Abstract
Aims
Two studies were conduced to assess the effects of ketoconazole, a CYP3A4/5 inhibitor; fluconazole, a CYP2C9 inhibitor; and paroxetine, a CYP2D6 inhibitor, on lasofoxifene pharmacokinetics.
Methods
The first parallel group study was conducted in 45 healthy postmenopausal women (15 per group) to compare the pharmacokinetics of a single dose of lasofoxifene (0.25 mg) administered alone and in combination with ketoconazole (400 mg daily × 20 days) or fluconazole (400 mg daily × 20 days). Lasofoxifene was administered on day 2 and blood samples were collected serially for up to 456 h postdose (20 days). The second study enrolled 20 healthy postmenopausal women (10 per group) to compare the pharmacokinetics of a single dose of lasofoxifene (0.25 mg) alone and in combination with paroxetine (30 mg qd × 21 days). Lasofoxifene was given on day 8 of paroxetine treatment and blood samples were collected serially for up to 336 h postdose.
Results
All subjects completed the study and the treatments were well tolerated. Lasofoxifene Cmax and AUC ratios [90% confidence interval (CI)] with/without ketoconazole were 111% (98.4, 127) and 120% (105, 136), respectively, and were 91.3% (80.3, 104) and 104% (91.4, 118), respectively, with/without fluconazole. Lasofoxifene Cmax and AUC ratios (90% CI) with/without paroxetine were 118% (95.4, 146) and 135% (120, 152), respectively.
Conclusions
Coadministration of potent inhibitors of CYP3A4/5 and CYP2D6, but not CYP2C9, resulted in a moderate increase in lasofoxifene exposure. No dosage adjustment should be required when lasofoxifene is coadministered with ketoconazole, fluconazole, paroxetine or other agents that inhibit these CYP enzymes.
Keywords: CYP inhibitor, drug interactions, fluconazole, ketoconazole, lasofoxifene, paroxetine
Introduction
Postmenopausal women have an increased risk of developing osteoporosis as their levels of endogenous oestrogens decline [1, 2]. In the past, osteoporosis has been effectively treated with oestrogen-based hormone therapy, but recent findings from the Women’s Health Initiative study have shown that the risks of long-term oestrogen therapy may outweigh the benefits [3]. The bisphosphonates are effective antiosteoporosis agents but they do not possess any of the other beneficial effects associated with oestrogen, such as those on vaginal atrophy. The selective oestrogen receptor modulators (SERMs) are being investigated for the treatment of several menopause-associated conditions, including osteoporosis, and have both oestrogen-receptor agonist and antagonist activity depending on the tissue type. These agents have the potential to affect multiple organ systems beneficially without the negative activities that oestrogen has demonstrated on breast and uterine tissue. Lasofoxifene is a next-generation SERM which is being developed for the prevention and treatment of osteoporosis, as well as other menopause-related conditions, such as vaginal atrophy. In preclinical studies, lasofoxifene significantly increased bone mineral density in ovariectomized rats [4, 5]. In this study, lasofoxifene was shown not to have detrimental effects on the endometrium, which is a significant advantage over oestrogen [5]. Multiple doses ranging from 0.01 to 1 mg daily were administered in postmenopausal women [6]. Lasofoxifene pharmacokinetics were linear and the treatments were well tolerated [6]. The effects on bone have been studied in Phase 2 clinical trials in humans [7–10] and lasofoxifene is currently undergoing evaluation in Phase 3 clinical trials.
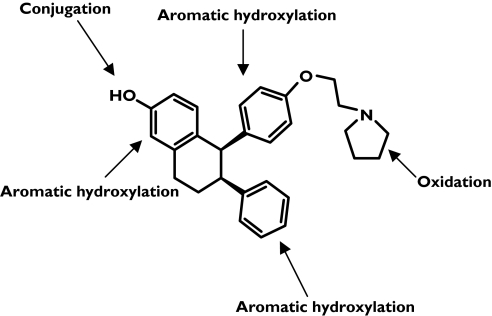
Lasofoxifene is subject to extensive metabolism with <2% of the dose recovered unchanged in the urine [11]. It has a long t1/2 in humans (6–7 days) [6], which may be partly due to enterohepatic recirculation of its glucuronide/sulphate conjugates. Five main pathways of lasofoxifene metabolism have been identified in humans, involving both oxidation and conjugation (Figure 1).
Figure 1.
