Abstract
Aims
To investigate the relationship between changes in plasma deoxynucleoside concentrations and response and toxicity in patients treated with capecitabine.
Methods
Twenty-six patients received 2 g capecitabine twice daily orally for 2 weeks of a 3-week cycle. Blood samples were collected on day 0 (baseline), day 8, day 15 and day 22 of the first cycle for the determination of plasma thymidine (TdR) and deoxyuridine (UdR) concentrations. Patients were reviewed weekly during the first cycle, then 3-weekly for toxicity assessment. Response was assessed according to Response Evaluation Criteria in Solid Tumours (RECIST) criteria.
Results
The plasma UdR and UdR/TdR ratios were significantly elevated (P < 0.001) compared with baseline (49.3 ± 20.8 nmol l−1) for the entire 3-week treatment period. In contrast, the plasma TdR concentrations of these patients were significantly reduced only on day 8 (P < 0.01) compared with baseline (12.1 ± 3.83 nmol l−1), but returned gradually to basal levels by day 15. There were no significant correlations demonstrated between pretreatment or maximal post-treatment plasma nucleoside ratio and either toxicity or response. The TSER genotype frequencies of homozygous TSER*2, TSER*3 and heterozygous TSER*2/*3 were 7.7%, 42.3% and 50%, respectively. These preliminary data also indicate no direct relationship between thymidylate synthase (TS) genotype and plasma nucleoside levels.
Conclusions
Capecitabine mimics continuous infusion of 5-FU to achieve sustained cellular TS inhibitory effects and suggests the antiproliferative mechanism of capectabine is at least partly due to TS inhibition through its active metabolite FdUMP. Although plasma UdR and TdR concentrations and the UdR/TdR ratio can provide some pharmacodynamic indication of TS inhibition, they are unlikely to predict therapeutic response or toxicity accurately following capecitabine treatment in cancer patients.
Keywords: capecitabine, deoxyuridine, nucleosides, pharmacodynamic marker, thymidine, TS inhibition
Introduction
Two main intracellular mechanisms of action have been suggested for the cytotoxic effects of fluoropyrimidines such as 5-FU and capecitabine (N4-pentyloxycarbonyl-5′-deoxy-5-fluorocytidine): inhibition of thymidylate synthase (TS) and hence of pyrimidine de novo synthesis; and incorporation of fraudulent bases into RNA and DNA. Protracted infusional regimens of 5-FU provide superior activity with reduced toxicity compared with bolus schedules and have been hypothesized to favour TS inhibition as their principal cytotoxic mechanism [1, 2]. Inhibition of TS should provide selectivity to DNA synthesis and limit toxicities caused by other mechanisms. Infusional regimens are difficult to administer, as they require central venous catheters and the use of infusion devices. Orally bioavailable 5-FU prodrugs have been developed to mimic infusional regimens. Capecitabine is a novel fluoropyrimidine carbamate designed to generate 5-FU preferentially in tumour tissues [3]. The final stage of its enzymatic conversion is mediated by thymidine phosphorylase (TP), which is upregulated in tumour compared with normal tissues. This is proposed to enhance drug activation in tumour cells and reduce systemic toxicity [4, 5]. Preclinical studies have shown that capecitabine is more effective over a wider dose range and has a broader spectrum of antitumour activity than either 5-FU or another 5-FU prodrug, UFT, against human cancer xenografts [6]. Results from two large Phase III trials have shown that capecitabine produced higher response rates and equivalent survival to an intravenous bolus schedule of 5-FU/LV (Mayo Clinic regimen) as first-line treatment for metastatic colorectal cancer (CRC) [7, 8] and provided superior safety and tolerability [9].
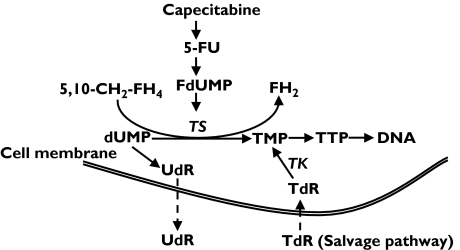
Inhibition of TS in tissues leads to accumulation of deoxyuridine monophosphate (dUMP) with subsequent efflux of deoxyuridine (UdR) into the circulation (Figure 1). Recent evidence suggests that increased plasma levels of UdR could be an important pharmacodynamic marker of antifolate TS inhibitors (TSI) such as AG377 [10–13], ZD 9331 [14–17] and ZD1694 [18, 19] and might enable rational dose adjustment in patients. In one study, plasma UdR was as used as a pharmacodynamic (PD) marker with 5-FU treatment (bolus and infusion) in CRC patients. The improved therapeutic response with the infusional regimen was associated with prolonged elevation of plasma UdR, suggesting more prolonged inhibition of TS with this schedule [20].
Figure 1.
