Abstract
Aim
We aimed to investigate the effect of the ABCB1 gene on the pharmacokinetics of amlodipine.
Methods
Based on polymorphisms of the ABCB1 gene at positions 2677 and 3435, 26 healthy male participants were divided into three groups: subjects with 2677GG/3435CC (n = 9), 2677GT/3435CT (n = 9) and 2677TT/3435TT (n = 8). After a single-dose administration of 5 mg amlodipine, plasma concentrations of amlodipine were measured and its pharmacokinetic characteristics were compared according to ABCB1 genotype.
Results
The area under the plasma concentration–time curve was significantly lower in subjects with 2677TT/3435TT (140.8 ± 35.6 ng h−1 ml−1) and 2677GT/3435CT (149.8 ± 40.1 ng h−1 ml−1) than in those with 2677GG/3435CC (208.6 ± 39.2 ng h−1 ml−1) [95% confidence interval (CI) on the difference, 2677GG/3435CC vs. 2677GT/3435CT 12.0, 105.6, P < 0.01; 2677GG/3435CC vs. 2677TT/3435TT 19.6, 116.0, P < 0.01; 2677GT/3435CT vs. 2677TT/3435TT −39.2, 57.2, P > 0.05]. The peak plasma concentrations were highest in subjects with 2677GG/3435CC (3.8 ± 0.5 ng ml−1), lower in subjects with 2677GT/3435CT (3.2 ± 0.5 ng ml−1) and 2677TT/3435TT (2.7 ± 0.5 ng ml−1) in rank and showed a significant difference between those with 2677GG/3435CC and with 2677TT/3435TT (95% CI on the difference 0.4, 2.0, P < 0.01). However, the oral clearance was higher in subjects with 2677TT/3435TT (37.7 ± 10.2 l h−1) than in those with 2677GT/3435CT (35.7 ± 9.9 l h−1) and with 2677GG/3435CC (24.8 ± 5.4 l h−1) and exhibited a significant difference between ABCB1 genotype groups (95% CI on the difference, 2677GG/3435CC vs. 2677GT/3435CT −21.5, −0.3, P < 0.05; 2677GG/3435CC vs. 2677TT/3435TT −23.8, −2.0, P < 0.05).
Conclusion
Amlodipine pharmacokinetics was affected by the genetic polymorphisms of the ABCB1 gene in humans. These findings may provide a plausible explanation for interindividual variation in the disposition of amlodipine, although our study could not explain the exact mechanism(s) by which the polymorphic ABCB1 gene paradoxically reduces the plasma levels of amlodipine. Further evaluation is thus warranted.
Keywords: ABCB1 (MDR1), amlodipine, P-glycoprotein, pharmacogenetics, pharmacokinetics, polymorphism
Introduction
The 170-kDa membrane protein P-glycoprotein (P-GP) is an adenosine triphosphate (ATP)-dependent drug efflux pump that is constitutively expressed in several human tissues [1, 2]. Recent pharmacogenomic studies have shown that differential expression of the ABCB1 (MDR1) gene can influence the activity and bioavailability of drugs [3].
Amlodipine, a third-generation dihydropyridine calcium channel blocker, is prescribed for the management of angina and hypertension [4–6]. Recent evidence suggests that amlodipine acts as a substrate of P-GP [7, 8]. Furthermore, grapefruit juice and diltiazem, known inhibitors of P-Gp [9–11], affect the pharmacokinetics of amlodipine [12, 13]. We can speculate that amlodipine acts as a substrate of P-GP, the activity of which thus influences the pharmacokinetic characteristics of amlodipine as a factor of interindividual variation.
We therefore investigated the effect of genetic polymorphisms of the ABCB1 gene, especially at positions 2677 and 3435, on the pharmacokinetics of amlodipine in healthy subjects.
Materials and methods
Subjects
Among the previously genotyped 160 subjects for ABCB1 exons, exon 21 G2677T/A and exon 26 C3435T polymorphisms, we enrolled 26 men into this study: nine with 3435CC/2677GG (mean age ± SD 24.7 ± 2.2 years; mean weight ± SD 67.8 ± 7.1 kg), nine with 3435CT/2677GT (mean age ± SD 24.7 ± 2.2 years; mean weight ± SD 70.7 ± 5.4 kg) and eight with 3435TT/2677TT (mean age ± SD 26.9 ± 2.6 years; mean weight ± SD 68.9 ± 4.5 kg). The study protocol was approved by the Institutional Review Board (IRB) of Gil Medical Centre, Incheon, Korea and all subjects provided written informed consent.