Lasofoxifene chemical structure and sites of metabolism
Due to its long t1/2 and low hepatic extraction, studies to identify the CYP isoforms responsible for lasofoxifene oxidative metabolism have been complicated by low turnover rates. More recently, the results from in vitro experiments with recombinant CYP isoforms and inhibition studies with isoform-selective inhibitors have suggested that lasofoxifene is primarily metabolized by CYP3A4, CYP3A5 and CYP2D6. Because CYP3A, CYP2C9 and CYP2D6 are the CYP enzymes most frequently involved in oxidative drug metabolism [12], the effects of coadministration of inhibitors of these three CYPs with lasofoxifene were examined in vivo. The change in exposure with these CYP inhibitors would give insight into which pathways are clinically relevant to lasofoxifene metabolism.
An ideal inhibitory probe needs to show adequate selectivity for the enzyme, potency and safety in healthy volunteers. Although each probe has its limitations, ketoconazole, fluconazole and paroxetine were selected as inhibitors of CYP3A, CYP2C9 and CYP2D6, respectively. In vitro studies have shown that these drugs may also be inhibitors of other CYPs [13–16].
In the first study, lasofoxifene was coadministered with either ketoconazole or fluconazole, while the second study investigated coadministration of lasofoxifene with paroxetine. Ketoconazole and fluconazole, two antifungal agents, are potent inhibitors of CYP3A and CYP2C9 enzyme subtypes, respectively [17–20]. Administration of ketoconazole results in significant interaction with drugs metabolized by CYP3A such as midazolam and is generally recommended as an inhibitory probe for that enzyme [17, 21, 22]. Fluconazole is a potent CYP2C9 inhibitor and has been shown to increase exposure to warfarin [20, 23]; however, high doses of fluconazole also inhibit CYP3A but to a lesser extent than ketoconazole [20]. Paroxetine, a selective serotonin reuptake inhibitor, was selected because of its potency and specificity for CYP2D6 [22, 24, 25]. Although not as potent as quinidine, it is safer in healthy volunteers and has also been shown to convert CYP2D6 extensive metabolizers (EMs) into poor metabolizers (PMs) [26]. Approximately 7–10% of White people are PMs of drugs metabolized by CYP2D6 [27]. Poor CYP2D6 metabolizers have increased concentrations of drugs metabolized via this pathway relative to subjects with normal CYP2D6 activity (referred to as EMs). Only EMs were enrolled to maximize the magnitude of the change, if any.
Methods and materials
Two Phase 1, open-label, randomized, parallel-group, clinical studies were conducted to determine the effects of different CYP inhibitors on single-dose lasofoxifene pharmacokinetics. The first study investigated coadministration of lasofoxifene with either ketoconazole or fluconazole (Study 1) and the second study tested coadministration of lasofoxifene with paroxetine (Study 2). For both studies, a parallel design was used to account for the long t1/2 of lasofoxifene.
Subjects
Healthy postmenopausal women were eligible if they were aged ≥40 years (Study 1) or 40–70 years (Study 2) and weighed ≥50 kg, with a normal electrocardiogram (ECG), including corrected QT interval ≤470 ms and an estimated creatinine clearance value at screening of ≥50 ml min−1 as determined by the Cockcroft–Gault equation. Women were excluded if they had a history or clinical evidence of significant respiratory, cardiovascular (including thromboembolic disorders), gastrointestinal, hepatic, renal, endocrine, haematological, neurological, psychiatric or other chronic disease, alcoholism or drug abuse. In both studies, administration of any medication, including herbal supplements and over-the-counter medications without the approval of a clinical investigator, was prohibited from screening to closeout. In Study 2, subjects had to possess the genotype for extensive CYP2D6 metabolism.
These studies were conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice, the Declaration of Helsinki, and in compliance with United States Food and Drug Administration (FDA) regulations. Written informed consent was required from each subject who participated, or her authorized representative, prior to the subject’s enrolment. The Ethics Committees were MDS Pharma Services Inc. Institutional Review Board, Lincoln, NE and Pfizer Research Clinic Institutional Review Board, Ann Arbor, MI for Studies 1 and 2, respectively.
