Abstract
Aims
To evaluate the effects of gender, age, diabetes mellitus, renal and hepatic impairment on tadalafil pharmacokinetics and tolerability.
Methods
Six single-dose (5, 10 or 20 mg orally) clinical pharmacology studies were conducted in the UK, Belgium, Poland and Germany in healthy male and female subjects, elderly subjects and subjects with diabetes mellitus, renal impairment, end-stage renal failure (ESRF) or hepatic impairment. The gender study also incorporated administration of 10 mg tadalafil daily for 10 days.
Results
Systemic exposure in the elderly was 25% greater than in young subjects (mean AUC ratio 1.25; 90% confidence interval 0.972, 1.61). The AUC was 19% lower in subjects with diabetes mellitus than in healthy age/gender-matched controls. Pharmacokinetics in female subjects were essentially similar to those in males. Exposure in subjects with mild or moderate renal insufficiency was approximately twice that in healthy subjects. The mean AUC for the major metabolite (total methylcatechol glucuronide) in the presence of ESRF was three times the mean for healthy subjects. Haemodialysis contributed negligibly to elimination of tadalafil or the metabolite. Hepatic impairment had negligible effects on exposure. The most common adverse events in these six studies were headache, back pain and myalgia. A 10-mg dose was not well tolerated by subjects with moderate renal dysfunction in this study.
Conclusions
No clinically significant effect of gender, age, diabetes mellitus or hepatic impairment on tadalafil pharmacokinetics was observed. Renal insufficiency resulted in increased systemic exposure. Tadalafil was not associated with any serious clinically significant adverse events or study discontinuations due to adverse events.
Keywords: erectile dysfunction, pharmacokinetics, phosphodiesterase, tadalafil
Introduction
Tadalafil (Cialis®; Lilly ICOS LLC, Indianapolis, IN, and Bothell, WA, USA) is a selective inhibitor of phosphodiesterase 5 (PDE5) with demonstrated efficacy and safety as an oral therapy for erectile dysfunction (ED) [1–6]. The recommended dose in most patients is 10 mg, taken with or without food prior to anticipated sexual activity, with adjustment to 5 or 20 mg based on individual efficacy and tolerability [7, 8]. The maximum recommended dosing frequency is once daily. Following single-dose oral administration to healthy subjects, tadalafil is rapidly absorbed, with a median time (tmax) to maximum plasma concentration (Cmax) of 2 h. Absorption is not affected by food [7–9]. Approximately 94% of tadalafil in plasma is bound to albumin or α1-acid glycoprotein. Absolute bioavailability relative to intravenous administration has not been determined. Linear pharmacokinetic behaviour across dose level and time has been demonstrated, with reference mean parameter values (for a 10-mg dose) from an integrated statistical analysis of: Cmax = 189 µg l−1; oral clearance (CL/F) = 2.48 l h−1; and apparent distribution volume (Vz/F) = 62.6 l [9]. The mean half-life (t1/2) of 17.5 h following a single dose and a duration of efficacy of up to 36 h have been demonstrated [10].
Tadalafil clearance is predominantly via hepatic metabolism by CYP3A to a catechol which is extensively conjugated to form the major circulating metabolite, a methylcatechol glucuronide, with a minor amount of the unconjugated metabolite also detected in plasma [11, 12]. Approximately 36% of an oral radiolabelled dose is excreted in urine, nearly entirely as metabolites [7, 8, 12]. Based on low affinity for PDE5 in vitro, the metabolites are expected to have no clinical effect at plasma concentrations typically observed at therapeutic doses [13].
Clinical pharmacology studies on tadalafil have been conducted in several special populations, as well as in healthy, young or middle-aged male subjects [9]. ED is associated with advancing age and age-related chronic diseases [14, 15]. Largely as a result of population ageing, the number of men with ED around the world is projected to increase from 152 million in 1995 to 322 million by 2025 [16]. Approximately 19 million American adults have chronic kidney disease and 80 000 individuals receive a diagnosis of chronic kidney failure each year [17, 18]. The number of individuals with diabetes mellitus across the world is projected to rise from about 171 million in 2000 to 366 million in 2030 [19]. Given the prevalence of these conditions, it was important to understand tadalafil disposition and tolerability in these special populations. In addition, tadalafil is being investigated for conditions affecting both males and females, including pulmonary arterial hypertension and systemic hypertension; therefore, it was important to evaluate pharmacokinetics and tolerability in both genders. Some of the findings reported here have been presented in abstract form or appear in a review on tadalafil [20,21].
Methods
Subjects
Subject demographics are summarized in Table 1. Studies were conducted, and subject written informed consent was obtained prior to screening, in conformity with the ethical principles of the Declaration of Helsinki (Somerset West, 1996) and applicable European laws. Ethics committees of all participating institutions approved the final protocol.
Table 1.
Demographics by study
| Effect of gender (UK) | Effect of age (Germany) | Effect of diabetes mellitus (Belgium) | Effect of renal impairment (Poland) | Effect of hepatic impairment (Germany) | Effect of renal dialysis (Poland and UK) | |
|---|---|---|---|---|---|---|
| Number of subjects | 24 | 24 (12 young, 12 elderly) | 24 (12 healthy) | 28 (12 healthy) | 33 (8 healthy) | 16 |
| Males/females | 12/12 | 24/0 | 18/6 | 25/3 | 21/12 | 13/3 |
| Mean age (years) ± SD (range) | Males: 38 ± 6.8 | Young: 36.3 ± 7.8 (19–45) | 42.1 ± 10.4 (27–59) | 47.7 ± 9.6 (30–65) | 43.3 ± 10.3 (25–63) | 48.1 ± 13.2 (28–74) |
| Females: 45 ± 10.8 | Elderly: 70.7 ± 3.9 (65–78) | |||||
| Tadalafil dose | 10 mg | 10 mg | 10 mg | 5 and 10 mg | 10 mg | 5, 10 and 20 mg |
SD, Standard deviation.
Study designs
Six single-dose (5, 10 or 20 mg) clinical pharmacology studies were conducted in the UK, Belgium, Poland and Germany in the following groups: healthy males and females (Lilly ICOS study identifier H6D-EW-LVAD), elderly (H6D-EW-LVBW), subjects with diabetes mellitus (H6D-EW-LVAS), subjects with renal impairment (H6D-EW-LVAJ), subjects with end-stage renal failure (ESRF; H6D-EW-LVDT) and subjects with hepatic impairment (H6D-EW-LVAK). The gender comparison also entailed once-daily, 10-mg dosing for 10 days.
