Briefly, seven histopathologic parameters of radiation injury (mucosal ulcerations, epithelial atypia, thickening of subserosa, vascular sclerosis, intestinal wall fibrosis, ileitis cystica profunda, and lymph congestion) were graded from 0–3. The sum of the scores for the individual alterations constitutes the RIS. All specimens were evaluated in a blinded fashion by two separate researchers, and discrepancies in scores were resolved by consensus.
Mucosal surface area
Radiation-induced decrease in mucosal surface area is a sensitive parameter of small bowel radiation injury. Mucosal surface area was measured in vertical sections using the stereologic projection/cycloid method described by Baddeley (15), adapted to our model system (16). This technique does not require assumptions about the shape or orientation distribution of the specimens and circumvents problems associated with most other procedures for surface area measurement.
Thickness of the intestinal wall and subserosa
Intestinal wall thickening is a measure of both reactive intestinal wall fibrosis and intestinal smooth muscle cell hyperplasia. In contrast, subserosal thickening mainly reflects reactive fibrosis. Intestinal wall thickness (submucosa, muscularis externa, and subserosa) and subserosal thickness were measured with a 10X objective and computer-assisted image analysis (Image-Pro Plus, Media Cybernetics, Silver Spring, MD). In each of 5 areas, 500 μm apart, 3 vertical lines were drawn, centered in the left, middle, and right third. The average of the 3 measurements was calculated, and the average across all 5 areas was used as a single value for statistical calculations.
Quantitative immunohistochemistry and image analysis
Immunohistochemistry and computer-assisted image analysis were used to assess 1) neutrophil infiltration (myeloperoxidase); 2) proliferation rate of intestinal smooth muscle cells (proliferating cell nuclear antigen, PCNA); 3) deposition of collagen types I and III in the intestinal wall, and 4) extracellular matrix-associated transforming growth factor-β (TGFβ) (12, 17).
Immunohistochemical staining was performed with standard avidin-biotin complex (ABC) technique, diaminobenzidine chromogen, and hematoxylin counterstaining. Appropriate positive and negative controls were included. The primary antibodies, incubation times, dilutions, and sources were as follows: polyclonal anti-myeloperoxidase antibody (A0398, 2 hrs, 1:100, Dako, Carpinteria, CA); monoclonal antibody against proliferating cell nuclear antigen (PCNA, NA03, 2 hrs, 1:100, Calbiochem, Cambrige, MA); polyclonal antibodies against collagen I and III (1310–01, 2 hrs, 1:100, and 1330–01, 2 hrs, 1:100 dilution, Southern Biotechnology Associates, Birmingham, AL); and polyclonal antibody against TGFβ (AB-100-NA, 2 hrs, 1:300 dilution, R&D, Minneapolis, MN).
Myeloperoxidase
Myeloperoxidase enzymatic activity in leukocytes correlate directly with neutrophil number (r=0.99) and myeloperoxidase activity in tissue extract correlates directly with cellular infiltration (r=0.94) (18). The number of myeloperoxidase-positive cells was determined by color thresholding and counting in twenty 40X fields, selected according to a predetermined grid pattern.
Smooth muscle cell proliferation
In the intestine, most collagen is produced by intestinal smooth muscle cells, rather than by fibroblasts. Intestinal smooth muscle cell proliferation rate is very low at baseline, but increases steeply after irradiation (19). The number of total smooth muscle cells and PCNA-positive smooth muscle cells was determined in twenty 40X fields using color thresholding and normalized to PCNA-positive smooth muscle cells per thousand.
Collagens
Fibrosis is a common post-radiation manifestation in normal tissues. Areas positive for collagen I and collagen III were determined in twenty 40X fields according to Raviv et al (20), as modified by us (21).
TGFβ
TGFβ is overexpressed in many fibrotic conditions (22), and is mechanistically involved in radiation enteropathy (12). The relative areas positive for TGFβ were determined in twenty 40X fields as described and validated previously (23).
Statistical Methods
In the rat experiment, differences as a function of drug treatment (simvastatin vs. control) and observation time (2 weeks vs. 26 weeks) were assessed using fixed-factor analysis of variance ANOVA using NCSS2004 for Windows (NCSS, Kaysville, UT). In the TM mutant mouse experiment, genotype (TMPro/− vs. TM+/+) was included in the analysis. Appropriate post-hoc comparisons of irradiated intestines were performed with the Mann-Whitney U test, using exact non-parametric inference (StatXact7, Cytel Software, Cambridge, MA). P-values less than 0.05 were considered statistically significant.