Study design: Study 1
Subjects were randomly assigned to one of three treatment groups, with 15 subjects per group. Group 1 (lasofoxifene alone control) received a single 0.25-mg oral dose of lasofoxifene on day 2 with no other medication for the remainder of the study. This dose was selected because it is the anticipated therapeutic dose for the prevention and treatment of osteoporosis. Group 2 was administered ketoconazole (400 mg day−1) on days 1–20 plus a single 0.25-mg oral dose of lasofoxifene on day 2. Group 3 received fluconazole (400 mg day−1) on days 1–20 along with a single 0.25-mg oral dose of lasofoxifene on day 2. All medications were administered at approximately the same time of day and the day 2 lasofoxifene dose was coadministered with the ketoconazole or fluconazole dose. Subjects remained at the clinic for the first 2 days of the study. Before the subjects left the clinic on day 3, they were given the remainder of the medication for self-administration on days 4–20. To assess compliance, subjects recorded dosing times for ketoconazole and fluconazole in a daily medication diary.
The women were required to fast overnight for 8 h before each clinical laboratory assessment and before lasofoxifene administration on day 2. Subjects remained fasting for 4 h after the lasofoxifene dose. They were also required to fast for 2 h prior to and 2 h after ketoconazole or fluconazole administration on day 1. Identical lunches and identical dinners were served 4 and 10 h, respectively, following the ketoconazole or fluconazole dose on day 1 and following the lasofoxifene dose on day 2. Ketoconazole or fluconazole could be administered without regard to meals on all days other than day 1. Grapefruit juice or food products containing grapefruit were prohibited for 7 days before day 1 until closeout.
Study design: Study 2
Women who fulfilled the entry criteria received a single 0.25-mg lasofoxifene dose on day 8. From days 1 to 21, half of the subjects were randomly assigned to receive an additional 30 mg paroxetine daily. The paroxetine dose was taken at approximately the same time each day, without regard to meals. Doses of paroxetine on days 1, 4, 6 to 12, 15 and 18 were also administered while subjects were in the clinic. To assess compliance, subjects recorded dosing times for paroxetine in a daily medication diary.
The subjects were required to fast overnight for 8 h before clinical laboratory measurements and before the lasofoxifene dose on day 8 and to remain fasted for 4 h after receiving the lasofoxifene dose on day 8. Lunches and dinners were served in the clinic 4 and 10 h, respectively, after drug administration on day 8.
Pharmacokinetic assessments
Pharmacokinetic sampling was performed by collecting 10 ml of venous blood in glass vacuum blood collection tubes containing sodium heparin. Blood samples were withdrawn before lasofoxifene dosing and at 1, 2, 4, 8, 12, 24, 48, 72, 120, 168, 216, 264, 336 and 456 h after the dose on day 2 (Study 1) or at 1, 2, 4, 6, 8, 10, 12, 24, 48, 72, 96, 168, 240 and 336 h after dose administration on day 8 (Study 2). Following each collection, blood samples were centrifuged as soon as possible and the separated plasma was stored frozen at ≤−20 °C until assayed. Plasma concentrations of lasofoxifene were measured using a validated liquid chromatography/mass spectrometry/mass spectrometry (LC/MS/MS) method at CEDRA Corporation (Austin, TX, USA) [6]. The analytical range was 0.025–6 ng ml−1, with a lower limit of quantification of 0.025 ng ml−1. Precision (expressed as percent coefficient of variation) was determined between days using quality control samples of low, medium and high concentrations and was within 3.5%. Accuracy of these quality controls ranged from 85.3 to 102%.
Lasofoxifene pharmacokinetic parameters including maximum plasma concentration (Cmax), time to maximum plasma concentration (Tmax), terminal half-life (t1/2) and area under the curve (AUC) values were determined using standard noncompartmental methods. Analysis of variance (anova) of log-transformed Cmax and AUC was used to construct 90% confidence intervals (CIs) for the ratio of least squares mean values of lasofoxifene coadministered with ketoconazole, fluconazole or paroxetine to those of lasofoxifene alone. Mean values for all other pharmacokinetic parameters were least squares means obtained from anova. Ratios and CIs for these parameters were based on untransformed values. Pharmacokinetic and statistical analyses were conducted using WinNonlin Pro (Pharsight Corp., Mountain View, CA, USA). Absence of an interaction was concluded if the 90% CIs for Cmax and AUC were within the 80–125% range.
Genotyping procedure (CYP2D6)
Blood collection for genotyping of CYP2D6 was done within 30 days of day 1 in Study 2. Venous blood (3 ml) was withdrawn into a plastic vacuum blood tube containing ethylenediamine tetraaceticacid. Genomic DNA was isolated using the QIAamp 96 DNA Blood Kit (Qiagen, Valencia, CA, USA). Genotyping was performed using TaqMan allelic discrimination assays (Applied Biosystems Inc., Foster City, CA, USA) for CYP2D6 (*3, *4, *6, *7, *8).