Study procedures
All subjects were admitted to the clinical trial centre the evening before the day of drug administration. The following morning, they were given a single oral dose of 5 mg amlodipine. Blood samples were collected immediately prior to drug administration and then at 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 24, 48, 96 and 144 h after drug administration.
Genotyping of ABCB1 polymorphism
DNA was extracted from peripheral whole blood of each subject using a Qiagen DNA extraction kit (Qiagen, Hilden, Germany). The genotypes of ABCB1, G2677T, G2677A and C3435T were identified by polymerase chain reaction-restriction fragment length polymorphism analysis as described previously [14–16] and results were confirmed for randomly selected individuals for each genotype by direct sequence analysis.
Drug analysis and pharmacokinetic analysis
Plasma amlodipine concentrations were analysed using validated high-performance liquid chromatography with fluorescence detection as described previously with a slight modification [17]. A linearity calibration curve in the range of 0.1–10 ng ml−1 was established for amlodipine (r2 = 0.9997). Intraday and interday coefficients of variation (CV) were <8% and <10%, respectively,
The software WinNONLIN professional version 4.1 (Pharsight Corp. Inc., Mountain View, CA, USA) was used for pharmacokinetic analysis and simulations. The peak plasma concentration (Cmax) and the time to reach Cmax (tmax) were estimated directly from the observed plasma concentration–time data. The area under the plasma concentration–time curve from time 0–144 h (AUClast) was calculated using the linear trapezoidal rule. The AUC from time 0 to infinity (AUCinf) was calculated as AUCinf = AUClast + Ct/ke, where Ct is the last plasma concentration measured and ke is the elimination rate constant; ke was determined using linear regression analysis of the logarithm-linear part of the plasma concentration–time curve. The half-life (t1/2) of amlodipine was calculated as t1/2 = ln2/ke. The oral clearance (CL/F) of amlodipine was calculated as CL/F = dose/AUCinf.
Statistical analysis
Statistical comparisons among ABCB1 genotypes were made with one-way anova, followed by a posteriori testing with the Bonferroni test. Statistical analyses were performed using the statistical software package Sigmastat for Windows (version 3.1; Systat Software Inc., Richmond, CA, USA). A P-value ≤0.05 was considered to be significant.
Results
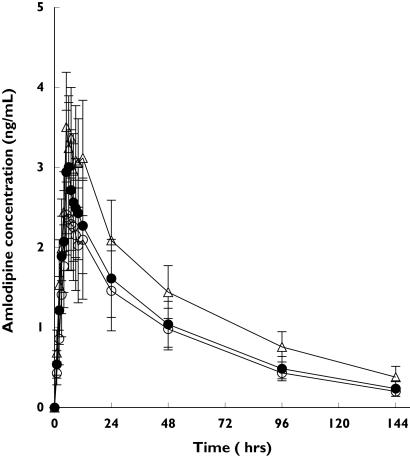
The plasma concentration–time profiles of amlodipine were compared among subjects with different ABCB1 genotypes after a single-dose administration of 5 mg amlodipine and it was found that the plasma concentrations in subjects with 2677GT/3435CT and 2677TT/3435TT were lower than in those with 2677GG/3435CC (Figure 1). In addition, the average values of AUCinf were highest in subjects with 2677GG/3435CC (208.6 ± 39.2 ng h−1 ml−1) and lower in subjects with 2677GT/3435CT (149.8 ± 40.1 ng*h/ml) and 2677TT/3435TT (140.8 ± 35.6 ng h−1 ml−1) in rank with statistical significance [95% confidence interval (CI) on the difference, 2677GG/3435CC vs. 2677GT/3435CT 12.0, 105.6, P < 0.01; 2677GG/3435CC vs. 2677TT/3435TT 19.6, 116.0, P < 0.01; 2677GT/3435CT vs. 2677TT/3435TT −39.2, 57.2, P > 0.05] (Table 1). In addition, subjects with 2677GG/3435CC showed a significant difference in Cmax values from those with 2677TT/3435TT [95% CI on the difference (l h−1) 0.4, 2.0, P < 0.01]. However, oral clearance of amlodipine was lower in subjects with 2677GG/3435CC than in those with 2677GT/3435CT [95% CI on the difference (ng ml−1) 0.4, 2.0, P < 0.01] and 2677TT/3435TT [95% CI on the difference (ng ml−1) 0.4, 2.0, P < 0.01] by 44% and 52%, respectively (P = 0.01) (Table 1). Pharmacokinetic parameters of amlodipine according to ABCB1 genotypes are summarized in Table 1.