Results
Effect of Simvastatin on the Intestinal Radiation Response of Rats
Radiation-induced histopathologic changes in this study were similar to those observed in other studies performed in our laboratory (16, 22). There were no obvious toxic effects of simvastatin administration and no early complications among irradiated rats in either treatment group. In 3 rats, all in the control chow group, small localized intestinal perforations were found at the time of euthanasia at 26 weeks.
Early alterations (2 weeks after irradiation) consisted mainly of mucosal injury and ulcerations, reactive bowel wall thickening, and inflammatory cell infiltration. Vascular sclerosis, chronic mucosal ulcers, and intestinal wall fibrosis predominated at the late observation time (26 weeks). There was significant loss of mucosal surface area, increase in the number of myeloperoxidase-positive cells, increased smooth muscle cell proliferation, and increased deposition of collagens and TGFβ. The differences between unirradiated and irradiated intestines were highly statistically significant for all parameters (p<0.001 for all parameters, data not shown).
Overall, simvastatin attenuated the delayed intestinal radiation response to a much greater extent than it affected early intestinal radiation toxicity. While the difference in histological injury (RIS) between controls and simvastatin-fed rats was only borderline significant at 2 weeks (p=0.05), the difference at 26 weeks post-irradiation was highly significant (p=0.002, Figure 1A). Simvastatin also did not attenuate mucosal injury (loss of mucosal surface area) at 2 weeks, but was associated with highly significant preservation of the mucosa at 26 weeks post-irradiation (p=0.004, Figure 1B). Similarly, simvastatin reduced radiation-induced thickening of the intestinal wall and subserosa at 26 weeks (p=0.007 and p=0.01, respectively), whereas, no difference was observed at 2 weeks (Figure 1C and 1D). There was a borderline significant reduction in neutrophil infiltration in simvastatin-treated animals (Figure 2A), both at 2 weeks (p=0.05) and at 26 weeks (0.05). Compared to controls, irradiated intestine from simvastatin-treated animals exhibited decreased accumulation of collagen I at both 2 weeks (p=0.04) and 26 weeks (p=0.001) post-irradiation (Figure 2B). However, simvastatin administration did alter the accumulation of collagen III at either observation time (data not shown). Although the ANOVA suggested a borderline significance (F=4.51, p=0.04), simvastatin did not influence TGFβ immunoreactivity at either observation time after irradiation upon post hoc analysis (Figure 2C). Finally, simvastatin did not influence post-irradiation smooth muscle cell proliferation overall (F=2.41, p=0.12) although univariate analysis revealed a slight attenuating effect on this parameter at 26 weeks (p=0.02, Figure 2D).
Figure 1. Effect of simvastatin administration on structural alterations after localized, fractionated irradiation of rat small intestine.
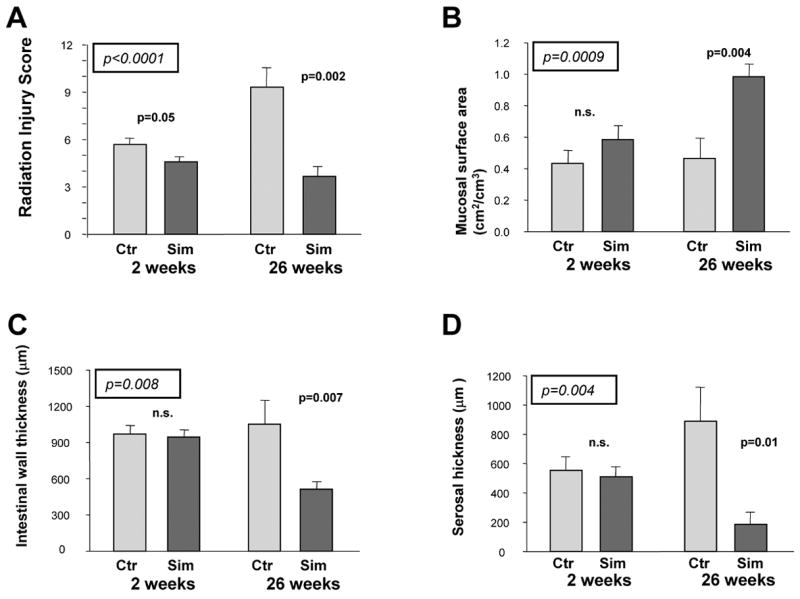
A) Radiation injury score; B) Mucosal surface area; C) Intestinal wall thickness; D) Serosal thickness
The significance levels from the analysis of variance, with observation time and simvastatin administration as factors, are enclosed in a box and italicized. Significance levels for the univariate comparison at each time point are also provided.