Within 3 weeks before the study, all subjects underwent screening procedures, including a 12-lead electrocardiogram (ECG); laboratory tests; physical examination including vital signs; and a urine drug screen and alcohol breath or blood test. Subjects were admitted to the research clinic 1 day before receiving tadalafil. Xanthine-containing drinks were restricted and alcohol and grapefruit juice (a CYP3A inhibitor) were not permitted within 24–48 h of dosing. Each subject received a single oral dose as 5-, 10- or 20-mg tablets (final clinical formulation), routinely taken with approximately 200 ml water. Each film-coated tablet contained these inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, hypromellose, iron oxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, sodium lauryl sulphate, talc, titanium dioxide and triacetin. A prototype 10-mg tablet used solely for the gender-effect study (early clinical development) was bioequivalent to the final clinical tablet with respect to extent of absorption, although its lower (21%) Cmax indicated slower absorption.
Pharmacokinetic assessments via venous blood collections (∼ 5 ml) were made at prespecified times and subjects were discharged between 24 and 48 h later. Subjects returned as ‘outpatients’ up to 13 days later for safety assessments and additional pharmacokinetic sampling. During the intervening days, alcohol intake and smoking were restricted.
Effect of gender
A total of 12 males and 12 females, aged 29–64 years and healthy according to medical history and physical examination, were enrolled. Women of childbearing potential were excluded, as were subjects with a history or presence of significant cardiovascular, respiratory, hepatic, renal, gastrointestinal or neurological disorders capable of altering the absorption, metabolism or elimination of drugs, or constituting a risk factor when taking tadalafil. Also excluded were subjects with a body mass index of ≤18 or ≥30 kg m−2; a history of diabetes mellitus with fasting blood glucose >10 mmol l−1 (182 mg dl−1); a history of glaucoma or cataracts; active haematological disease; use of hormone replacement therapy by women; 6-month history of excessive methylxanthine use, smoking of >10 cigarettes daily or regular weekly alcohol intake of >28 units in men or 21 units in women (1 unit = 8 g ethanol); and/or positive HIV, hepatitis B or hepatitis C test results. Intended use of prescription medication within 14 days and over-the-counter medication use within 7 days prior to dosing were also excluded. In the single-dose period, blood samples were collected predose and from 0.25 to 144 h postdose for determination of plasma tadalafil concentrations. Following a 7-day wash-out period, each subject received 10 mg tadalafil once daily for 10 consecutive days. Serial blood samples were collected predose and for 24 h following the first and fifth doses, as well as for 168 h after the tenth dose for analysis of tadalafil.
Effect of age
A total of 12 healthy subjects (aged 18–45 years inclusive) and 12 elderly subjects (aged 65–80 years inclusive) were selected and matched according to body weight, height and smoking habit (Table 1). Exclusion criteria were essentially as noted above, with the added exclusion of a history of ED within 6 months of study initiation. Subjects received a single 10-mg dose and remained in the hospital until approximately 36 h postdose. Serial blood samples were collected predose and from 0.25 to 144 h postdose for the tadalafil assay.
Effect of diabetes mellitus
A total of 12 healthy male or female subjects and 12 subjects with Type 1 or Type 2 diabetes mellitus, a haemoglobin Alc level ≤13.0% at screening and who were currently receiving insulin therapy were enrolled. Exclusion criteria included uncontrolled diabetes mellitus, defined as two or more episodes of ketoacidosis within 1 year prior to day 1 of the study; one episode of ketoacidosis within 3 months; and/or three or more episodes of hypoglycaemia requiring assistance as defined by the Diabetes Control and Complications Trial within 3 months [22]. Concomitant medications other than insulin injections were kept to a minimum. After an American Diabetes Association recommended breakfast [50 g bread, jam for diabetics, 60 g cheese, orange juice (75 kcal) and noncaffeinated coffee], subjects received a single 10-mg tadalafil tablet and remained in the hospital until 24 h postdose. Serial blood samples were collected predose and from 0.5 to 144 h postdose.
Effect of renal impairment
Creatinine clearance (CLcr) was estimated by the Cockroft–Gault formula [23]. Male or female subjects with stable renal impairment that was mild (CLcr = 51–80 ml min−1) or moderate (CLcr = 31–50 ml min−1) for ≥3 months prior to the start of the study were enrolled. Exclusion criteria included: renal transplantation; concomitant use of thyroid hormones and/or iodide (except for the prevention of euthyroid goitre); antacids; cimetidine, proton pump inhibitors or nitrates; use of phosphate binders, cholestyramine, colestipol or ranitidine, or nizatidine within 10 h prior to dosing and within 6 h after dosing; or use of medications known to interfere with hepatic metabolism or alter major organs or systems, within 30 days prior to admission on day −1.
A total of 16 subjects with renal impairment and 12 healthy subjects were recruited for this study. Following a review of tolerability data at the 10-mg dose level, it was decided not to administer tadalafil to subjects with severe renal impairment and to investigate tadalafil 5 mg. Six subjects with moderate renal impairment, five with mild impairment and eight healthy subjects received tadalafil 10 mg. Six subjects with moderate renal impairment and three with mild impairment, as well as four healthy subjects, received tadalafil 5 mg. Four subjects with moderate impairment received both 10 and 5 mg tadalafil. Screening was performed in the 14-day period prior to study admission to determine serum creatinine concentrations.
Study participants were admitted to the research unit on day −1 and fasted (including no water) from 22.00 h the preceding evening until 4 h postdose. Subjects received a single dose of tadalafil 5 or 10 mg on day 1. Participants were discharged approximately 48 h postdose and returned on days 4–8 for pharmacokinetic blood sampling and vital sign assessments. A poststudy assessment was conducted on day 8. Serial blood samples for determination of tadalafil and of total metabolite (total methylcatechol in hydrolysed plasma; predominantly the methylcatechol glucuronide) were collected predose and up to 168 h postdose.
Effect of ESRF
Male or female subjects with ESRF and receiving three haemodialysis sessions per week were eligible. Exclusion criteria were as noted above for healthy subjects. Six subjects were to be recruited for each tadalafil dose group (5, 10 and 20 mg). Initially, six subjects received tadalafil 5 mg. Plasma tadalafil and total metabolite concentration–time profiles, safety and tolerability were reviewed before proceeding to the 10-mg dose with the same study schedule. The same process was followed before proceeding to the 20-mg dose.