Thymidylate synthase (TS) inhibitory effects on the biosynthetic pathway of thymidine nucleotides
Inhibition of TS and hence de novo pyrimidine synthesis may be offset by salvage pathways. However, the clinical relevance of thymidine (TdR) salvage is unknown and much less attention has been given to this alternative pathway in cancer chemotherapy. Early in vitro and animal studies have shown that inhibition of TdR salvage potentiates the cytotoxicity of TSI. Coadministration of exogenous TdR effectively reverses the cytotoxic effects of TSI [21–26]. Therefore, significant interpatient differences in pretreatment plasma TdR concentrations could lead to differences in response and toxicity after treatment with TS inhibitors. Also, tracking the extent and duration of the fall in TdR after treatment with a TSI may help identify patients more likely to respond to and/or experience undue toxicity following treatment and could help to improve drug scheduling. Consequently, like UdR, plasma TdR could also be a useful surrogate PD marker of TS inhibition. The measurement of plasma TdR concentration has been difficult and to date no clinical study has successfully been carried out to assess the response of plasma TdR to treatment with TSI.
There is also emerging evidence that genetic polymorphisms of the TS gene (18p11.32) could predict toxicity and tumour response to 5-FU-based therapies in patients with metastatic CRC. In particular, a polymorphism of the variable number of tandem repeat (VNTR) in the 5′-promoter/enhancer region (TSER) of the gene, mainly TSER*2 and TSER*3, has been shown to modulate TS mRNA expression and translational efficiency [27–29]. Several clinical studies have demonstrated that the presence of this 28-bp tandem repeat polymorphism was able to predict the clinical response to 5-FU-based chemotherapy [30–34]. Recently, a common G→C single nucleotide polymorphism (SNP) has also been identified in the 12th nucleotide of the second repeat of TSER*3 allele and has been related to the TS transcriptional activation [35]. Observations from Kawakami and Watanabe showed that coevaluation of both polymorphic loci may provide a more effective prediction of the clinical outcome of 5-FU-based chemotherapy [36].
On the basis of these observations, the major objectives of the present study were to investigate the relationship between pretreatment (baseline) and post-treatment plasma deoxynucleoside concentrations and response and toxicity in metastatic CRC patients treated with capecitabine twice daily for 2 weeks of a 3-week cycle. In addition, it was planned to undertake a preliminary exploration of the relationship between polymorphisms of the TSER of the TS gene and plasma nucleosides levels.
Patients and methods
All patients included in this study were recruited from Sydney Cancer Centre at Royal Prince Alfred and Concord Hospitals. Eligibility criteria for patient recruitment included histological confirmation of advanced CRC, less than two prior other chemotherapy regimens, performance status 0–2 and a life expectancy of at least 12 weeks. Exclusion criteria were uncontrolled cerebral metastases, inability to be changed to low-molecular-weight heparins if on warfarin and the presence of severe comorbidities or pregnancy/lactation. The patients received a fixed dose of 2 g capecitabine twice daily orally for 2 weeks of a 3-week cycle. They were reviewed weekly during first cycle, then 3-weekly for toxicity assessment. Response was assessed every two cycles according to Response Evaluation Criteria in Solid Tumours (RECIST) criteria [37] and toxicities were graded according to the National Cancer Institute Common Toxicity Criteria (CTC) v2.0 [38]. The human ethics committee of the Central Sydney Area Health Service and the University of Sydney approved these studies.
Sample preparation
Blood samples were collected from the patients on days 0 (baseline), 8, 15 and 22 of the first cycle for the determination of plasma TdR and UdR concentrations. Blood samples were also obtained from six normal volunteers in the same period of time. Whole blood was collected into a prechilled heparinized blood tube and placed on ice immediately. Plasma was rapidly separated from cellular components by centrifuging at 3300 g at 4 °C for 15 min. Plasma was then stored at −80 °C and thawed just prior to analysis. Additional whole blood was also collected for TSER genotyping.
Sample analysis
Plasma UdR and TdR levels were analysed using a validated LC-MS method which we have previously reported [39, 40]. Briefly, plasma samples were extracted with strong anion-exchange solid-phase extraction (SAX-SPE) columns followed by high-performance liquid chromatography separation and atmospheric pressure chemical ionization mass spectrometry detection (APCI-MS) in a selected-ion monitoring (SIM) mode. Values for nucleosides were expressed as a percentage of pretreatment baseline levels and ratios of UdR/TdR. For TSER genotyping, genomic DNA was extracted from whole blood using the Qiagen DNeasy extraction kit according to the manufacturer’s instructions (QIAGEN, Clifton Hill, Australia). The TSER VNTR polymorphism was analysed by polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) analysis as previously described [41]. The forward primer was 5′-GTG GCT CCT GCG TTT CCC-3′ and the reverse 5′-GCT CCG AGC CGG CCA CAG GCA TGG CGG-3′. Analysis of the G→C (12th nucleotide/TSER*3) SNP in the second repeat was performed by digesting PCR products with HaeIII restriction enzyme as previously reported [35].
Statistical analysis
The percentage values for UdR and TdR at each time point are expressed as mean ± SD. Statistical analysis of data was achieved by Student’s t-tests and regression correlation (GraphPad Prism program, San Diego, CA, USA). Significant differences between response in patients and plasma nucleoside levels were assessed by unpaired t-test. One-way anova test was used to assess significant differences between plasma nucleoside levels and TSER genotyping. The relationship between toxicity profiles and plasma nucleoside levels were also carried out by one-way anova test. Hardy–Weinberg equilibrium analysis was used to assess the allele frequency. P-values ≤0.05 were considered to denote statistical significance.