Safety evaluations
All symptoms or adverse events (AEs) following drug administration were recorded. In Study 2, AEs may have been counted twice: during the paroxetine-only phase (days 1–7) and during administration of the lasofoxifene–paroxetine combination.
In both studies, physical examinations, vital signs and ECG measurements were performed at screening and at closeout. In Study 1, vital signs and ECGs were also measured predose and 1–2 h postdose on day 1, and predose and 6–8 h postdose on day 2. Fasting blood and/or urine samples for clinical laboratory measurements were collected during screening, on days 9 and 16 (haematology and clinical chemistry only in Study 1) and at closeout.
Results
The number of subjects participating in Study 1 and Study 2 is summarized in Table 1 along with demographic characteristics. There were no premature study discontinuations.
Table 1.
Demographic characteristics of the participants for both studies
| Study 1 | Study 2 | ||||
|---|---|---|---|---|---|
| Lasofoxifene alone | Lasofoxifene + ketoconazole | Lasofoxifene + fluconazole | Lasofoxifene alone | Lasofoxifene + paroxetine | |
| No. of subjects | 15 | 15 | 15 | 10 | 10 |
| Age, years, mean (range) | 60 (50–75) | 62 (43–81) | 58 (45–69) | 54 (48–67) | 58 (50–69) |
| Weight, kg, mean (range) | 70 (50–99) | 71 (58–87) | 77 (63–124) | 71 (57–88) | 71 (59–88) |
| Height, cm, mean (range) | 166 (157–174) | 165 (157–175) | 165 (157–174) | 164 (157–174) | 166 (160–172) |
Pharmacokinetics
Study 1
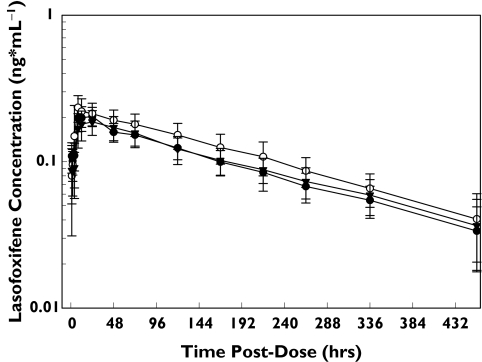
Mean lasofoxifene plasma concentration–time profiles following administration of 0.25 mg lasofoxifene alone and during daily dosing with 400 mg ketoconazole or 400 mg fluconazole are shown in Figure 2. A summary of pharmacokinetic parameters is provided in Table 2. Lasofoxifene exposure, as measured by AUC0–∞, was 20% higher when coadministered with ketoconazole (Table 3). The 90% CI for the treatment ratio of AUC0–∞ values was outside of the 80–125% range. The effect on Cmax was smaller with an 11% increase with concomitant ketoconazole. Lasofoxifene pharmacokinetic parameters following coadministration with fluconazole were equivalent to those with lasofoxifene alone. The 90% CI for both Cmax and AUC were within the 80–125% range (Table 3), indicating the absence of an interaction between fluconazole and lasofoxifene. Given the slow absorption and the limited sampling scheme, mean lasofoxifene Tmax varied for the different treatment groups, ranging from approximately 10 h to 21 h. Lasofoxifene t1/2-values were similar across each treatment group.
Figure 2.
Mean ± SD lasofoxifene plasma concentration–time profiles following administration of a single 0.25-mg lasofoxifene dose alone and during daily dosing with 400 mg ketoconazole or with 400 mg fluconazole. Lasofoxifene (alone) (•), lasofoxifene + ketoconazole (○), lasofoxifene + fluconazole (▾)
Table 2.
Summary of pharmacokinetic parameters (mean ± SD) for lasofoxifene alone or when coadministered with ketoconazole, fluconazole (Study 1) or alone or with paroxetine (Study 2)
| Study 1 | Study 2 | ||||
|---|---|---|---|---|---|
| Parameter | Lasofoxifene alone | Lasofoxifene + ketoconazole | Lasofoxifene + fluconazole | Lasofoxifene alone | Lasofoxifene + paroxetine |
| N | 15 | 15 | 15 | 10 | 10 |
| Tmax, h | 15.4 ± 7.4 | 9.6 ± 4.5 | 21.3 ± 12.4 | 13.0 ± 6.0 | 11.8 ± 6.6 |
| Cmax, ng ml−1 | 0.218 ± 0.033 | 0.249 ± 0.074 | 0.200 ± 0.037 | 0.203 ± 0.050 | 0.243 ± 0.083 |
| AUC0–∞, ng h ml−1 | 52.7 ± 9.5 | 63.6 ± 14.0 | 55.3 ± 12.7 | 41.4 ± 4.5 | 56.4 ± 10.2 |
| t1/2, h | 196 ± 45.3 | 190 ± 40.0 | 204 ± 30.6 | 168 ± 31.7 | 202 ± 37.2 |
Cmax, Maximum plasma concentration; AUC0–∞, area under plasma concentration–time profile from time zero extrapolated to infinite time; Tmax, time to reach Cmax; t1/2, terminal half-life.