Figure 2. Effect of simvastatin administration on mucosal inflammation and intestinal wall remodeling.
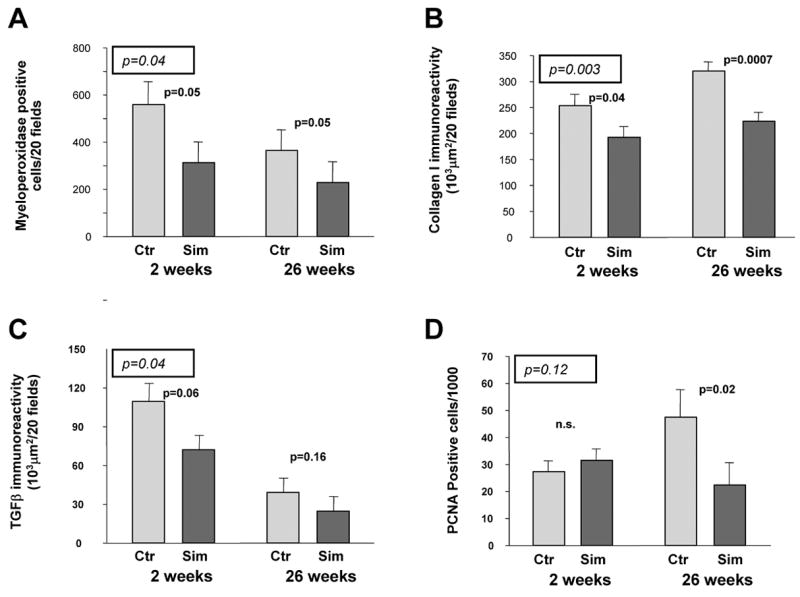
A) Myeloperoxidase-positive cells; B) Collagen I immunoreactivity; C) Extracellular matrix-associated TGFβ immunoreactivity; D) PCNA-positive smooth muscle cells.
The significance levels from the analysis of variance, with observation time and simvastatin administration as factors, are enclosed in a box and italicized. Significance levels for the univariate comparison at each time point are also provided.
Simvastatin did not influence intestinal wall thickness, serosal thickness, mucosal surface area, intestinal smooth muscle cell proliferation rate, and collagen accumulation in unirradiated intestine after 2-week and 26-week of simvastatin administration (data not shown). However, simvastatin administration was associated with a reduced number of myeloperoxidase-positive cells at the 26 weeks observation time point (p=0.02).
Effect of Simvastatin on the Intestinal Radiation Response of TM Mutants
Similar to the rat experiments, there were no obvious toxic effects of simvastatin administration. The difference in the development of post-radiation intestinal complications between TMPro/− mice and TM+/+ mice (all were complications before the 2-week observation time point, mainly intestinal perforations) did not reach statistical significance (Fisher’s Exact Test p=0.07) Simvastatin administration did not affect the complication rate in either genotype. Five of 34 TM+/+ mice (14.7%) in the regular chow group and 4 of 31 TM+/+ mice (12.9%) in the simvastatin chow group developed radiation-induced intestinal complications. In the TMPro/− group, 9 of 31 mice (29%) in the regular chow group and 7 of 23 mice (30%) in the simvastatin chow group developed such complications.
The results of multivariate analysis of the influence of observation time (2 weeks versus 26 weeks), genotype (TMPro/− mice versus TM+/+ mice), and simvastatin (simvastatin versus no simvastatin) on structural injury is shown in Table 1. Genotype had little or no influence on the various parameters of injury after localized intestinal irradiation. In contrast, simvastatin consistently exhibited a highly significant effect in the analysis of variance. Therefore, in contrast to what is observed after total body irradiation, the present study indicates that increased protein C activation is unlikely to be responsible for the amelioration of the intestinal radiation response by simvastatin after localized irradiation.
Table 1. Effect of simvastatin treatment on the intestinal radiation response in TM+/+ and TMPro/− mice.
Influence of observation time (2 weeks vs. 26 weeks), genotype (TM+/+ vs. TMPro/−), and simvastatin (simvastatin vs. no simvastatin) on radiation injury score, mucosal surface area, intestinal wall thickness, and subserosal thickness after localized intestinal irradiation mice.