Screening was performed approximately 1 month before study onset and subjects underwent a final evaluation for entry on day 1 (the day of dosing). Study participants were required to fast for 2 h before dosing. Each subject received a single 5-, 10- or 20-mg dose approximately 24–30 h before their first haemodialysis session in the study period. After dosing, subjects returned to the study site in the morning, afternoon or evening of days 2, 4 and 6 for haemodialysis and pharmacokinetic sampling according to each subject’s normal routine. Subjects also attended the study site on days 5 and 7 to provide pharmacokinetic samples. Blood samples were collected from each subject for the analysis of plasma protein binding before dosing and at 4 h postdose on day 1. A poststudy assessment was conducted on approximately day 13, when laboratory samples were taken before haemodialysis (if applicable). For subjects who participated in two dosing groups, a wash-out period of ≥13 days was considered appropriate for eliminating any residual plasma tadalafil or metabolite from the previous dosing occasion. Subjects could participate in no more than two dosing groups. Pharmacokinetics were evaluated relative to pooled data from the healthy subjects (10-mg dose) in the renal impairment study (Table 2) and four healthy subjects in that study at the 5-mg dose level, as well as 16 healthy subjects who received single 5-, 10- and 20-mg tadalafil doses in a crossover dose-proportionality study [9].
Table 2.
Geometric mean (CV%) pharmacokinetic parameters for tadalafil after administration of a single 10-mg dose
| Parameter | Male (N = 12)* | Female (N = 12)* | Elderly (N = 12) | Young (N = 12) | Healthy subjects (N = 12) | Diabetic (N = 12) | Healthy subjects (renal) (N = 8) | Mild renal impair. (N = 5) | Moderate renal impair. (N = 6) | Healthy subjects (hepatic) (N = 8) | Very mild hepatic impair. (N = 8) | Mild hepatic impair. (N = 8) | Moderate hepatic impair. (N = 8) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC (μg h l−1) | 3565 (23) | 4097 (28) | 4881 (32) | 3896 (43) | 4249 (36) | 3458 (38) | 2868 (44) | 6280 (46) | 4911 (50) | 5823 (74) | 3961 (34) | 5760 (52) | 4049 (56) |
| Cmax (μg l−1) | 142 (26) | 140 (21) | 196 (27) | 183 (25) | 193 (22) | 184 (27) | 183 (31) | 217 (21) | 220 (22) | 180 (38) | 133 (21) | 146 (23) | 101 (39) |
| tmax (h)† | 3.5 (1–8) | 3.5 (2–8) | 2.0 (1–4) | 2.5 (1–6) | 2.0 (1–8) | 3.0 (1–4) | 1.0 (0.5–3.0) | 2.0 (2–4) | 2.0 (0.5–3) | 2.50 (0.5–4) | 3.0 (0.5–4) | 2.0 (0.5–6) | 2.5 (0.5–4) |
| t1/2 (h) | 16.6 (21) | 19.8 (26) | 21.6 (39) | 16.9 (29) | 17.1 (27) | 13.8 (33) | 14 (46) | 26 (33) | 22 (43) | 24.2 (53) | 24.7 (43) | 34.9 (48) | 37.8 (62) |
| CL/F (l h−1) | 2.81 (23) | 2.44 (28) | 2.05 (32) | 2.57 (43) | 2.35 (36) | 2.89 (38) | 3.49 (44) | 1.59 (46) | 2.04 (50) | 1.72 (74) | 2.52 (34) | 1.74 (52) | 2.47 (55) |
| Vz/F (l) | 67.2 (16) | 69.8 (19) | 63.9 (25) | 62.5 (17) | 58.2 (23) | 57.4 (18) | 71.8 (40) | 59.2 (16) | 65.9 (17) | 59.9 (30) | 90.1 (19) | 87.5 (25) | 135 (55) |
Multiple-dose pharmacokinetics are discussed in text.
Median (min–max) data. CV%, Coefficient of variation.
Effect of hepatic impairment
Male or female subjects were eligible provided that they had stable liver cirrhosis (alcoholic, posthepatitis, biliary cirrhosis and cryptogenic). Exclusion criteria included evidence of severe hepatorenal syndrome as demonstrated by CLcr <40 ml min−1; presence of hepatocellular carcinoma; concomitant use of any drugs except those necessary for the treatment of liver disease or related complications, especially antacids, cimetidine and related compounds, proton pump inhibitors and cyclosporin, or other immunosuppressants, except for cortisone derivatives; and use of medications known to interfere with hepatic metabolism or alter other major organs or systems, within 30 days prior to admission. Eight healthy subjects, eight subjects with very mild hepatic impairment (fatty liver detected by sonography), eight with mild impairment (Child-Pugh Class A; 5–6 points), eight with moderate impairment (Class B; 7–9 points) and one subject with severe impairment (Class C; 10–15 points) were recruited for this study. Individuals with a Child-Pugh score >12 were excluded.
Subjects received tadalafil 10 mg in the morning of day 1 following an overnight fast, were discharged from the hospital approximately 48 h postdose and returned on days 4–8 for pharmacokinetic sampling and vital sign assessments. A poststudy assessment was conducted before discharge on day 8 or upon a participant’s discontinuation from the study. Serial blood samples were collected predose and from 0.25 to 168 h postdose for the tadalafil assay. The individual with severe hepatic impairment participated while in the hospital and remained hospitalized after completion of the study.
Pharmacokinetic and statistical analyses
Noncompartmental parameters were calculated from plasma tadalafil concentration–time data using WinNonlin Professional (Pharsight Corp., Mountain View, CA, USA). These included the area under the plasma concentration–time curve from zero to infinite time (AUC), maximum observed concentration (Cmax), time to Cmax (tmax), terminal rate constant (λz), half-life (t1/2), oral clearance (CL/F) and apparent volume of distribution in the terminal elimination phase (Vz/F). For some comparisons of interest, Cmax and AUC values were normalized to a 10-mg dose, e.g. a value for 5 mg was doubled. Statistical analysis of AUC and Cmax was performed using an analysis of variance (anova) model. Estimates of the ratio of means for two subject groups were made along with the corresponding 90% confidence intervals (90% CI). Values of tmax were analysed nonparametrically [24].
Bioanalytical methods
Tadalafil concentrations were measured using a validated high-performance liquid chromatography tandem mass spectrometry (HPLC/MS/MS) assay with a lower limit of quantification of 0.5 μg l−1 essentially as described [25]. Intra-assay accuracy (% relative error) and precision (% relative SD) were <12.88% and <8.84%, respectively. Interassay accuracy and precision were <6.68% and <7.09%, respectively. The major metabolite, methylcatechol glucuronide, was quantified as total methylcatechol in hydrolysed (β-glucuronidase) plasma using a validated HPLC/MS/MS assay with a lower limit of quantification of 1 μg l−1. Intra-assay accuracy and precision were <18.64% and <3.51%, respectively, and interassay accuracy and precision were <16.23% and <3.19%, respectively.