Results
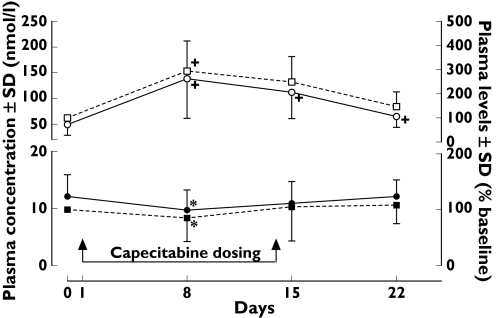
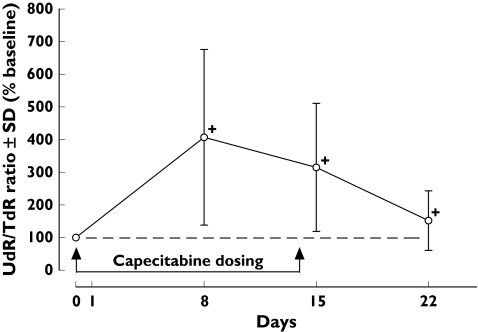
Full deoxynucleoside analyses were performed in 26 patients. The plasma UdR and TdR concentrations in patients treated with capecitabine are shown in Figure 2. The mean pretreatment plasma UdR and TdR concentrations were 49.3 ± 20.8 nmol l−1 and 12.1 ± 3.83 nmol l−1, respectively. There was no correlation between plasma TdR and UdR concentrations in pretreatment (R2 = 0.0543) or post-treatment (R2 = 0.0093) samples. The plasma UdR levels of treated patients were significantly increased on day 8 (294 ± 125%, P < 0.001), day 15 (249 ± 105%, P < 0.001) and day 22 (147 ± 61%, P < 0.001) compared with baseline (Figure 2). In contrast, the plasma TdR concentrations of these patients were significantly reduced only on day 8 (85.7 ± 42.4%, P < 0.01) compared with baseline, but returned gradually to basal levels by days 15 (105 ± 60.7%) and 22 (108 ± 33.3%). As a result, percentage ratios of plasma UdR/TdR concentrations were all elevated significantly during the 3-week cycle of treatment (Figure 3). The maximum peak ratio was on day 8 (fourfold, P < 0.001). The overall pattern of percentage elevation from UdR/TdR ratios was similar to plasma UdR alone, on day 8 (407 ± 269%, P < 0.001), day 15 (315 ± 196%, P < 0.001) and day 22 (152 ± 91%, P < 0.001). There were no significant differences in the basal plasma UdR (56.2 ± 18.2 and 49.3 ± 20.8 nmol l−1) and TdR (9.98 ± 3.85 and 12.1 ± 3.83 nmol l−1) concentrations in normal volunteers and cancer patients, respectively.
Figure 2.
Plasma deoxyuridine (UdR) and thymidine (TdR) levels in cancer patients following capecitabine 2 g twice daily for 14 days (n = 26). Data are mean ± SD. †P < 0.001 and *P < 0.01 compared with baseline value (paired t-test)
Figure 3.
Percentage ratio changes of plasma deoxyuridine (UdR)/thymidine (TdR) during the course of capecitabine treatment (n = 26). Data are mean ± SD. †P < 0.001 when compared with baseline value (paired t-test)
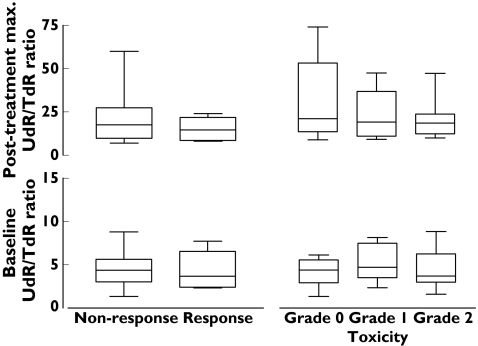
Tumour response (complete response (CR) + partial response (PR)) was observed in 23% (6/26) of patients. The most common nonhaematological toxicities were nausea (8/26), hand–foot syndrome (4/26), stomatitis (3/26) and vomiting (2/26). Toxicity was generally mild with grade I and grade II toxicities seen in 8/26 and 9/26 patients, respectively. There were no grade III or IV toxicities observed. There were no significant differences between baseline or maximum increased post-treatment plasma nucleoside ratios and toxicity (baseline, F = 0.40, P < 0.67; post-treatment, F = 0.38, P < 0.69) or response (pretreatment t = 0.29, P < 0.77; post-treatment, t = 0.97, P < 0.34) as shown in Figure 4.
Figure 4.