Note that the p-values for the influence of genotype are not significant for any of the parameters, whereas, all p-values for the effect of simvastatin are highly significant.
| Term | F-ratio | p-value | |
|---|---|---|---|
| Radiation injury score | Observation time | 2.36 | 0.13 |
| Genotype | 0.0 | 0.95 | |
| Simvastatin | 9.89 | 0.002 | |
| Mucosal surface area | Observation time | 45.28 | <0.0001 |
| Genotype | 0.38 | 0.54 | |
| Simvastatin | 6.33 | 0.01 | |
| Intestinal wall thickness | Observation time | 17.49 | <0.0001 |
| Genotype | 2.35 | 0.13 | |
| Simvastatin | 11.60 | 0.001 | |
| Subserosal thickness | Observation time | 5.83 | 0.02 |
| Genotype | 1.18 | 0.28 | |
| Simvastatin | 8.28 | 0.005 |
Discussion
The pathophysiological manifestations of radiation toxicity in the intestine and other normal tissues are the result of a complex interplay among different components and cell types, in which endothelial injury appear to play a central role. We hypothesized that statins, because of their vasculoprotective properties, would reduce the adverse effects of radiation in the intestine. Therefore, we examined the effects of simvastatin on early and delayed structural and molecular manifestations of intestinal radiation injury in a validated, clinically relevant rat model of fractionated small bowel irradiation. In this model, simvastatin conferred significant protection against early radiation enteropathy, and particularly delayed radiation enteropathy. However, unexpectedly, the enteroprotective effect of simvastatin did not appear to be related to activation of protein C.
There has been considerable debate regarding the relative roles of endothelial cells versus intestinal stem cells (crypt cells) in the mechanism of intestinal radiation injury (3). In general, however, endothelial cells are relatively resistant to apoptosis after low to moderate radiation doses. On the other hand, it is well known that even low doses of ionizing radiation alter many of the functional characteristics of the vascular endothelium. In the acute phase, radiation increases endothelial cell permeability and expression of chemokines and adhesion molecules, and causes loss of vascular thromboresistance (3, 4). Radiation-induced loss of vascular thromboresistance is a result of decreased fibrinolytic activity (24), increased expression of tissue factor (TF) (25, 26) and von Willebrand factor (27), and decreased expression of prostacyclin (28) and TM (5, 29–31). TM is a transmembrane glycoprotein, located on the luminal surface of endothelial cells in most normal blood vessels. TM forms a complex with thrombin, and converts thrombin from a pro-coagulant to an anticoagulant by changing its substrate specificity to where thrombin no longer cleaves fibrinogen to form fibrin and no longer activates PARs, but instead activates protein C.
The non-lipid related effects of inhibitors of statins counteract many of the radiation-induced changes in vascular endothelium. Statins reduce isoprenylation of small GTP-binding proteins (eg, Rho, Ras, Rac) and influence many metabolic and physiological processes, including cell proliferation, apoptosis, immune function, inflammation, coagulation, and fibrinolysis. The endothelium is a major effector cell compartment for the pleiotropic effects of statins. Statins increase eNOS activity (32), decrease oxidative stress (33), downregulate expression of TF (34), endothelin-1 (35) and plasminogen activator inhibitor-1 (PAI-1) (36), and upregulate prostacyclin (37), tissue-type plasminogen activator (tPA) (36), and endothelial TM (38, 39). Statins exert anti-inflammatory and anti-thrombotic effects on irradiated endothelial cells in vitro (40) and reduce lung injury in mice after irradiation of the chest (41).
Simvastatin was chosen for the present study because we and others have found the pleiotropic effects to be similar for most statins in most endothelial cell types (38) and because simvastatin is commercially available. The dose used in our study is higher than that used by the Rochester group (5 mg/kg/d) to ameliorate pneumonitis after whole lung irradiation (42). First, however, the 80 mg/kg/d dose was well tolerated in a pilot study with localized intestinal irradiation (43). Second, long term studies in humans receiving 30 mg/kg/d for more than 1 year have shown no obvious toxicity (44). Third, pharmacological studies show that significantly higher statin doses are needed in rodents than in humans to achieve similarly effective concentrations (45). Finally, long term administration of 80 mg/kg/d to rodents has been tested and found to be safe (personal communication, 1/19/2005, Tian-Quan Cai, Ph.D., Merck & Company, Statin Development Team leader).