Safety assessment
Routine safety data (adverse events, vital signs and clinical laboratory tests) were monitored throughout the studies. In addition, ECG data were collected for all studies at visit 1 and at the end of study. Vital signs (blood pressure, heart rate) and clinical laboratory tests (serum chemistry, haematology) were measured at all visits for all studies. An urinalysis was conducted at the screening and final visits. Adverse events were coded using the Medical Dictionary for Regulatory Affairs (MedDRA).
Results
A total of 149 subjects (36 female) were enrolled in the six studies (Table 1). All subjects were White, except for one Afro-Caribbean. In the diabetes mellitus study, the mean haemoglobin Alc level at screening was 7.7% (± 1.3%).
Effect of gender
Pharmacokinetics following a single 10-mg dose in female subjects were essentially similar to results in males (Table 2). Steady state was attained at least by day 5 of once-daily dosing (by inspection of daily trough concentrations) and linear pharmacokinetic behaviour was observed across dosing duration. Accumulation (mean and 90% CI) from the first to tenth day of dosing in female subjects was 1.94-fold (1.70, 2.18) and 1.65-fold (1.48, 1.82) in males. The ratio (female/male) of least-squares AUC means was 1.13 (0.89, 1.44) based on the 24-h intervals following the fifth and tenth once-daily doses; i.e. exposure was approximately 13% higher in female subjects. The corresponding ratio for weight-normalized AUC means was 0.97 (0.74, 1.28). The ratio of mean t1/2 values following the tenth dose was 1.14 (0.91, 1.42). These results supported inclusion of female subjects in subsequent clinical pharmacokinetic studies and were later corroborated by the integrated statistical analysis of 13 clinical pharmacology studies.
Effect of age
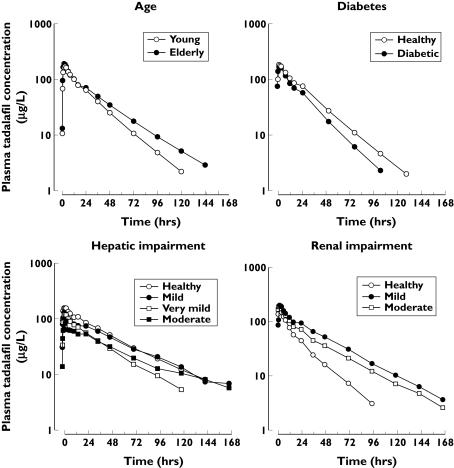
Only small effects of age on pharmacokinetics were observed following administration of a 10-mg dose to 12 elderly and 12 younger male subjects (Table 2; Figure 1). The ratio (elderly/young) of geometric mean AUC values (90% CI) was 1.25 (0.97, 1.61). On average, t1/2 in the elderly was prolonged by approximately 5 h.
Figure 1.
Effects of age, diabetes mellitus and hepatic and renal impairment on arithmetic mean tadalafil concentration–time profiles. (Reprinted from Porst H. European Urology Supplement 1:19–24, Copyright 2002 Elsevier Science B.V. with permission from the European Association of Urology.)
Effect of diabetes mellitus
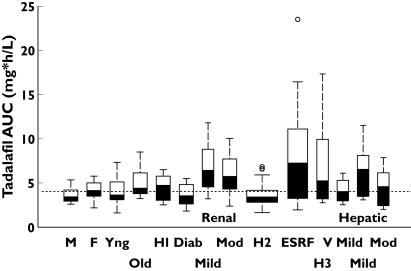
A small effect of diabetes mellitus was detectable in concentration–time profiles (Figure 1) and corresponding noncompartmental parameters (Table 2). The AUC ratio (diabetes/healthy) was 0.81 (0.63, 1.06) and the range of individual AUC values was similar across cohorts (Figure 2). The mean t1/2 was approximately 3 h shorter in the diabetic cohort; 144 h postdose, plasma tadalafil concentrations were quantifiable in four of 12 subjects with diabetes compared with seven of 12 healthy individuals.
Figure 2.
Tadalafil systemic exposures by subject group. Each box defines the 25th, 50th (median) and 75th percentiles of individual AUC values normalized to a 10-mg dose. Box width is proportional to the square root of sample size. The ‘whiskers’ extend from each box to the farthest value within an interquartile range (box height); symbols (○) indicate extreme values. H1, H2 and H3 denote the healthy subject control values for the renal impairment, end-stage renal failure (ESRF) and hepatic impairment studies, respectively. The reference line denotes the historical mean AUC of 4033 μg h−1 ml−1
Effect of renal impairment
Initially, tadalafil 10 mg was administered to eight healthy subjects (CLcr >80 ml min−1), five with mild impairment and six subjects with moderate renal impairment. A high incidence of back pain and myalgia was observed in subjects with moderate impairment in this early clinical pharmacology study. Subsequently, a 5-mg dose was well tolerated following administration to four healthy subjects, three subjects with mild and six with moderate impairment. Pharmacokinetics for 5 mg were approximately dose-proportional to results at the 10-mg dose level.
Renal impairment was associated with relatively high exposure to tadalafil and its major glucuronide metabolite (Figure 1). The mean tadalafil AUC in subjects with mild and moderate renal impairment was approximately twice the value for the healthy cohort and the mean Cmax was 20% higher (Table 2). Contrasts across cohorts for 5-mg doses were consistent with those stated here for 10 mg and dose-normalized data (AUC values at 5 mg multiplied by 2) from all healthy and impaired subjects in the study are included in Figure 2. The effect on AUC was greater than on Cmax, and t1/2 was substantially prolonged (26 or 22 vs. the 14-h control value). Only small effects on distribution volume were observed. Protein binding in individual plasma samples (3 h after dosing) was essentially similar (94–97%) across cohorts and dose levels.
Renal impairment had a greater effect on disposition of the major metabolite than on parent drug and there was a stronger nonlinear association between AUC and CLcr, as expected for a renally eliminated glucuronide. The mean AUC ratio (impaired/healthy) for exposure to total methylcatechol glucuronide was 2.6 for mild and 3.6 for moderate impairment. Corresponding metabolite Cmax ratios were 1.3 and 1.6. An increase in the geometric mean t1/2 from 20 to 44 to 55 h was observed with increasing degree of renal impairment (control, mild, moderate).
ESRF subjects on dialysis
This study was conducted much later than those above, after extensive pharmacokinetic, tolerability and safety information was available. A total of 16 subjects entered the study, eight of whom entered on two occasions (distinct dose levels) for a total of 24 dosing occasions on which a single 5-, 10- or 20-mg dose was administered. A marked difference in the plasma concentration–time profiles was observed between the Polish and UK cohorts, with the latter showing markedly higher exposures (Table 3). This difference, reflected by the mean pharmacokinetic parameters, could not be explained by differences in clinical chemistry, haematology or concomitant medication data. All doses were well tolerated by these subjects.
Table 3.