Table 3.
Least square mean ratio (90% confidence interval) Cmax and AUC of lasofoxifene administered with ketoconazole, fluconazole or paroxetine using lasofoxifene alone as reference
| Treatment | Parameter | Ratio (%) | 90% CI |
|---|---|---|---|
| Ketoconazole | Cmax | 111 | 98.4, 127 |
| AUC0–∞ | 120 | 105, 136 | |
| Fluconazole | Cmax | 91.3 | 80.3, 104 |
| AUC0–∞ | 104 | 91.4, 118 | |
| Paroxetine | Cmax | 118 | 95.6, 146 |
| AUC0–∞ | 135 | 120, 152 |
Cmax, Maximum plasma concentration; AUC0–∞, area under plasma concentration–time profile from time zero extrapolated to infinite time.
Study 2
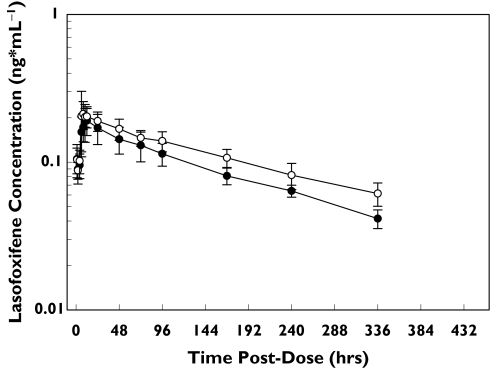
Figure 3 shows the mean lasofoxifene plasma concentration–time profiles following administration of 0.25 mg lasofoxifene alone and during daily dosing with 30 mg paroxetine. The lasofoxifene AUC0–∞ was 35% greater during daily dosing with 30 mg paroxetine (Table 3), outside the 80–125% range. Consistent with these results, the lasofoxifene Cmax was increased by 18% during coadministration with paroxetine. Lasofoxifene t1/2 was 34 h greater when coadministered with paroxetine (168 vs. 202 h).
Figure 3.
Mean ± SD lasofoxifene plasma concentration–time profiles following administration of a single 0.25-mg lasofoxifene dose alone and during daily dosing with 30 mg paroxetine. Lasofoxifene (alone) (•), lasofoxifene + paroxetine (○)
Adverse events
Administration of lasofoxifene alone and in combination with ketoconazole, fluconazole or paroxetine was generally well tolerated. Table 4 summarizes the number of AEs reported in Study 1 and Study 2. For Study 1, all AEs were mild in intensity and generally short in duration, resolving within 1 day. For Study 2, the majority of AEs were mild or moderate; one AE (nausea) was rated as severe and occurred during paroxetine-only dosing. There were no clinically relevant laboratory abnormalities.
Table 4.
Overview of adverse events (AEs)
| Study 1 | Study 2 Lasofoxifene + paroxetine | |||||
|---|---|---|---|---|---|---|
| Lasofoxifene alone | Lasofoxifene + ketoconazole | Lasofoxifene + fluconazole | Lasofoxifene alone | Paroxetine only days 1–7 | Combination days 8–21 | |
| Number of AEs | ||||||
| All AEs | 8 | 29 | 28 | 8 | 34 | 30 |
| Associated AEs | 1 | 2 | 1 | 4 | 31 | 27 |
| Number of subjects reporting AEs | ||||||
| All AEs | 6 | 12 | 8 | 5 | 10 | 10 |
| Associated AEs | 1 | 1 | 1 | 2 | 10 | 10 |
All AEs are defined as all observed or volunteered AEs regardless of treatment group or suspected causal relationship to study drug. Associated AEs are defined as AEs that were evaluated by the investigator as being definitely, probably or possibly related to study drug.
Discussion
Lasofoxifene, a next-generation SERM developed for the prevention and treatment of osteoporosis, is currently undergoing Phase 3 clinical trials. As stated in the FDA Guidance for Industry on Drug Metabolism [21], it is necessary to determine the metabolic pathways and routes of elimination of new drugs to ensure their safety and to understand the potential for drug–drug interactions.