The thrombin-endothelial TM-protein C pathway likely regulate radiation responses in the intestine and other organs (46 and unpublished observations, 2007). Therefore, experiments in TM+/+ and TMPro/− mice were performed to assess the requirement for protein C activation in the mechanisms by which simvastatin protects against tissue injury after localized intestinal irradiation. Although the mouse experiments were performed with single dose irradiation, the observation that there was little difference between TM+/+ and TMPro/− mice in terms of post-radiation structural injury and that the protective effect of simvastatin was retained in TMPro/− mice indicates that, in contrast to the situation after total body irradiation, the enteroprotective effect of statins after localized irradiation may not be mediated by increased protein C activation.
It is possible that the protective effects of statins may be related to other aspects of endothelial TM function and/or to other pleiotropic properties of statins. Hence, while the Gly(404)→Pro substitution affects protein C activation, other aspects of TM function are retained. These include activation of TAFI, that plays important roles in coagulation, inflammation, and extracellular matrix remodeling (47, 48), and binding of the highly pro-inflammatory HMGB1 DNA binding protein (sometimes referred to as the “nuclear weapon in the immune system’s arsenal”). HMGB1 binds to the cell surface receptor for advanced glycation endproducts (RAGE), which is involved in induction of fibrosis and intestinal inflammation and mucosal barrier loss (49–51). It is interesting to speculate that HMGB1 may be a particularly important aspect of TM in regulating the transition from acute inflammation to chronic inflammation and intestinal fibrosis.
While the results of the present study are not in any way contradictory with these previous studies, that showed post-radiation loss of endothelial TM, they do suggest that the effect of simvastatin on intestinal radiation fibrosis may be related to other aspects of TM or, alternatively, is unrelated to TM. Other pleiotropic statin effects that may confer an enteroprotective effect include a reduction of oxidative stress, upregulation of tPA, or downregulation of TF, PAI-1, and connective tissue growth factor (CTGF). CTGF has been implicated in the development of intestinal radiation fibrosis (52–54). An effect mediated through CTGF downregulation might explain why the protective effect of statins against delayed toxicity was greater than protection against early toxicity . CTGF, in addition to being regulated by TGFβ, is also subject to regulation by thrombin in a TGFβ-independent manner (55). Additional studies are needed to elucidate the mechanisms by which statins influence normal tissue radiation injury.
In conclusion, simvastatin confers prominent protective effects on development of early and particularly delayed intestinal radiation injury. These data provide a basis for future clinical studies to assess statins as a method to ameliorate intestinal toxicity in patients undergoing radiation therapy. These results may also be relevant to the development of medical countermeasures after accidental radiation exposure or in the radiological terrorism setting. Further mechanistic studies to elucidate the molecular underpinnings of the enteroprotective effects of statins are required.
Acknowledgments
Financial Support: National Institutes of Health (Grant CA-83719) and Veterans Healthcare Administration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.DeVita VT, Hellman S, Rosenberg SA. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 2.Denham JW, Hauer-Jensen M. The radiotherapeutic injury - a complex "wound". Radiother Oncol. 2002;63:129–145. doi: 10.1016/s0167-8140(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 3.Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 4.Fajardo LF. The unique physiology of endothelial cells and its implication in radiobiology. Radiation Tolerance of Normal Tissues. In: Vaeth JM, Meyer JL, editors. Front Ther Oncol. Karger: Basel; 1989. pp. 96–112. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Zheng H, Ou X, et al. Deficiency of microvascular thrombomodulin and upregulation of protease-activated receptor 1 in irradiated rat intestine: possible link between endothelial dysfunction and chronic radiation fibrosis. Am J Pathol. 2002;160:2063–2072. doi: 10.1016/S0002-9440(10)61156-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauer-Jensen M, Poulakos L, Osborne JW. Effects of accelerated fractionation on radiation injury of the small intestine: a new rat model. Int J Radiat Oncol Biol Phys. 1988;14:1205–1212. doi: 10.1016/0360-3016(88)90399-9. [DOI] [PubMed] [Google Scholar]