Geometric mean (CV%) pharmacokinetic parameters for tadalafil and its major metabolite following administration of 5, 10 and 20 mg to subjects with end-stage renal failure
| Parameter | Tadalafil 5 mg (Polish; N = 6) | Tadalafil 10 mg (Polish cohort; N = 6) | Tadalafil 10 mg (UK cohort; N = 6) | Tadalafil 20 mg (UK; N = 6) |
|---|---|---|---|---|
| Tadalafil | ||||
| AUC (μg h l−1) | 1633 (63) | 4023 (38) | 13 749 (37) | 18 090 (39) |
| Cmax (μg l−1) | 78.6 (22) | 186 (17) | 394 (21) | 621 (27) |
| tmax (h)* | 3.00 (2.00–4.00) | 4.00 (2.00–4.00) | 2.04 (1.98–3.98) | 2.04 (1.85–2.15) |
| t1/2 (h) | 13.8 (51) | 15.2 (42) | 24.8 (38) | 18.7 (35) |
| CL/F (l h−1) | 3.06 (63) | 2.49 (38) | 0.73 (37) | 1.11 (39) |
| Vz/F (l) | 60.9 (18) | 55.0 (20) | 26.1 (23) | 29.8 (17) |
| Total methylcatechol glucuronide | ||||
| AUC (μg h l−1) | 6051 (34) | 16837 (18) | –† | – |
| AUC (0–last sample time) (μg h l−1) | 5392 (39) | 9983 (29) | 15 981 (35) | 32 229 (25) |
| Cmax (μg l−1) | 64.2 (33) | 109 (36) | 158 (34) | 300 (26) |
| tmax (h)* | 55.3 (27.9–96.0) | 52.4 (24.0–81.7) | 77.5 (29.2–96.1) | 74.1 (30.3–80.8) |
| t1/2 (h) | 51.6 (52) | 66.2 (41) | – | – |
Median (min–max).
Not calculated. CV%, Coefficient of variation.
An overall mean AUC value calculated across cohorts and dose levels was 2.09 (90% CI 1.74, 2.56) times the mean value for 28 healthy subjects (Figure 2; H2) in two reference studies. The corresponding mean Cmax ratio (ESRF/healthy) was 1.41 (1.23, 1.62). Geometric mean t1/2 values were comparable to historical values in healthy subjects. For the total metabolite, the overall mean AUC ratio (ESRF/healthy) was 3.15 (2.25, 4.41). Haemodialysis contributed negligibly to the elimination of either tadalafil or the metabolite. The overall mean protein binding in ESRF subjects was approximately 96% irrespective of dose and time relative to dialysis compared with a reference value of 94% for healthy subjects.
Effect of hepatic impairment
Tadalafil 10 mg was administered to subjects with very mild, mild or moderate hepatic impairment, as well as to age-matched healthy subjects (Figure 1). There was a trend towards lower, not higher, Cmax values and a prolongation in t1/2 with increasing severity of impairment. No consistent or statistically significant association between CL/F or AUC and disease severity was observed (Table 2). The mean Vz/F value increased significantly with increasing severity of impairment. Pharmacokinetic parameters for the single subject with severe hepatic impairment fell in the range of individual values in the other cohorts. The range of tadalafil AUC values in the healthy cohort (Figure 2; H3) reflected anomalously high between-subject variability (74%) relative to other studies and to an historical reference value (39%) based on exposures in 237 healthy subjects (186 males) in 13 studies [9]. In that integrated statistical analysis, within-subject variability was 13%, indicating consistent exposure in a given individual from occasion to occasion.
Safety
Tadalafil was well tolerated, although the 10-mg dose was judged as not well tolerated in subjects with moderate renal impairment in this early clinical pharmacology study. In spite of the increased incidence of back pain/myalgia in the moderately impaired cohort, onset and duration were similar to those in healthy subjects that experienced back pain or myalgia. This finding in moderate renal impairment was not replicated in subjects with ESRF at doses up to 20 mg. Most drug-related, treatment-emergent adverse events were mild or moderate. There were no serious adverse events or discontinuations due to adverse events. The most commonly reported adverse events across all six studies were headache, back pain and myalgia (Table 4). In the multiple-dose pharmacokinetic evaluation involving females and males, drug-related adverse events occurring in ≥20% of subjects included headache in 15 subjects (62.5%), back pain in nine (37.5%) and myalgia or eye pain in five (20.8%) each.
Table 4.
Drug-related treatment-emergent adverse events (Medical Dictionary for Regulatory Activities; MedDRA) reported on one or more occasions by two or more subjects (in any group) receiving tadalafil
| Number of subjects | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gender effect study* | Age effect study | Diabetes effect study | Renal Impairment study | |||||
| Adverse event | Male (N = 12) | Female (N = 12) | Elderly (N = 12) | Young (N = 12) | Healthy (N = 12) | Diabetes (N = 12) | Healthy (N = 12) | Impaired (N = 20) |
| Headache | 3 | 4 | 2 | 4 | 3 | 3 | 1 | 2 |
| Back pain | 6 | 5 | 3 | 4 | 1 | 6 | 0 | 3 |
| Myalgia | 5 | 3 | 4 | 3 | 1 | 1 | 2 | 4 |
| Musculoskeletal pain | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| Asthenia | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 |
| Somnolence | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Pain in extremity | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Number of subjects | |||||
|---|---|---|---|---|---|
| Hepatic impairment study | Renal dialysis study† | ||||
| Adverse event | Healthy (N = 8) | Hepatically impaired (N = 25) | Tadalafil 5 mg (N = 6) | Tadalafil 10 mg (N = 12) | Tadalafil 20 mg (N = 6) |
| Headache | 4 | 4 | 2 | 2 | 0 |
| Back pain | 0 | 1 | 0 | 0 | 0 |
| Myalgia | 2 | 3 | 0 | 0 | 0 |
| Musculoskeletal pain | 0 | 0 | 0 | 0 | 0 |
| Asthenia | 0 | 0 | 0 | 0 | 0 |
| Somnolence | 0 | 0 | 0 | 3 | 0 |
| Pain in extremity | 0 | 0 | 0 | 0 | 0 |
Single-dose pharmacokinetic data only. Multiple-dose pharmacokinetic data are discussed in text.
Data for treatment-emergent adverse events.
There were no clinically significant laboratory abnormalities or changes in 12-lead ECGs. There were no clinically significant trends in vital sign measurements during the study, although, as expected, several patients with renal impairment were taking antihypertensive medication. Isolated increases and decreases in blood pressure and heart rate were recorded for several subjects. Blood pressure decreases were greater in those individuals with high predose values and occurred more often for standing compared with supine readings, but were not considered to be clinically significant.