Changes of plasma nucleoside ratios on drug response and toxicity (n = 26). The box extends from the 25th percentile to the 75th percentile, with a line at the median (the 50th percentile). The whiskers extend above and below the box to show the highest and lowest values
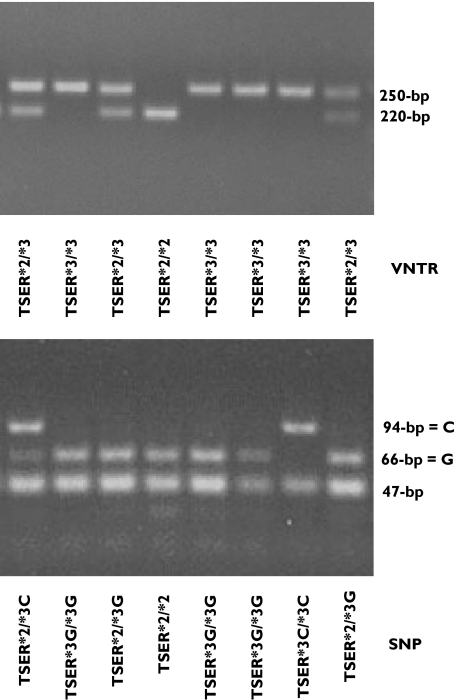
The PCR-RFLP analyses of the VNTR and G→C SNP of 12th nucleotide of the second repeat of TSER*3 allele are shown in Figure 5. Individuals homozygous for the presence of the TSER*2 or TSER*3 were characterized by a single fragment of 220 or 250 bp, respectively. For the SNP (12th nucleotide/TSER*3), HaeIII digestion produced 66- and 47-bp bands for the *3G allele and 94- and 47-bp bands for the *3C allele. The genotype and allele frequencies of TSER polymorphism in the study patients are shown in Table 1. The TSER genotype frequencies of TSER*2/TSER*2, TSER*2/TSER*3 and TSER*3/TSER*3 were 7.7%, 50% and 42.3%, respectively, which are similar to those previously reported in White populations [41, 42]. The SNP allele frequencies for TSER*2, TSER*3G and TSER*3C were 32.7%, 44.2% and 23.1%, respectively. There were no significant differences in either pretreatment or maximum post-treatment plasma nucleoside ratios as a function of TSER genotyping for VNTR (pretreatment, F = 0.58, P < 0.57; post-treatment, F = 0.04, P < 0.96). Statistical analysis was not carried out on TSER SNP because of inadequate sample numbers.
Figure 5.
Polymerase chain reaction–restriction fragment length polymorphism analysis of VNTR and SNP in patients (n = 26)
Table 1.
Genotype frequency of TSER polymorphism in colorectal cancer patients (number within parentheses indicates percentage)
| TSER*2/*2 | TSER*2/*3 | TSER*3/*3 | ||||
|---|---|---|---|---|---|---|
| *2/*2 | *2*/3G | *2/*3C | *3G/*3G | *3G/*3C | *3C/*3C | |
| Male | 0 | 5 (31.2) | 3(18.8) | 4(25) | 3 (18.8) | 1 (6.3) |
| Female | 2 (20) | 3 (30) | 2 (20) | 1 (10) | 2 (20) | 0 |
| Total | 2 (7.7) | 8 (30.8) | 5 (19.2) | 5 (19.2) | 5 (19.2) | 1 (3.8) |
| 7.7% | 50% | 42.3% | ||||
Discussion
The present findings are the first to describe the metabolic relationship of endogenous nucleoside concentration during capecitabine treatment in CRC patients. The plasma levels of UdR in CRC patients treated with 2 g capecitabine twice daily orally for 2 weeks of a 3-week cycle were significantly elevated for the entire 3-week treatment period. These results suggest that TS is inhibited during the course of capecitabine treatment and support the use of plasma UdR as a surrogate marker of TS inhibition [14, 16, 17, 20, 43, 44]. The data also support the view that the antiproliferative mechanism of capecitabine is at least partly due to the inhibitory effects on TS of its active metabolite FdUMP and that inhibition of TS by the standard regimen of capecitabine is durable. This may help to explain its superior response rates compared with standard intravenous 5-FU/LV (Mayo Clinic regimen) as first-line treatment for metastatic CRC [7–9]. Early preclinical studies have demonstrated that the duration of TS inhibition was significantly longer following treatment with FUdR than with 5-FU and resulted in improved antitumour effect without increased toxicity [45]. This suggests that, in order to improve the therapeutic efficacy of TS inhibitors, it is necessary to provide sustained inhibition. Therefore, the duration of TS inhibition, as demonstrated by sustained elevation of plasma UdR, may be an important determinant of the activity of TSI. This finding has recently been supported by clinical studies of different schedules of 5-FU regimens in cancer patients. Ford and colleagues reported that plasma UdR concentrations were elevated for <8 days in CRC patients receiving 5-FU 425 mg m−2 day−1 with leucovorin (LV) 20 mg m−2 day−1 (Mayo Clinic regimen) as a daily bolus dose for the first 5 days of a 4-week cycle [20]. Although TS inhibition was more prolonged with a continuous infusion of 5-FU 300 mg m−2 day−1 for 12 weeks, there was less toxicity than with the Mayo Clinic regimen. This suggests that part of the toxicity may not be due to TS inhibition but possibly result from fraudulent base incorporation. Therefore, on the basis of these previous observations, the present findings provide additional evidence that the improved therapeutic index of capecitabine might result from prolonged TS inhibition. These data also suggest that capecitabine mimics continuous infusion of 5-FU to achieve sustained cellular TS inhibitory effects.