Lasofoxifene elimination is slow, with a t1/2 of 5–6 days [6]. Renal excretion of unchanged lasofoxifene accounts for only 2% of the dose, whereas metabolism, including oxidation and conjugation pathways, appears to play a more important role. In vitro metabolic studies to elucidate the potential routes of lasofoxifene metabolism have been complicated by low rates of metabolism, consistent with the low hepatic extraction of lasofoxifene in vivo. We therefore used an in vivo approach to examine the clinical effects of various CYP inhibitors on the pharmacokinetic profile of lasofoxifene.
In vitro study results suggest that CYP3A4/5 and CYP2D6 may be involved in lasofoxifene metabolism. Consistent with these results, lasofoxifene exposure was increased following coadministration with ketoconazole (20%) and paroxetine (35%), but not fluconazole. Although the changes in lasofoxifene Cmax and AUC0–∞ observed during the coadministration of lasofoxifene and ketoconazole were statistically significant, the differences were relatively small, suggesting a minor role for CYP3A. Coadministration of ketoconazole with midazolam, a compound metabolized by CYP3A, results in an 7.7-fold increase in AUC [28]. A 67% increase is AUC is noted with zolpidem, a compound with a predicted CYP3A-mediated clearance of 61%[29].
The effect with paroxetine was larger than that with ketoconazole, suggesting CYP2D6 may play a role in lasofoxifene metabolism greater than CYP3A in this extensive CYP2D6-metabolizer population. Drugs such as desipramine, imipramine and metoprolol (R- and S-combined), which are metabolized by CYP2D6, have shown an increase in AUC ratios of 7.4, 1.74 and 6.1, respectively, when coadministered with paroxetine. These values are considerably greater than those observed with lasofoxifene [30–32], thus no clinically significant interactions are expected to occur between lasofoxifene and CYP2D6 inhibitors such as paroxetine. In addition, lasofoxifene pharmacokinetic data show that lasofoxifene exposure does not exhibit a bimodal distribution in the general population as would be expected if CYP2D6 were the predominant enzyme involved in lasofoxifene metabolism.
Lasofoxifene is generally safe and well tolerated when given to postmenopausal women at doses as high as 10 mg daily for up to 1 year [8]. The interactions observed when coadministered with either ketoconazole or paroxetine are not considered clinically significant. Thus, no dosage adjustment should be required when lasofoxifene is coadministered with ketoconazole, paroxetine or other CYP3A and CYP2D6 inhibitors. The impact of the administration of multiple inhibitors on lasofoxifene has not been studied and an additive inhibitory effect cannot be excluded. Lasofoxifene was generally well tolerated when administered alone or with any of the enzyme inhibitors.
In conclusion, coadministration of potent inhibitors of CYP3A and CYP2D6, but not CYP2C9, resulted in a moderate increase in lasofoxifene exposure. No dosage adjustment should be required when lasofoxifene is coadministered with ketoconazole, paroxetine or other agents that inhibit these CYP enzymes.
References
- 1.Sowers MR, Finkelstein JS, Ettinger B, Bondarenko I, Neer RM, Cauley JA, Sherman S, Greendale GA. The association of endogenous hormone concentrations and bone mineral density measures in pre- and perimenopausal women of four ethnic groups: SWAN. Osteoporos Int. 2003;14:44–52. doi: 10.1007/s00198-002-1307-x. [DOI] [PubMed] [Google Scholar]
- 2.Sowers MR, Greendale GA, Bondarenko I, Finkelstein JS, Cauley JA, Neer RM, Ettinger B. Endogenous hormones and bone turnover markers in pre- and perimenopausal women: SWAN. Osteoporos Int. 2003;14:191–7. doi: 10.1007/s00198-002-1329-4. [DOI] [PubMed] [Google Scholar]