Discussion
Six clinical pharmacology studies were conducted to evaluate the effects of gender, age, diabetes mellitus and renal and hepatic impairment on the pharmacokinetics and tolerability of tadalafil. The gender-effect study was completed early in development and the finding of essentially similar pharmacokinetics to those in males supported inclusion of female subjects in some later studies. An integrated statistical analysis of 13 clinical pharmacology studies subsequently [9] indicated that AUC was 14% higher in females even after normalization for body mass index, suggesting that the small gender effect is not necessarily due to body size. Age >65 years and diabetes mellitus had no clinically significant effects on tadalafil pharmacokinetics; thus, dosage adjustment for these factors in patients with ED is not warranted on this basis alone and no adjustment is specified in product labelling [7, 8]. These findings are consistent with population analyses in two efficacy trials which indicated pharmacokinetics in patients with ED and in healthy subjects were essentially similar. Age, weight, diabetic status and renal status (creatinine clearance) had no statistically significant effects on tadalafil clearance or distribution volume [26]. Furthermore, no effect of age was found in pharmacodynamic modelling of ED outcome measures as a function of tadalafil dose or systemic exposure [27]. Diabetic status was confounded with ED severity at baseline, possibly masking an independent effect of this condition in that report.
Age generally affects hepatic blood flow to a greater extent than intrinsic metabolic clearance mediated by CYP3A [28–30]. For tadalafil, age was associated with a negligible effect on Cmax and a slight decrease in CL/F and prolongation of t1/2. By comparison, Cmax of the PDE5 inhibitor sildenafil is 70% higher in the elderly, CL/F is approximately one-half that in younger subjects and t1/2 is prolonged (3.8 vs. 2.6 h) [31]. This pattern suggests a diminution in both presystemic and systemic clearance of sildenafil citrate with advancing age. Both of these PDE5 inhibitors are cleared predominantly via CYP3A4; however, systemic clearance (CL) of sildenafil citrate is at least 15-fold higher than CL of tadalafil [9]. A greater effect of age on the higher clearance drug is consistent with a greater effect of age on hepatic blood flow than on CYP3A metabolism.
Because tadalafil is cleared predominantly via CYP3A4, it was important to evaluate the effect of hepatic impairment in altering tadalafil pharmacokinetics, especially given that metabolic clearance of some drugs by CYP3A4 is reduced by 30–50% in impaired subjects [32]. Mild or moderate hepatic impairment did not decrease CL/F, as would be expected from a decrement of tadalafil intrinsic clearance and/or an increment in its bioavailability (F) as the predominant mechanism. Indeed, maximal (Cmax) and average (AUC) plasma tadalafil concentrations were actually lower in the impaired subjects compared with healthy volunteers. Population analyses showed that serum albumin, alanine aminotransferase, aspartate aminotransferase and bilirubin had no statistically significant effects on clearance in patients with ED [26]. In one analysis, CL/F decreased with increasing serum γ-glutamyl transaminase; however, this hepatic enzyme was not a significant covariate in the other analysis, explained negligible patient-to-patient variability in clearance, and was considered clinically unimportant. Thus, there is no pharmacokinetic basis to consider dose reduction. Product labelling states that the tadalafil dose in mild and moderate hepatic impairment should not exceed the 10-mg dose that was studied [7, 8]. Because of recruitment difficulties limiting data to one subject, no inference about severe hepatic impairment can be supported.
The only consistent and statistically significant difference across groups was an increase in apparent distribution volume (Vz/F) with increasing severity of hepatic impairment. An increase in Vz/F as the primary impact would be expected to lower Cmax and prolong t1/2 values, with negligible consistent effect on CL/F or AUC, as was observed. A higher Vz/F might in theory [33] arise from an increase in unbound fraction in plasma, due perhaps to reduced albumin concentrations; however, this mechanism does not account for the pharmacokinetic pattern observed (Table 2) for tadalafil. Tadalafil is a low (restrictive) clearance drug; therefore, one would expect CL/F to increase (and AUC to decrease) with increasing unbound fraction in plasma (assuming no compensatory decrease in intrinsic clearance). Interestingly, the observed pattern (increased Vz/F, prolonged t1/2 and presumably no change in CL/F) is consistent with the theoretical expectations for an increase in hepatic tissue binding as the predominant impact of hepatic impairment [34].
Clearance of tadalafil is reduced by renal insufficiency, with a corresponding prolongation of t1/2 and exposure approximately twice that in healthy subjects. Interpretation of the pharmacokinetics was hampered by inconsistencies in findings within and across studies. For example, the mean AUC (2868 μg h l−1) for the healthy subject (10-mg cohort) was anomalously low relative to the reference mean (4033 μg h l−1) from the integrated statistical analysis of 13 clinical pharmacology studies [9]. The rank order of mean AUC values was not consistent across degree of renal impairment, presumably due to high between-subject variability and small sample size, nor across the Polish and UK cohorts in the ESRF study. Regardless, the weight of evidence clearly indicates that for a given dose, tadalafil concentrations will be relatively high in the presence of moderate impairment, and especially in ESRF, thereby providing a pharmacokinetic basis for dose adjustment as specified in product labelling. In the European Union, a 10-mg dose is recommended in the case of moderate impairment and careful individual benefit/risk evaluation in the case of severe impairment (creatinine clearance <30 ml min−1). Labelling in the USA recommends a starting dose of 5 mg (not exceeding 10 mg per 48 h) and a dose not to exceed 5 mg in patients with severe impairment on haemodialysis. No dose adjustment is required in patients with mild renal impairment [7, 8].
It is not obvious why exposure to tadalafil, which has negligible renal clearance, is increased by renal impairment, especially given that changes in plasma protein binding were ruled out. There is ample precedence for renal impairment increasing F (decreased first-pass metabolism) or decreasing systemic clearance (CL), and thereby increasing Cmax, AUC and t1/2, of drugs (e.g. reboxetine) extensively metabolized by various CYP forms [35–37]. These effects are not presently understood at a mechanistic level. The fact that AUC was increased more than Cmax and t1/2 was prolonged strongly suggests that the predominant impact of renal impairment was on CL (diminished intrinsic clearance by CYP3A4) rather than F. Renal impairment increased exposure to the major metabolite, total methylcatechol glucuronide, and prolonged its elimination half-life to a greater extent than tadalafil itself, as expected for a glucuronide conjugate cleared by the kidney. This metabolite, like most ether (as opposed to acyl) glucuronides, is presumed to be pharmacologically inactive [38, 39].