Figure 1.
Plasma concentration–time curve of amlodipine after administration of 5 mg amlodipine orally according to ABCB1 genetic polymorphisms at positions of G2677T and C3435T. Values are given as mean ± SD. 2677GG/3435CC (▵); 2677GT/3435CT (•); 2677TT/3435TT (○)
Table 1.
Pharmacokinetic profiles of amlodipine after administration of a single dose of 5 mg amlodipine according to the genetic polymorphisms of the ABCB1 gene at positions 2677 and 3435
| Difference between genotypes | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | 2677GG/3435CC | 2677GT/3435CT | 2677TT/3435TT | 2677GG/3435CC & 2677GT/3435CT | 2677GG/3435CC & 2677TT/3435TT | 2677GT/3435CT & 2677TT/3435TT | P-value |
| Cmax (ng ml−1) | 3.8 ± 0.5 | 3.2 ± 0.5 | 2.7 ± 0.5 | 0.6 | 1.2† | 0.5 | 0.006 |
| (95% CI) | (3.5, 4.1) | (2.6, 3.8) | (2.3, 3.1) | (−0.2, 1.4) | (0.4, 2.0) | (−0.3, 1.3) | |
| tmax (h) | 5 | 6 | 6 | 0.9 | −0.3 | −1.2 | 0.828 |
| (range) | (5.0–12.0) | (5.0–8.0) | (5.0–12.0) | (−1.7, 3.5) | (−3.0, 2.4) | (−3.9, 1.5) | |
| Half-life (h) | 51.8 ± 10.9 | 46.3 ± 7.1 | 43.0 ± 5.6 | 5.5 | 8.8 | 3.3 | 0.132 |
| (95% CI) | (44.7, 58.9) | (41.7, 50.9) | (39.1, 46.9) | (−4.6, 15.6) | (−1.6, 19.2) | (−7.1, 13.7) | |
| AUClast (ng h−1 ml−1) | 180.9 ± 29.0 | 133.6 ± 33.1 | 125.1 ± 33.0 | 47.3* | 55.9† | 8.5 | 0.003 |
| (95% CI) | (161.9, 199.9) | (111.9, 155.3) | (102.2, 148.0) | (8.7, 85.9) | (16.1, 95.7) | (−31.3, 48.3) | |
| AUCinf (ng h−1 ml−1) | 208.6 ± 39.2 | 149.8 ± 40.1 | 140.8 ± 35.6 | 58.8† | 67.8† | 9.0 | 0.002 |
| (95% CI) | (183.0, 234.2) | (123.6, 176.0) | (116.1, 165.5) | (12.0, 105.6) | (19.6, 116.0) | (−39.2, 57.2) | |
| CL/F (l h−1) | 24.8 ± 5.4 | 35.7 ± 9.9 | 37.7 ± 10.2 | −10.9* | −12.9* | −2.1 | 0.010 |
| (95% CI) | (21.3, 28.3) | (29.2, 42.2) | (30.6, 44.8) | (−21.5, −0.3) | (−23.8, −2.0) | (−13.0, 8.8) | |
Data are shown as mean ± SD. tmax is given as median (range). Cmax, Peak plasma concentration; tmax, time to Cmax; AUClast, area under the time–concentration curve from 0 to 144 h; AUCinf, area under the time–concentration curve from 0 to infinity; CL/F, oral clearance.
P < 0.05 by anova with Bonferroni correction a posteriori for wild-type group
P < 0.01 by anova with Bonferroni correction a posteriori for wild-type group.
Discussion
The results of the present study suggest that polymorphisms of the ABCB1 gene affect the disposition of amlodipine in humans. Subjects with mutant alleles (2677TT/3435TT) of the ABCB1 gene, especially, showed an increase in the oral clearance of amlodipine with its lower plasma concentrations compared with those with heterozygote (2677GT/3435CT) or wild type (2677GG/3435CC). We first hypothesized that if P-GP plays a crucial role in the disposition of amlodipine, subjects with polymorphic ABCB1 gene might show higher plasma concentrations of amlodipine compared with those with wild-type allele. On the supposition that amlodipine is a substrate of P-GP [7, 8] and that coadministration of grapefruit juice [9] or diltiazem [11], known inhibitors of P-GP, increased the concentrations of amlodipine in humans [12, 13], polymorphisms of the ABCB1 gene would cause increases in the plasma concentrations of amlodipine. However, on the contrary, subjects with the mutant ABCB1 gene showed a decrease in plasma concentrations of amlodipine and an increase in the oral clearance in this study. AUC values were 33% lower in subjects with 2677GG/3435CC and 28% lower in those with 2677GT/3435CT compared with those with 2677TT/3435TT. Despite the belief that the polymorphic ABCB1 gene should be an important determinant of interindividual variations in the disposition of amlodipine, contrary to our results, no association [18] or higher concentrations of other P-GP substrates in 2677TT/3435TT compared with 2677GG/3435CC have been reported in other studies [19, 20].