- 7.Langberg CW, Waldron JA, Baker ML, et al. Significance of overall treatment time for the development of radiation-induced intestinal complications. An experimental study in the rat. Cancer. 1994;73:2663–2668. doi: 10.1002/1097-0142(19940515)73:10<2663::aid-cncr2820731031>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Healy AM, Rayburn HB, Rosenberg RD, et al. Absence of the blood-clotting regulator thrombomodulin causes embryonic lethality in mice before development of a functional cardiovascular system. Proc Natl Acad Sci USA. 1995;92:850–854. doi: 10.1073/pnas.92.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiler-Guettler H, Christie PD, Beeler DL, et al. A targeted point mutation in thrombomodulin generates viable mice with a prethrombotic state. J Clin Invest. 1998;101:1983–1991. doi: 10.1172/JCI2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiler-Guettler H, Aird WC, Husain M, et al. Targeting of transgene expression to the vascular endothelium of mice by homologous recombination at the thrombomodulin locus. Circ Res. 1996;78:180–187. doi: 10.1161/01.res.78.2.180. [DOI] [PubMed] [Google Scholar]
- 11.Weiler H, Lindner V, Kerlin B, et al. Characterization of a mouse model for thrombomodulin deficiency. Arterioscler Thromb Vasc Biol. 2001;21:1531–1537. doi: 10.1161/hq0901.094496. [DOI] [PubMed] [Google Scholar]
- 12.Zheng H, Wang JR, Koteliansky VE, et al. Recombinant soluble transforming growth factor beta type II receptor ameliorates radiation enteropathy in mice. Gastroenterology. 2000;119:1286–1296. doi: 10.1053/gast.2000.19282. [DOI] [PubMed] [Google Scholar]
- 13.Langberg CW, Sauer T, Reitan JB, et al. Tolerance of rat small intestine to localized single dose and fractionated irradiation. Acta Oncol. 1992;31:781–787. doi: 10.3109/02841869209083871. [DOI] [PubMed] [Google Scholar]
- 14.Hauer-Jensen M, Sauer T, Devik F, et al. Late changes following single dose roentgen irradiation of rat small intestine. Acta Radiol Oncol. 1983;22:299–303. doi: 10.3109/02841868309134045. [DOI] [PubMed] [Google Scholar]
- 15.Baddeley AJ, Gundersen HJ, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc. 1986;142:259–276. doi: 10.1111/j.1365-2818.1986.tb04282.x. [DOI] [PubMed] [Google Scholar]
- 16.Langberg CW, Sauer T, Reitan JB, et al. Relationship between intestinal fibrosis and histopathologic and morphometric changes in consequential and late radiation enteropathy. Acta Oncol. 1996;35:81–87. doi: 10.3109/02841869609098484. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Zheng H, Ou X, et al. Deficiency of microvascular thrombomodulin and up-regulation of protease-activated receptor-1 in irradiated rat intestine: possible link between endothelial dysfunction and chronic radiation fibrosis. Am J Pathol. 2002;160:2063–2072. doi: 10.1016/S0002-9440(10)61156-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boughton-Smith NK, Wallace JL, Whittle, et al. Relationship between arachidonic acid metabolism, myeloperoxidase activity and leukocyte infiltration in a rat model of inflammatory bowel disease. Agents Actions. 1988;25:115–123. doi: 10.1007/BF01969102. [DOI] [PubMed] [Google Scholar]
- 19.Zheng H, Wang J, Hauer-Jensen M. Role of mast cells in early and delayed radiation injury in rat intestine. Radiat Res. 2000;153:533–539. doi: 10.1667/0033-7587(2000)153[0533:romcie]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Raviv G, Kiss R, Vanegas JP, et al. Objective measurement of the different collagen types in the corpus cavernosum of potent and impotent men: an immunohistochemical staining with computerized-image analysis. World J Urol. 1997;15:50–55. doi: 10.1007/BF01275157. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Zheng H, Hauer-Jensen M. Influence of short-term octreotide administration on chronic tissue injury, transforming growth factor beta (TGF-beta) overexpresion, and collagen accumulation in irradiated rat intestine. Journal of Pharmacology & Experimental Therapeutics. 2001;297:35–42. [PubMed] [Google Scholar]