Tadalafil 5–20 mg was well tolerated, with no serious adverse events or discontinuations due to adverse events in these studies. Unlike the observations in subjects with moderate renal impairment administered 10 mg tadalafil, single oral doses of tadalafil up to 20 mg were well tolerated by subjects with ESRF undergoing haemodialysis. The high incidence of reports of back pain and myalgia by subjects with moderate renal impairment may be attributable to the small sample size and is consistent with incidences reported in clinical pharmacology studies with healthy subjects. The incidence of back pain and myalgia ranged from 0 to 100% of subjects across 43 single-dose studies (5-, 10- and 20-mg doses), with a mean incidence of approximately 30% in placebo-controlled efficacy/safety trials.
The most common adverse events were headache, back pain and myalgia, which are among the most common treatment-emergent adverse events observed during multiple-dose, Phase 3 studies, dyspepsia and nasopharyngitis being the others [4, 7, 8]. In integrated analyses of Phase 3 studies, the most frequent treatment-emergent adverse event was headache, occurring in 15% of patients randomized to tadalafil 20 mg (taken as needed prior to sexual intercourse), in 12% of patients taking tadalafil 10 mg and in 5% of placebo patients. Corresponding values for 20 mg, 10 mg and placebo, respectively, were: dyspepsia (8%, 7% and 1%), back pain (5%, 6% and 2%), nasopharyngitis (2%, 8% and 4%) and myalgia (3%, 5% and 1%) [4]. No association between the incidence of all treatment-emergent adverse events related to back pain and myalgia (including back stiffness, buttock pain, coccydynia, loin pain, sacral pain and pain not otherwise specified) and mild/moderate renal impairment was found. The multiple-dose (gender effect) study was conducted in a small number of subjects and was not placebo controlled. Frequencies of headache (62.5%) and back pain (37.5%) in the multiple-dose assessment in the gender study were much higher than has been observed in other multiple-dose clinical pharmacology studies. For example, in a study in which otherwise healthy men with or without mild ED received tadalafil once daily for 6 months, headache occurred in 17.5% of subjects randomized to 10 mg (vs. 5% on placebo) and in 19.8% of subjects randomized to 20 mg (vs. 3.8%). Back pain occurred in 11.7% of subjects receiving 10 mg (vs. 4.0%) and in 10.8% of those receiving 20 mg (vs. 1.9%) [40].
Acknowledgments
The authors thank Alison Mackie, Isabelle Pouliquen, Dr Alexander Staab and Nathalie Toublanc for their technical contributions, and acknowledge Stephen Gutkin (Rete Biomedical Communications Corp., Ridgewood, NJ, USA) and Diane Stothard PhD (Eli Lilly and Co., Indianapolis, IN, USA) for writing and editorial assistance. Funding: Lilly ICOS LLC (Indianapolis, IN, and Bothell, WA, USA).
References
- 1.Brock GB, McMahon CG, Chen KK, Costigan T, Shen W, Watkins V, Anglin G, Whitaker S. Efficacy and safety of tadalafil for the treatment of erectile dysfunction: results of integrated analyses. J Urol. 2002;168:1332–6. doi: 10.1016/S0022-5347(05)64442-4. [DOI] [PubMed] [Google Scholar]
- 2.Montorsi F, Verheyden B, Meuleman E, Junemann KP, Moncada I, Valiquette L, Casabe A, Pacheco C, Denne J, Knight J, Segal S, Watkins VS. Long-term safety and tolerability of tadalafil in the treatment of erectile dysfunction. Eur Urol. 2004;45:339–44. doi: 10.1016/j.eururo.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Montorsi F, Nathan HP, McCullough A, Brock GB, Broderick G, Ahuja S, Whitaker S, Hoover A, Novack D, Murphy A, Varanese L. Tadalafil in the treatment of erectile dysfunction following bilateral nerve sparing radical retropubic prostatectomy: a randomized, double-blind, placebo controlled trial. J Urol. 2004;172:1036–41. doi: 10.1097/01.ju.0000136448.71773.2b. [DOI] [PubMed] [Google Scholar]
- 4.Carson CC, Rajfer J, Eardley I, Carrier S, Denne JS, Walker DJ, Shen W, Cordell WH. The efficacy and safety of tadalafil: an update. Br J Urol Int. 2004;93:1276–81. doi: 10.1111/j.1464-410X.2004.04819.x. [DOI] [PubMed] [Google Scholar]
- 5.Carson C, Shabsigh R, Segal S, Murphy A, Fredlund P. Efficacy, safety, and treatment satisfaction of tadalafil versus placebo in patients with erectile dysfunction evaluated at tertiary-care academic centers. Urology. 2005;65:353–9. doi: 10.1016/j.urology.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 6.Sáenz de Tejada I, Anglin G, Knight JR, Emmick JT. Effects of tadalafil on erectile dysfunction in men with diabetes. Diabetes Care. 2002;25:2159–64. doi: 10.2337/diacare.25.12.2159. [DOI] [PubMed] [Google Scholar]
- 7.Tadalafil (Cialis®) Summary of Product Characteristics. [6 July 2006]; Available at http://www.emea.eu.int/humandocs/Humans/EPAR/cialis/cialis.htm.
- 8.Lilly ICOS LLC. Tadalafil (Cialis®) US Prescribing Information. [6 July 2006]; Available at http://pi.lilly.com/us/cialis-pi.pdf.