The TS inhibitory effects on de novo thymidine triphosphate (TTP) synthesis might be expected to result in the activation of the TdR salvage pathway by phosphorylating extracellular TdR to make thymidine monophosphate (TMP) available for DNA synthesis (Figure 1). However, the importance of TdR salvage in humans is unknown. Early reports indicated that plasma levels of TdR in normal subjects and advanced solid tumour patients exhibited marked variation (>20-fold) in the range of 50–900 nmol l−1[46–51]. However, using sophisticated mass spectrometry techniques, we have shown no significant difference in basal plasma TdR concentrations among normal subjects and CRC cancer patients [39]. Interestingly, human plasma TdR levels are much lower than previously indicated, in the range of 7–15 nmol l−1. This is in marked contrast to the 100-fold and 300-fold higher concentrations observed in rats and mice, respectively [39]. The present study has shown that TdR concentrations fall modestly after treatment with capecitabine to a maximum of 86% of baseline on day 8, but return to basal concentrations on day 15. These data concur with previous studies in mice, which demonstrated a 50% reduction of plasma TdR 24 h after five daily injections of RTX (5 mg kg −1 × 5 days) [18]. The small number of patients in the current study precludes any major conclusions being drawn from statistical analysis of the data. In particular, the incidences of tumour response and severe toxicity with single agent capecitabine were relatively low and did not appear correlated with either plasma TdR concentration or the UdR/TdR ratio. Also, the depletion of endogenous plasma TdR in the first week did not correlate with elevations in plasma UdR concentration. The unsustained reduction of plasma TdR observed in this study is contrary to what might have been expected from the prolonged elevation in plasma UdR concentration. Nevertheless, it may indicate differences in the relative contributions of de novo synthesis and salvage synthesis in different tissue pyrimidine nucleotide pools [52]. The large differences in the basal plasma TdR observed between man and rodent [39] indicate that rodent tumour models (or human tumour xenografts) might not be suitable models in which to evaluate antitumour activity or host toxicity of anticancer drugs designed to inhibit the de novo synthesis of TS prior to clinical studies. Furthermore, the TSER VNTR genotype, which has previously been proposed as a predictor for TS activity [30–33], was not significantly associated with either baseline or post-treatment levels of either nucleoside.
In summary, we have shown PD changes consistent with TS inhibition following capecitabine. Although plasma UdR and TdR concentrations and the UdR/TdR ratio can provide some pharmacodynamic indication of TS inhibition, they are unlikely to predict therapeutic response or toxicity accurately of capecitabine treatment in patients. Capecitabine mimics continuous infusion of 5-FU to achieve sustained cellular TS inhibitory effects and suggests the antiproliferative mechanism of capecitabine is at least partly due to TS inhibition through its active metabolite FdUMP. The superior therapeutic index of capecitabine compared with intravenous regimens of 5-FU/LV might result from prolonged tumoral TS inhibition during the course of treatment, as much as the more selective inhibition mediated by differential levels of thymidine phosphorylase between tumour and normal tissues. The data described in this study may assist in the evaluation and dose scheduling of TS inhibitors.
References
- 1.Lokeich JJ, Ahlgren JD, Gullo JJ. A prespective randomized trial of continuous infusion fluorouracil with a conventional bolus schedule in metastatic colorectal carcinoma: a mid-Atlantic oncology program study. J Clin Oncol. 1989;7:425–32. doi: 10.1200/JCO.1989.7.4.425. [DOI] [PubMed] [Google Scholar]
- 2.Meta-Analysis. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. Meta-analysis Group In Cancer. J Clin Oncol. 1998. pp. 301–8. [DOI] [PubMed]
- 3.Ishikawa T, Utoh M, Sawada N, Nishida M, Fukase Y, Sekiguchi F, Ishitsuka H. Tumor selective delivery of 5-fluorouracil by capecitabine, a new oral fluoropyrimidine carbamate, in human cancer xenografts. Biochem Pharmacol. 1998;55:1091–7. doi: 10.1016/s0006-2952(97)00682-5. [DOI] [PubMed] [Google Scholar]
- 4.Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, Shimma N, Umeda I, Ishitsuka H. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998;34:1274–81. doi: 10.1016/s0959-8049(98)00058-6. [DOI] [PubMed] [Google Scholar]