- 22.Richter KK, Langberg CW, Sung CC, et al. Association of transforming growth factor beta Immunoreactivity with specific histopathologic lesions in subacute and chronic experimental radiation enteropathy. Radiother Oncol. 1996;39:243–251. doi: 10.1016/0167-8140(95)01735-6. [DOI] [PubMed] [Google Scholar]
- 23.Richter KK, Sung C-C, Langberg CW, et al. Association of transforming growth factor β (TGF-β) immunoreactivity with specific histopathologic lesions in subacute and chronic experimental radiation enteropathy. Radiother Oncol. 1996;39:243–251. doi: 10.1016/0167-8140(95)01735-6. [DOI] [PubMed] [Google Scholar]
- 24.Åstedt B, Bergentz S-E, Svanberg L. Effect of irradiation on the plasminogen activator content in rat vessels. Experientia. 1974;30:1466–1467. doi: 10.1007/BF01919698. [DOI] [PubMed] [Google Scholar]
- 25.Verheij M, Dewit LGH, van Mourik JA. The effect of ionizing radiation on endothelial tissue factor activity and its cellular localization. Thromb Haemost. 1995;73:894–895. [PubMed] [Google Scholar]
- 26.Wang J, Zheng H, Ou X, et al. Hirudin ameliorates intestinal radiation toxicity in the rat: support for thrombin inhibition as strategy to minimize side effects after radiation therapy and as countermeasure against radiation exposure. J Thromb Haemost. 2004;2:2027–2035. doi: 10.1111/j.1538-7836.2004.00960.x. [DOI] [PubMed] [Google Scholar]
- 27.Jahroudi N, Ardekani AM, Greenberger JS. Ionizing radiation increases transcription of the von Willebrand factor gene in endothelial cells. Blood. 1996;88:3801–3814. [PubMed] [Google Scholar]
- 28.Rubin DB, Drab EA, Ts'ao C, et al. Prostacyclin synthesis in irradiated endothelial cells cultured from bovine aorta. J Appl Physiol. 1985;58:592–597. doi: 10.1152/jappl.1985.58.2.592. [DOI] [PubMed] [Google Scholar]
- 29.Richter KK, Fink LM, Hughes BM, et al. Is the loss of endothelial thrombomodulin involved in the mechanism of chronicity in late radiation enteropathy? Radiother Oncol. 1997;44:65–71. doi: 10.1016/s0167-8140(97)00063-7. [DOI] [PubMed] [Google Scholar]
- 30.Richter KK, Fink LM, Hughes BM, et al. Differential effect of radiation on endothelial cell function in rectal cancer and normal rectum. Am J Surg. 1998;176:642–647. doi: 10.1016/s0002-9610(98)00280-3. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Q, Zhao Y, Li P, et al. Thrombomodulin as a marker of radiation-induced endothelial cell injury. Radiat Res. 1992;131:285–289. [PubMed] [Google Scholar]
- 32.Laufs U, La Fata V, Plutzky J, et al. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 33.Rikitake Y, Kawashima S, Takeshita S, et al. Anti-oxidant properties of fluvastatin, an HMG-CoA reductase inhibitor, contribute to prevention of atherosclerosis in cholesterol-fed rabbits. Atherosclerosis. 2001;154:87–96. doi: 10.1016/s0021-9150(00)00468-8. [DOI] [PubMed] [Google Scholar]
- 34.Eto M, Kozai T, Cosentino F, et al. Statin prevents tissue factor expression in human endothelial cells. Role of Rho/Rho-kinase and Akt pathways. Circulation. 2002;105:1756–1759. doi: 10.1161/01.cir.0000015465.73933.3b. [DOI] [PubMed] [Google Scholar]
- 35.Hernandez-Perera O, Perez-Sala D, Navarro-Antolin J, et al. Effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, atorvastatins and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J Clin Invest. 1998;101:2711–2719. doi: 10.1172/JCI1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Essig M, Nguyen G, Prie D, et al. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors increase fibrinolytic activity in rat aortic endothelial cells. Role of geranylgeranylation and Rho proteins. Circ Res. 1998;83:683–690. doi: 10.1161/01.res.83.7.683. [DOI] [PubMed] [Google Scholar]
- 37.Seeger H, Mueck AO, Lippert TH. Fluvastatin increases prostacyclin and decreases endothelin production by human umbilical vein endothelial cells. Int J Clin Pharmacol Ther. 2000;38:270–272. doi: 10.5414/cpp38270. [DOI] [PubMed] [Google Scholar]
- 38.Shi J, Wang J, Zheng H, et al. Statins increase thrombomodulin expression and function in human endothelial cells by a nitric oxide-dependent mechanism and counteract tumor necrosis factor alpha-induced thrombomodulin downregulation. Blood Coagul Fibrinolysis. 2003;14:575–585. doi: 10.1097/00001721-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Masamura K, Oida K, Kanehara H, et al. Pitavastatin-Induced Thrombomodulin Expression by Endothelial Cells Acts Via Inhibition of Small G Proteins of the Rho Family. Arterioscler Thromb Vasc Biol. 2003;23:512–513. doi: 10.1161/01.ATV.0000060461.64771.F0. [DOI] [PubMed] [Google Scholar]