- 9.Forgue ST, Patterson BE, Bedding AW, Payne CD, Phillips DL, Wrishko RE, Mitchell MI. Tadalafil pharmacokinetics in healthy subjects. Br J Clin Pharmacol. 2006;61:280–8. doi: 10.1111/j.1365-2125.2005.02553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porst H, Padma-Nathan H, Giuliano F, Anglin G, Varanese L, Rosen R. Efficacy of tadalafil for the treatment of erectile dysfunction at 24 and 36 hours after dosing: a randomized controlled trial. Urology. 2003;62:121–5. doi: 10.1016/s0090-4295(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 11.Ring BJ, Gillespie JS, Mullen JH, Wrighton SA. Identification of the human cytochrome P450 (CYP) responsible for the formation of the major oxidative metabolite of tadalafil (Cialis) Drug Metab Rev. 2004;36(S1):70. [Google Scholar]
- 12.Phillips DL, Smith RL, Patterson BE, Parker N, Mitchell M, Wheeler WJ, Watkins VS, Barbuch RJ. Metabolism and excretion of tadalafil in healthy men after oral administration of 100 mg [14C]-tadalafil. The AAPS J. 2004;6:W5308. [Google Scholar]
- 13.Francis SH, Corbin JD. Molecular mechanisms and pharmacokinetics of phosphodiesterase-5 antagonists. Cur Urol Reports. 2003;4:457–65. doi: 10.1007/s11934-003-0027-x. [DOI] [PubMed] [Google Scholar]
- 14.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 15.National Institutes of Health. NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA. 1993;270:83–90. [PubMed] [Google Scholar]
- 16.Aytaç IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. Br J Urol Int. 1999;84:50–6. doi: 10.1046/j.1464-410x.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- 17.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 18.Collins AJ, Kasiske B, Herzog C, Chen SC, Everson S, Constantini E, Grimm R, McBean M, Xue J, Chavers B, Matas A, Manning W, Louis T, Pan W, Liu J, Li S, Roberts T, Dalleska F, Snyder J, Ebben J, Frazier E, Sheets D, Johnson R, Li S, Dunning S, Berrini D, Guo H, Solid C, Arko C, Daniels F, Wang X, Forrest B, Gilbertson D, St Peter W, Frederick P, Eggers P, Agodoa L. Excerpts from the United States Renal Data System 2003 annual data report: atlas of end-stage renal disease in the United States. Am J Kidney Dis. 2003;42:S1–S226. [PubMed] [Google Scholar]
- 19.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 20.Patterson B, Bedding A, Jewell H, Payne C, Mitchell M. Proceedings of the 4th Congress of the European Society for Sexual and Impotence Research. Rome, Italy: 2001. The effect of intrinsic and extrinsic factors on the pharmacokinetic properties of tadalafil (IC351) [Google Scholar]
- 21.Curran MP, Keating GM. Tadalafil. Drugs. 2003;63:2203–12. doi: 10.2165/00003495-200363200-00004. [DOI] [PubMed] [Google Scholar]
- 22.The Diabetes Control and Complications Trial Research Group. Influence of intensive diabetes treatment on body weight and composition of adults with type 1 diabetes in the Diabetes Control and Complications Trial. Diabetes Care. 2001;24:1711–21. doi: 10.2337/diacare.24.10.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 24.Hauschke D, Steinijans VW, Diletti E. A distribution-free procedure for the statistical analysis of bioequivalence studies. Int J Clin Pharmacol Ther Toxicol. 1990;28:72–8. [PubMed] [Google Scholar]
- 25.Ring BJ, Patterson BE, Mitchell MI, Vandenbranden M, Gillespie J, Bedding AW, Jewell H, Payne CD, Forgue ST, Eckstein J, Wrighton SA, Phillips DL. Effect of tadalafil on cytochrome P450 3A4-mediated clearance: studies in vitro and in vivo. Clin Pharm Ther. 2005;77:63–75. doi: 10.1016/j.clpt.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Trocóniz IF, Tillmann C, Staab A, Rapado J, Mackie A, Mitchell M, Patterson B, Forgue ST. Tadalafil population pharmacokinetics in patients with erectile dysfunction. AAPS Pharmsci. 2003;5:T2341. doi: 10.1007/s00228-007-0297-1. [DOI] [PubMed] [Google Scholar]
- 27.Staab A, Tillmann C, Forgue ST, Mackie A, Allerheiligen SRB, Rapado J, Trocóniz IF. Population dose–response model for tadalafil in the treatment of male erectile dysfunction. Pharm Res. 2004;21:1463–70. doi: 10.1023/b:pham.0000036922.03519.40. [DOI] [PubMed] [Google Scholar]
- 28.Cotreau MM, von Moltke LL, Greenblatt DJ. The influence of age and sex on the clearance of cytochrome P450 3A substrates. Clin Pharmacokinet. 2005;44:33–60. doi: 10.2165/00003088-200544010-00002. [DOI] [PubMed] [Google Scholar]
- 29.Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmaco-dynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57:6–14. doi: 10.1046/j.1365-2125.2003.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmucker DL. Liver function and phase I drug metabolism in the elderly: a paradox. Drugs Aging. 2001;18:837–51. doi: 10.2165/00002512-200118110-00005. [DOI] [PubMed] [Google Scholar]
- 31.Muirhead GJ, Wilner K, Colburn W, Haug-Pihale G, Rouviex B. The effects of age and renal and hepatic impairment on the pharmacokinetics of sildenafil. Br J Clin Pharmacol. 2002;53(Suppl. 1):21S–30S. doi: 10.1046/j.0306-5251.2001.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodighiero V. Effects of liver disease on pharmacokinetics. An update. Clin Pharmacokinet. 1999;37:399–431. doi: 10.2165/00003088-199937050-00004. [DOI] [PubMed] [Google Scholar]
- 33.MacKichan J. Influence of protein binding and of unbound (free) drug concentrations. In: Evans WE, Schentag JJ, Jusko WJ, editors. Applied Pharmacokinetics. Principles of Therapeutic Drug Monitoring. 3. Vancouver WA: Applied Therapeutics; 1992. pp. 5-1–5-48. [Google Scholar]
- 34.Wedlund PJ, Wilkinson GR. Hepatic tissue binding and the oral first-pass effect. J Pharm Sci. 1984;73:422–5. doi: 10.1002/jps.2600730340. [DOI] [PubMed] [Google Scholar]
- 35.Nolin TD, Frye RF, Matzke GR. Hepatic drug metabolism and transport in patients with kidney disease. Am J Kidney Dis. 2003;42:906–25. doi: 10.1016/j.ajkd.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Yuan R, Venitz J. Effect of chronic renal failure on the disposition of highly hepatically metabolized drugs. Int J Clin Pharmacol Ther. 2000;38:245–53. doi: 10.5414/cpp38245. [DOI] [PubMed] [Google Scholar]
- 37.Coulomb F, Ducret F, Laneury JP, Fiorentini F, Poggesi I, Jannuzzo MG, Fleishaker JC, Houin G, Duchene P. Pharmacokinetics of single-dose reboxetine in volunteers with renal insufficiency. J Clin Pharmacol. 2000;40:482–7. doi: 10.1177/00912700022009251. [DOI] [PubMed] [Google Scholar]
- 38.Ritter JK. Roles of glucuronidation and UDP-glucuronosyltransferases in xenobiotic bioactivation reactions. Chem-Biol Interact. 2000;129:171–93. doi: 10.1016/s0009-2797(00)00198-8. [DOI] [PubMed] [Google Scholar]
- 39.Bailey MJ, Dickinson RG. Acyl glucuronide reactivity in perspective: biological consequences. Chem-Biol Interact. 2003;145:117–37. doi: 10.1016/s0009-2797(03)00020-6. [DOI] [PubMed] [Google Scholar]
- 40.Hellstrom WJG, Overstreet JW, Yu A, Saikali K, Shen W, Beasley CM, Jr, Watkins V. Tadalafil has no detrimental effect on human spermatogenesis or reproductive hormones. J Urol. 2003;170:887–91. doi: 10.1097/01.ju.0000081053.97792.da. [DOI] [PubMed] [Google Scholar]