Unfortunately, we do not fully understand the mechanism(s) of why the polymorphic ABCB1 gene paradoxically reduced the plasma concentrations of amlodipine in this study. Gender differences and interethnic differences in ABCB1 haplotypes have generally been suggested to explain the discrepancies in the findings [21]. However, we could rule out the potential role of gender difference because only male subjects were enrolled in this study. Regarding the ethnic difference in ABCB1 genotype, different patterns of linkage disequilibrium are found in different population groups [22, 23]. Therefore, a significant but unidentified single nucleotide polymorphism (SNP) might cause inconsistent results. In the current study, we recruited only the most frequently observed haplotypes in a Korean population. Among 160 subjects screened for SNPs of the ABCB1 gene in this study, major haplotypes 2677G/3435C, 2677G/3435T and 2677T/3435T constituted 42.5%, 13.8% and 28.1% of all haplotypes, respectively, adding up to a total of 84.4%, and the genotype frequencies observed in this study are similar to previous observations in Asian populations [22, 23]. However, remarkable ethnic differences in the frequencies of SNPs of the ABCB1 gene have been reported [24, 25]. Recently it has been reported that the combination of certain SNP variants into haplotypes might be of higher value in predicting P-GP activity [19]. The authors demonstrated that there are significant differences in the pharmacokinetics of digoxin, a substrate of P-GP, between carriers and noncarriers of haplotypes between G2677T and C3435T. Therefore, we recruited subjects with simultaneous wild, heterozygous, or mutant types of the MDR1 gene at positions 2677 and 3435. Even though our findings showed apparent discrepancies with the previous findings [18–20], our observations are similar to the results of studies using fexofenadine or digoxin as a substrate of P-GP, which showed that subjects with 2677GG/3435CC showed a lower concentration of P-GP substrate than those with 2677TT/3435GG [26–28]. Therefore, haplotype analysis rather than analysis of each genotype may be more crucial in determining the pharmacokinetic characteristics of amlodipine.
In conclusion, amlodipine pharmacokinetics was affected by the polymorphic ABCB1 gene in humans. These findings may provide a plausible explanation for interindividual variation in the disposition of amlodipine, although our study could not explain the exact mechanism(s) of why polymorphic ABCB1 gene paradoxically reduces the plasma levels of amlodipine; this warrants further evaluation.
Acknowledgments
This study was supported by a grant of the Korea Health 21 R&D Project. Ministry of Health & Welfare, R. O. K. (03-PJ10-PG13-GD01-0002).
References
- 1.Cordon-Cardo C, O’Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood–brain barrier sites. Proc Natl Acad Sci USA. 1989;86:695–8. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ieiri I, Takane H, Otsubo K. The MDR1 (ABCB1) gene polymorphism and its clinical implications. Clin Pharmacokinet. 2004;43:553–76. doi: 10.2165/00003088-200443090-00001. [DOI] [PubMed] [Google Scholar]
- 3.Eichelbaum M, Fromm MF, Schwab M. Clinical aspects of the MDR1 (ABCB1) gene polymorphism. Ther Drug Monit. 2004;26:180–5. doi: 10.1097/00007691-200404000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Park JY, Kim KA, Lee GS, Park PW, Kim SL, Lee YS, Lee YW, Shin EK. Randomized, open-label, two-period crossover comparison of the pharmacokinetic and pharmacodynamic properties of two amlodipine formulations in healthy adult male Korean subjects. Clin Ther. 2004;26:715–23. doi: 10.1016/s0149-2918(04)90071-9. [DOI] [PubMed] [Google Scholar]
- 5.Abernethy DR. The pharmacokinetic profile of amlodipine. Am Heart J. 1989;118:1100–3. doi: 10.1016/0002-8703(89)90834-x. [DOI] [PubMed] [Google Scholar]