- 40.Gaugler MH, Vereycken-Holler V, Squiban C, et al. Pravastatin limits endothelial activation after irradiation and decreases the resulting inflammatory and thrombotic responses. Radiat Res. 2005;163:479–487. doi: 10.1667/rr3302. [DOI] [PubMed] [Google Scholar]
- 41.Williams JP, Hernady E, Johnston CJ, et al. Effect of administration of lovastatin on the development of late pulmonary effects after whole-lung irradiation in a murine model. Radiat Res. 2004;161:560–567. doi: 10.1667/rr3168. [DOI] [PubMed] [Google Scholar]
- 42.Williams JP, Hernady E, Johnston CJ, et al. Effect of administration of lovastatin on the development of late pulmonary effects after whole-lung irradiation in a murine model. Radiat Res. 2004;161:560–567. doi: 10.1667/rr3168. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Qiu X, Zheng H, et al. Effect of statins on endothelial thrombomodulin in vitro and the intestinal radiation response in vivo (Abstr) Radiation Research Society. 2004;51:37. [Google Scholar]
- 44.Larner J, Jane J, Laws E, et al. A phase I–II trial of lovastatin for anaplastic astrocytoma and glioblastoma multiforme. Am J Clin Oncol. 1998;21:579–583. doi: 10.1097/00000421-199812000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Dostal LA, Whitfield LR, Anderson JA. Fertility and general reproduction studies in rats with the HMG-CoA reductase inhibitor, atorvastatin. Fundam Appl Toxicol. 1996;32:285–292. doi: 10.1006/faat.1996.0132. [DOI] [PubMed] [Google Scholar]
- 46.Hauer-Jensen M, Fink LM, Wang J. Radiation injury and the protein C pathway. Crit Care Med. 2004;32:S325–S330. doi: 10.1097/01.ccm.0000126358.15697.75. [DOI] [PubMed] [Google Scholar]
- 47.Mosnier LO, Bouma BN. Regulation of fibrinolysis by thrombin activatable fibrinolysis inhibitor, an unstable carboxypeptidase B that unites the pathways of coagulation and fibrinolysis. Arterioscler Thromb Vasc Biol. 2006;26:2445–2453. doi: 10.1161/01.ATV.0000244680.14653.9a. [DOI] [PubMed] [Google Scholar]
- 48.Bouma BN, Mosnier LO. Thrombin activatable fibrinolysis inhibitor (TAFI) at the interface between coagulation and fibrinolysis. Pathophysiol Haemost Thromb. 2003;33:375–381. doi: 10.1159/000083832. [DOI] [PubMed] [Google Scholar]
- 49.Oldfield MD, Bach LA, Forbes JM, et al. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE) J Clin Invest. 2001;108:1853–1863. doi: 10.1172/JCI11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andrassy M, Igwe J, Autschbach F, et al. Posttranslationally modified proteins as mediators of sustained intestinal inflammation. Am J Pathol. 2006;169:1223–1237. doi: 10.2353/ajpath.2006.050713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raman KG, Sappington PL, Yang R, et al. The role of RAGE in the pathogenesis of intestinal barrier dysfunction after hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol. 2006;291:G556–G565. doi: 10.1152/ajpgi.00055.2006. [DOI] [PubMed] [Google Scholar]
- 52.Boerma M, Burton GR, Wang J, et al. Comparative expression profiling in primary and immortalized endothelial cells: changes in gene expression in response to hydroxy methylglutaryl-coenzyme A reductase inhibition. Blood Coagul Fibrinolysis. 2006;17:173–180. doi: 10.1097/01.mbc.0000220237.99843.a1. [DOI] [PubMed] [Google Scholar]
- 53.Vozenin-Brotons MC, Milliat F, Sabourin JC, et al. Fibrogenic signals in patients with radiation enteritis are associated with increased connective tissue growth factor expression. Int J Radiat Oncol Biol Phys. 2003;56:561–572. doi: 10.1016/s0360-3016(02)04601-1. [DOI] [PubMed] [Google Scholar]
- 54.Watts KL, Spiteri MA. Connective tissue growth factor expression and induction by transforming growth factor-beta is abrogated by simvastatin via a Rho signaling mechanism. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1323–L1332. doi: 10.1152/ajplung.00447.2003. [DOI] [PubMed] [Google Scholar]
- 55.Chambers RC, Leoni P, Blanc-Brude OP, et al. Thrombin is a potent inducer of connective tissue growth factor production via proteolytic activation of protease-activated receptor-1. J Biol Chem. 2000;275:35584–35591. doi: 10.1074/jbc.M003188200. [DOI] [PubMed] [Google Scholar]