- 3.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 4.Ke HZ, Paralkar VM, Grasser WA, Crawford DT, Qi H, Simmons HA, Pirie CM, Chidsey-Frink KL, Owen TA, Smock SL, Chen HK, Jee WS, Cameron KO, Rosati RL, Brown TA, Dasilva-Jardine P, Thompson DD. Effects of CP-336,156, a new, nonsteroidal estrogen agonist/antagonist, on bone, serum cholesterol, uterus and body composition in rat models. Endocrinology. 1998;139:2068–76. doi: 10.1210/endo.139.4.5902. [DOI] [PubMed] [Google Scholar]
- 5.Ke HZ, Foley GL, Simmons HA, Shen V, Thompson DD. Long-term treatment of lasofoxifene preserves bone mass and bone strength and does not adversely affect the uterus in ovariectomized rats. Endocrinology. 2004;145:1996–2005. doi: 10.1210/en.2003-1481. [DOI] [PubMed] [Google Scholar]
- 6.Gardner M, Taylor A, Wei G, Calcagni A, Duncan B, Milton A. Clinical pharmacology of multiple doses of lasofoxifene in postmenopausal women. J Clin Pharmacol. 2006;46:52–8. doi: 10.1177/0091270005283280. [DOI] [PubMed] [Google Scholar]
- 7.Bolognese MA, Weiss SR, Ettinger MP, Moffett AH, Lee A. Lasofoxifene: a next generation selective estrogen receptor modulator (SERM) for the prevention of bone loss in postmenopausal women. Osteoporos Int. 2004;15(Suppl. 1):S11. [Abstract] [Google Scholar]
- 8.Ettinger M, Schwartz E, Emkey R, Moffett AH, Bolognese M, Weiss S, Lee A. Lasofoxifene, a Next Generation Selective Estrogen Receptor Modulator (SERM), in the Prevention of Bone Loss in Postmenopausal Women. 2004. Presented at ENDO 2004, New Orleans, USA. Abstract S35-2.16–19June.
- 9.McClung M, Omizo M, Weiss S, Moffett AH, Bolognese M, Civitelli R. Comparison of lasofoxifene and raloxifene for the prevention of bone loss in postmenopausal women. J Bone Miner Res. 2004;19(Suppl. 1):S96. [Abstract] [Google Scholar]
- 10.McClung M, Weiss S, Moffett AH, Bolognese M, Civitelli R, Somayagi V, Brunell R, Lee A. A study of lasofoxifene, a next generation SERM, versus raloxifene, in preventing bone loss in postmenopausal women. Arthritis Rheum. 2004;50(Suppl.):1791. [Abstract] [Google Scholar]
- 11.Johnson KA, Gardner MJ, Prakash C. In vivo and in vitro metabolism of a next-generation selective estrogen receptor modulator, lasofoxifene, in humans. Drug Metab Rev. 2004;36(S1):246. doi: 10.1124/dmd.108.020404. [Abstract] [DOI] [PubMed] [Google Scholar]
- 12.Ma MK, Woo MH, McLeod HL. Genetic basis of drug metabolism. Am J Health Syst Pharm. 2002;59:2061–9. doi: 10.1093/ajhp/59.21.2061. [DOI] [PubMed] [Google Scholar]
- 13.Atiba JO, Blaschke TP, Wilkinson GR. Effects of ketoconazole on the ploymorphic 4-hydroxylation of S-mephenytoin and debrisoquine. Br J Clin Pharmacol. 1989;28:216–22. doi: 10.1111/j.1365-2125.1989.tb05409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ong CE, Coulter S, Birkett DJ, Bhasker CR, Miners JO. The xenobiotic inhibitor profile of cytochrome P4502C8. Br J Clin Pharmacol. 2000;50:573–80. doi: 10.1046/j.1365-2125.2000.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wienkers LC, Wurden CJ, Storch E, Kunze KL, Rettie AE, Trager WF. Formation of (R)-8-ydroxywarfarin in human liver microsomes. A new metabolic marker for the (S)-mephenytoin hydroxylase, P4502C19. Drug Metab Dispos. 1996;24:610–4. [PubMed] [Google Scholar]
- 16.von Moltke LL, Greenblatt DJ, Harmatz JS, Duan SX, Harrel LM, Cotreau-Bibbo MM, Pritchard GA, Wright CE, Shader RI. Triazolam biotransformation by human liver microsomes in vitro: effects of metabolic inhibitors and clinical confirmation of a predicted interaction with ketoconazole. J Pharmacol Exp Ther. 1996;276:370–9. [PubMed] [Google Scholar]