- 5.Schuller J, Cassidy J, Dumont E, Roos B, Durston S, Banken L, Utoh M, Mori K, Weidekamm E, Reigner B. Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol. 2000;45:291–7. doi: 10.1007/s002800050043. [DOI] [PubMed] [Google Scholar]
- 6.Ishitsuka H. Capecitabine: preclinical pharmacology studies. Invest New Drugs. 2000;18:343–54. doi: 10.1023/a:1006497231579. [DOI] [PubMed] [Google Scholar]
- 7.Hoff PM, Ansari R, Batist G, Cox J, Kocha W, Kuperminc M, Maroun J, Walde D, Weaver C, Harrison E, Burger HU, Osterwalder B, Wong AO, Wong R. Comparision of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001;19:2282–92. doi: 10.1200/JCO.2001.19.8.2282. [DOI] [PubMed] [Google Scholar]
- 8.Van Cutsem E, Twelves C, Cassidy J, Allman D, Bajetta E, Boyer M, Bugat R, Findlay M, Frings S, Jahn M, McKendrick J, Osterwalder B, Perez-Manga G, Rosso R, Rougier P, Schmiegel WH, Seitz JF, Thompson P, Vieitez JM, Weitzel C, Harper P. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol. 2001;19:4097–106. doi: 10.1200/JCO.2001.19.21.4097. [DOI] [PubMed] [Google Scholar]
- 9.Van Cutsem E, Hoff PM, Harper P, Bukowski RM, Cunningham D, Dufour P, Graeven U, Lokich J, Madajewicz S, Maroun JA, Marshall JL, Mitchell F, Perez-Manga G, Rougier P, Schmiegel W, Schoelmerich J, Sobrero A, Schilsky RL. Oral capecitabine vs intravenous 5-fluorouracil and leucovorin: integrated efficacy data and novel analyses from two large, radomised, phase III trials. Br J Cancer. 2004;90:1190–7. doi: 10.1038/sj.bjc.6601676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rafi I, Taylor GA, Calvete JA, Boddy AV, Balmanno K, Bailey N, Lind M, Calvert AH, Webber S, Jackson RC. Clinical pharmacokinetic and pharmacodynamic studies with the nonclassical antifolate thymidylate synthase inhibitor 3, 4-dihydro-2-amino-6-methyl-4-oxo-5-(4-pyridylthio)-quinazolone dihydrochloride (AG337) given by 24-hour continuous intravenous infusion. Clin Cancer Res. 1995;1:1275–84. [PubMed] [Google Scholar]
- 11.Jodrell DI, Bowman A, Rye R, Byrne B, Boddy A, Rafi I, Taylor GA, Johnston A, Clendeninn NJ. A phase I study of the lipophilic thymidylate synthase inhibitor Thymitaq (nolatrexed dihydrochloride) given by 10-day oral administration. Br J Cancer. 1999;79:915–20. doi: 10.1038/sj.bjc.6690146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estlin EJ, Pinkerton CR, Lewis IJ, Lashford L, McDowell H, Morland B, Kohler J, Newell DR, Boddy AV, Taylor GA, Price L, Ablett S, Hobson R, Pitsiladis M, Brampton M, Clendeninn N, Johnston A, Pearson AD. A phase I study of nolatrexed dihydrochloride in children with advanced cancer. A United Kingdom Children’s Cancer Study Group Investigation. Br J Cancer. 2001;84:11–8. doi: 10.1054/bjoc.2000.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells P, Aboagye E, Gunn RN, Osman S, Boddy AV, Taylor GA, Rafi I, Hughes AN, Calvert AH, Price PM, Newell DR. 2-[11C]thymidine positron emission tomography as an indicator of thymidylate synthase inhibition in patients treated with AG337. J Natl Cancer Inst. 2003;95:675–82. doi: 10.1093/jnci/95.9.675. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell F, Lynn S, Jackman AL. Modified high-performance liquid chromatography assay for the measurement of 2′-deoxyuridine in human plasma and its application to pharmacodynamic studies of antimetabolite drugs. J Chromatogr B Biomed Sci Appl. 2000;744:351–8. doi: 10.1016/s0378-4347(00)00260-7. [DOI] [PubMed] [Google Scholar]
- 15.de Jonge MJ, Punt CJ, Sparreboom A, Planting AS, Peters ME, van De Schraaf J, Jackman A, Smith R, de Mulder PH, Verweij J. Phase I and pharmacologic study of oral ZD9331, a novel nonpolyglutamated thymidylate synthase inhibitor, in adult patients with solid tumors. J Clin Oncol. 2002;20:1923–31. doi: 10.1200/JCO.2002.07.057. [DOI] [PubMed] [Google Scholar]
- 16.Rees C, Beale P, Trigo JM, Mitchell F, Jackman A, Smith R, Douglass E, Judson I. Phase I trial of ZD9331, a nonpolyglutamatable thymidylate synthase inhibitor, given as a 5-day continuous infusion to patients with refractory solid malignancies. Clin Cancer Res. 2003;9:2049–55. [PubMed] [Google Scholar]
- 17.Plummer R, Rees C, Hughes A, Beale P, Highley M, Trigo J, Gokul S, Judson I, Calvert H, Jackman A, Mitchell F, Smith R, Douglass E. A phase I trial of ZD9331, a water-soluble, nonpolyglutamatable, thymidylate synthase inhibitor. Clin Cancer Res. 2003;9:1313–22. [PubMed] [Google Scholar]
- 18.Clarke SJ, Farrugia DC, Aherne GW, Pritchard DM, Benstead J, Jackman AL. Balb/c mice as a preclinical model for raltitrexed-induced gastrointestinal toxicity. Clin Cancer Res. 2000;6:285–96. [PubMed] [Google Scholar]