- 17.Venkatakrishnan K, von Moltke LL, Greenblatt DJ. Effects of the antifungal agents on oxidative drug metabolism: clinical relevance. Clin Pharmacokinet. 2000;38:111–80. doi: 10.2165/00003088-200038020-00002. [DOI] [PubMed] [Google Scholar]
- 18.Gibbs MA, Kunze KL, Howald WN, Thummel KE. Effect of inhibitor depletion on inhibitory potency: tight binding inhibition of CYP3A by clotrimazole. Drug Metab Dispos. 1999;27:596–9. [PubMed] [Google Scholar]
- 19.von Moltke LL, Greenblatt DJ, Schmider J, Duan SX, Wright CE, Harmatz JS, Shader RI. Midazolam hydroxylation by human liver microsomes in vitro: inhibition by fluoxetine, norfluoxetine, and by azole antifungal agents. J Clin Pharmacol. 1996;36:783–91. doi: 10.1002/j.1552-4604.1996.tb04251.x. [DOI] [PubMed] [Google Scholar]
- 20.Kunze KL, Wienkers LC, Thummel KE, Trager WF. Warfarin-fluconazole. I. Inhibition of the human cytochrome P450-dependent metabolism of warfarin by fluconazole: in vitro studies. Drug Metab Dispos. 1996;24:414–21. [PubMed] [Google Scholar]
- 21.FDA. Guidance for Industry. In Vivo Drug Metabolism/Drug Interaction StudiesStudy Design, Data Analysis, and Recommendations for Dosing and Labeling. Washington DC: Food and Drug Administration; 1999. [Google Scholar]
- 22.EMEA Committee for Proprietary Medicinal Products. Note for Guidance on the Investigation of Drug Interactions. 1997. CPMP/EWP/560/95. The European Agency for the Evaluation of Medicinal Products, London.
- 23.Kunze KL, Trager WF. Warfarin-Fluconazole III: a rationale approach to management of a metabolically based drug interaction. Drug Metab Dispos. 1996;24:429–35. [PubMed] [Google Scholar]
- 24.Belle DJ, Ernest CS, Sauer JM, Smith BP, Thomasson HR, Witcher JW. Effect of potent CYP2D6 inhibition by paroxetine on atomoxetine pharmacokinetics. J Clin Pharmacol. 2002;42:1219–27. doi: 10.1177/009127002762491307. [DOI] [PubMed] [Google Scholar]
- 25.Crewe HK, Lennard MS, Tucker GT, Woods FR, Haddock RE. The effect of selective serotonin re-uptake inhibitors on cytochrome P4502D6 (CYP2D6) activity in human liver microsomes. Br J Clin Pharmacol. 1992;34:262–5. doi: 10.1111/j.1365-2125.1992.tb04134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alfaro CL, Lam YW, Simpson J, Ereshefsky L. CYP2D6 status of extensive metabolizers after multiple-dose fluoxetine, fluvoxamine, paroxetine, or sertraline. J Clin Psychopharmacol. 1999;19:155–63. doi: 10.1097/00004714-199904000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Steiner E, Bertilsson L, Sawe J, Bertling I, Sjoqvist F. Polymorphic debrisoquin hydroxylation in 757 Swedish subjects. Clin Pharmacol Ther. 1988;44:431–5. doi: 10.1038/clpt.1988.176. [DOI] [PubMed] [Google Scholar]
- 28.Lam YWF, Alfaro CL, Ereshefsky L, Miller M. Pharmacokinetic and pharmacodynamic interactions of oral midazolam with ketoconazole, fluoxetine, fluvoxamine, and nefazodone. J Clin Pharmacol. 2003;43:1274–82. doi: 10.1177/0091270003259216. [DOI] [PubMed] [Google Scholar]
- 29.Hesse LM, von Moltke LL, Greenblatt DJ. Clinically important drug interactions with zopiclone, zolpidem and zaleplon. Drugs. 2003;17:513–37. doi: 10.2165/00023210-200317070-00004. [DOI] [PubMed] [Google Scholar]
- 30.Albers LJ, Reist C, Helmeste D, Vu R, Tang SW. Paroxetine shifts imipramine metabolism. Psychiatry Res. 1996;59:189–96. doi: 10.1016/0165-1781(95)02822-6. [DOI] [PubMed] [Google Scholar]
- 31.Bergstrom RF, Peyton AL, Lemberger L. Quantification and mechanism of the fluoxetine and tricyclic antidepressant interaction. Clin Pharmacol Ther. 1992;51:239–48. doi: 10.1038/clpt.1992.18. [DOI] [PubMed] [Google Scholar]
- 32.Hemeryck A, Lefebvre RA, De Vriendt C, Belpaire FM. Paroxetine affects metoprolol pharmacokinetics and pharmacodynamics in healthy volunteers. Clin Pharmacol Ther. 2000;67:283–91. doi: 10.1067/mcp.2000.104788. [DOI] [PubMed] [Google Scholar]