- 19.Horton TM, Blaney SM, Langevin A-M, Kuhn J, Kamen B, Berg SL, Bernstein M, Weitman S. Phase i trial and pharmacokinetic study of raltitrexed in children with recurrent or refractory leukemia: a Pediatric Oncology Group Study. Clin Cancer Res. 2005;11:1884–9. doi: 10.1158/1078-0432.CCR-04-1676. [DOI] [PubMed] [Google Scholar]
- 20.Ford HER, Mitchell F, Cunningham D, Farrugia DC, Hill ME, Rees C, Calvert AH, Judson IR, Jackman AL. Patterns of elevation of plasma 2′-deoxyuridine, a surrogate marker of thymidylate synthase (TS) inhibition, after administration of two different schedules of 5-fluorouracil and the specific TS inhibitors raltitrexed (Tomudex) and ZD9331. Clin Cancer Res. 2002;8:103–9. [PubMed] [Google Scholar]
- 21.Jackman AL, Taylor GA, Gibson W, Kimbell R, Brown M, Calvert AH, Judson IR, Hughes LR. ICI D1694, a quinazoline antifolate thymidylate synthase inhibitor that is a potent inhibitor of L1210 tumor cell growth in vitro and in vivo: a new agent for clinical study. Cancer Res. 1991;51:5579–86. [PubMed] [Google Scholar]
- 22.Yin MB, Guimaraes MA, Zhang ZG, Arredondo MA, Rustum YM. Time dependence of DNA lesions and growth inhibition by ICI D1694, a new quinazoline antifolate thymidylate synthase inhibitor. Cancer Res. 1992;52:5900–5. [PubMed] [Google Scholar]
- 23.Jackman AL, Farrugia DC, Clarke SJ, Aherne GW, Boyle FT, Seymour L, Azab M, Kennealey G. Delayed rescue of ZD1694 toxicity in Balb/c mice with thymidine (dThd) or leucovorin (LV) Pro Am Assoc Cancer Res. 1995;36:377. [Google Scholar]
- 24.McGuire JJ, Magee KL, Russell CA, Canestrare JM. Thymidylate synthase as a target for growth inhibition in metrotrexate-sensitive and -resistant human head and neck cancer and leukemia cell lines. Oncol Res. 1997;9:139–47. [PubMed] [Google Scholar]
- 25.Smith PG, Marshman E, Newell DR, Curtin NJ. Dipyridamole potentiates the in vitro activity of MTA ( LY231514) by inhibition of thymidine transport. Br J Cancer. 2000;82:924–30. doi: 10.1054/bjoc.1999.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehman NL, Daneneberg PV. Modulation of RTX cytotoxicity by thymidine and dipyridamole in vitro: implication for chemotherapy. Cancer Chemother Pharmacol. 2000;45:142–8. doi: 10.1007/s002800050022. [DOI] [PubMed] [Google Scholar]
- 27.Kaneda S, Takeishi K, Ayusawa D, Shimizu K, Seno T, Altman S. Role in translation of a triple tandemly repeated sequence in the 5′-untranslated region of human thymidylate synthase mRNA. Nucl Acids Res. 1987;15:1259–70. doi: 10.1093/nar/15.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horie N, Aiba H, Oguro K, Hojo H, Takeishi K. Functional analysis and DNA polymorphism of the tandemly repeated sequences in the 5′-terminal regulatory region of the human gene for thymidylate synthase. Cell Struct Funct. 1995;20:191–7. doi: 10.1247/csf.20.191. [DOI] [PubMed] [Google Scholar]
- 29.Kawakami K, Omura K, Kanehira E, Watanabe Y. Polymorphic tandem repeats in the thymidylate synthase gene are associated with its protein expression in human gastrointestinal cancers. Anticancer Res. 1999;19:3249–52. [PubMed] [Google Scholar]
- 30.Pullarkat ST, Stoehlmacher J, Ghaderi V, Xiong YP, Ingles SA, Sherrod A, Warren R, Tsao-Wei D, Groshen S, Lenz HJ. Thymidylate synthase gene polymorphism determines response and toxicity of 5-FU chemotherapy. Pharmacogenomics J. 2001;1:65–70. doi: 10.1038/sj.tpj.6500012. [DOI] [PubMed] [Google Scholar]
- 31.Marsh S, McKay JA, Cassidy J, McLeod HL. Polymorphism in the thymidylate synthase promoter enhancer region in colorectal cancer. Int J Oncol. 2001;19:383–6. doi: 10.3892/ijo.19.2.383. [DOI] [PubMed] [Google Scholar]
- 32.Iacopetta B, Grieu F, Joseph D, Elsaleh H. A polymorphism in the enhancer region of the thymidylate synthase promoter influences the survival of colorectal cancer patients treated with 5-fluorouracil. Br J Cancer. 2001;85:827–30. doi: 10.1054/bjoc.2001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villafranca E, Okruzhnov Y, Dominguez MA, Garcia-Foncillas J, Azinovic I, Martinez E, Illarramendi JJ, Arias F, Martinez Monge R, Salgado E, Angeletti S, Brugarolas A. Polymorphisms of the repeated sequences in the enhancer region of the thymidylate synthase gene promoter may predict downstaging after preoperative chemoradiation in rectal cancer. J Clin Oncol. 2001;19:1779–86. doi: 10.1200/JCO.2001.19.6.1779. [DOI] [PubMed] [Google Scholar]
- 34.Park DJ, Stoehlmacher J, Zhang W, Tsao-Wei D, Groshen S, Lenz HJ. Thymidylate synthase gene polymorphism predicts response to capecitabine in advanced colorectal cancer. Int J Colorectal Dis. 2002;17:46–9. doi: 10.1007/s003840100358. [DOI] [PubMed] [Google Scholar]
- 35.Mandola MV, Stoehlmacher J, Muller-Weeks S, Cesarone G, Yu MC, Lenz H-J, Ladner RD. A novel single nucleotide polymorphism within the 5′-tandem repeat polymorphism of the thymidylate synthase gene abolishes USF-1 binding and alters transcriptional activity. Cancer Res. 2003;63:2898–904. [PubMed] [Google Scholar]
- 36.Kawakami K, Watanabe G. Identification and functional analysis of single nucleotide polymorphism in the tandem repeat sequence of thymidylate synthase gene. Cancer Res. 2003;63:6004–7. [PubMed] [Google Scholar]
- 37.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 38.National Cancer Institute Common Toxicity Criteria, v. 2.0. [June 26, 2006]; http://ctep.cancer.gov/forms/CTCManual_v4_10-4-99.pdf. [PubMed]
- 39.Li KM, Clarke SJ, Rivory LP. Quantitation of plasma thymidine by high-performance liquid chromatography–atmospheric pressure chemical ionization mass spectrometry and its application to pharmacodynamic studies in cancer patients. Anal Chim Acta. 2003;486:51–61. doi: 10.1016/j.jchromb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Li KM, Rivory LP, Clarke SJ. Rapid quantitation of plasma 2′-deoxyuridine by high-performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry and its application to pharmacodynamic studies in cancer patients. J Chromatogr B. 2005;820:121–30. doi: 10.1016/j.jchromb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Marsh S, Collie-Duguid ESR, Li T, Liu X, McLeod HL. Ethnic variation in the thymidylate synthase enhancer region polymorphism among Caucasian and Asian populations. Genomics. 1999;58:310–2. doi: 10.1006/geno.1999.5833. [DOI] [PubMed] [Google Scholar]
- 42.Marsh S, Ameyaw MM, Githang’a J, Indalo A, Ofori-Adjei D, McLeod HL. Novel thymidylate synthase enhancer region alleles in African populations. Hum Mutat. 2000;16:528. doi: 10.1002/1098-1004(200012)16:6<528::AID-HUMU11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell F, Farrugia D, Rees C, Cunningham D, Judson I, Jackman AL. Estimation of 2′-deoxyuridine (dUrd) in human plasma by HPLC with fluorimetric detection: a pharmacodynamic marker for thymidylate synthase (TS) inhibition. Br J Cancer. 1997;75(Suppl. 1):25. [Google Scholar]
- 44.de Jonge MJA, Verweij J, van der Gaast A, Valota O, Mora O, Planting AST, Mantel MA, Bosch SVD, Lechuga MJ, Fiorentini F. Phase I and pharmacokinetic studies of PNU-159548, a novel alkycycline, administered intravenously to patients with advanced solid tumours. Eur J Cancer. 2002;38:2407–15. doi: 10.1016/s0959-8049(02)00492-6. [DOI] [PubMed] [Google Scholar]
- 45.van Laar JA, van der Wilt CL, Rustum YM, Noordhuis P, Smid K, Pinedo HM, Peters GJ. Therapeutic efficacy of fluoropyrimidines depends on the duration of thymidylate synthase inhibition in the murine colon 26-B carcinoma tumor model. Clin Cancer Res. 1996;2:1327–33. [PubMed] [Google Scholar]
- 46.Nottebrock H, Then R. Thymidine concentration in serum and urine of different animal species and man. Biochem Pharmacol. 1977;26:2175–9. doi: 10.1016/0006-2952(77)90271-4. [DOI] [PubMed] [Google Scholar]
- 47.Taylor GA, Dady PJ, Harrap KR. Quantitative high-performance liquid chromatography of nucleosides and bases in human plasma. J Chromatogr. 1980;183:421–31. doi: 10.1016/s0378-4347(00)81584-4. [DOI] [PubMed] [Google Scholar]
- 48.Holden L, Hoffbrand AV, Tattersall MH. Thymidine concentrations in human sera: variations in patients with leukaemia and megaloblastic anaemia. Eur J Cancer. 1980;16:115–21. doi: 10.1016/0014-2964(80)90116-4. [DOI] [PubMed] [Google Scholar]
- 49.Howell SB, Mansfield SJ, Taetle R. Significance of variation in serum thymidine concentration for the marrow toxicity of methotrexate. Cancer Chemother Pharmacol. 1981;5:221–6. doi: 10.1007/BF00434388. [DOI] [PubMed] [Google Scholar]
- 50.Dudman NPB, Deveski WB, Tattersall MHN. Radioimmunoassays of plasma thymidine, uridine, deoxyuridine and cytidine/deoxycytidine. Anal Biochem. 1981;115:428–37. doi: 10.1016/0003-2697(81)90029-4. [DOI] [PubMed] [Google Scholar]
- 51.Apinazzola A, Marti R, Nishino I, Andreu AL, Naini A, Tadesse S, Pela I, Zammarchi E, Donati A, Oliver A, Hirano M. Altered thymidine metabolism due to defects of thymidine phosphorylase. J Biol Chem. 2002;277:4128–33. doi: 10.1074/jbc.M111028200. [DOI] [PubMed] [Google Scholar]
- 52.Zaharevitz DW, Anderson LW, Malinowski NM, Hyman R, Strong JM, Cysyk RL. Contribution of de-novo and salvage synthesis to the uracil nucleotide pool in mouse tissues and tumors in vivo. Eur J Biochem. 1992;210:293–6. doi: 10.1111/j.1432-1033.1992.tb17420.x. [DOI] [PubMed] [Google Scholar]