- 6.Meredith PA, Elliott HL. Clinical pharmacokinetics of amlodipine. Clin Pharmacokinet. 1992;22:22–31. doi: 10.2165/00003088-199222010-00003. [DOI] [PubMed] [Google Scholar]
- 7.Kuzuya T, Kobayashi T, Moriyama N, Nagasaka T, Yokoyama I, Uchida K, Nakao A, Nabeshima T. Amlodipine, but not MDR1 polymorphisms, alters the pharmacokinetics of cyclosporine A in Japanese kidney transplant recipients. Transplantation. 2003;76:865–8. doi: 10.1097/01.TP.0000084873.20157.67. [DOI] [PubMed] [Google Scholar]
- 8.Katoh M, Nakajima M, Yamazaki H, Yokoi T. Inhibitory potencies of 1,4-dihydropyridine calcium antagonists to P-glycoprotein-mediated transport: comparison with the effects on CYP3A4. Pharm Res. 2000;17:1189–97. doi: 10.1023/a:1007568811691. [DOI] [PubMed] [Google Scholar]
- 9.Tian R, Koyabu N, Takanaga H, Matsuo H, Ohtani H, Sawada Y. Effects of grapefruit juice and orange juice on the intestinal efflux of P-glycoprotein substrates. Pharm Res. 2002;19:802–9. doi: 10.1023/a:1016100715125. [DOI] [PubMed] [Google Scholar]
- 10.Wang EJ, Casciano CN, Clement RP, Johnson WW. Inhibition of P-glycoprotein transport function by grapefruit juice psoralen. Pharm Res. 2001;18:432–8. doi: 10.1023/a:1011089924099. [DOI] [PubMed] [Google Scholar]
- 11.Saeki T, Ueda K, Tanigawara Y, Hori R, Komano T. P-glycoprotein-mediated transcellular transport of MDR-reversing agents. FEBS Lett. 1993;324:99–102. doi: 10.1016/0014-5793(93)81540-g. [DOI] [PubMed] [Google Scholar]
- 12.Josefsson M, Zackrisson AL, Ahlner J. Effect of grapefruit juice on the pharmacokinetics of amlodipine in healthy volunteers. Eur J Clin Pharmacol. 1996;51:189–93. doi: 10.1007/s002280050183. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki M, Maeda A, Fujimura A. Influence of diltiazem on the pharmacokinetics of amlodipine in elderly hypertensive patients. Eur J Clin Pharmacol. 2001;57:85–6. doi: 10.1007/s002280000241. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmoller J, Johne A, Cascorbi I, Gerloff T, Roots I, Eichelbaum M, Brinkmann U. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA. 2000;97:3473–8. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cascorbi I, Gerloff T, Johne A, Meisel C, Hoffmeyer S, Schwab M, Schaeffeler E, Eichelbaum M, Brinkmann U, Roots I. Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clin Pharmacol Ther. 2001;69:169–74. doi: 10.1067/mcp.2001.114164. [DOI] [PubMed] [Google Scholar]
- 16.Tanabe M, Ieiri I, Nagata N, Inoue K, Ito S, Kanamori Y, Takahashi M, Kurata Y, Kigawa J, Higuchi S, Terakawa N, Otsubo K. Expression of P-glycoprotein in human placenta: relation to genetic polymorphism of the multidrug resistance (MDR)-1 gene. J Pharmacol Exp Ther. 2001;297:1137–43. [PubMed] [Google Scholar]
- 17.Bahrami G, Mirzaeei S. Simple and rapid HPLC method for determination of amlodipine in human serum with fluorescence detection and its use in pharmacokinetic studies. J Pharm Biomed Anal. 2004;36:163–8. doi: 10.1016/j.jpba.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Drescher S, Schaeffeler E, Hitzl M, Hofmann U, Schwab M, Brinkmann U, Eichelbaum M, Fromm MF. MDR1 gene polymorphisms and disposition of the P-glycoprotein substrate fexofenadine. Br J Clin Pharmacol. 2002;53:526–34. doi: 10.1046/j.1365-2125.2002.01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johne A, Kopke K, Gerloff T, Mai I, Rietbrock S, Meisel C, Hoffmeyer S, Kerb R, Fromm MF, Brinkmann U, Eichelbaum M, Brockmoller J, Cascorbi I, Roots I. Modulation of steady-state kinetics of digoxin by haplotypes of the P-glycoprotein MDR1 gene. Clin Pharmacol Ther. 2002;72:584–94. doi: 10.1067/mcp.2002.129196. [DOI] [PubMed] [Google Scholar]
- 20.Chowbay B, Cumaraswamy S, Cheung YB, Zhou Q, Lee EJ. Genetic polymorphisms in MDR1 and CYP3A4 genes in Asians and the influence of MDR1 haplotypes on cyclosporin disposition in heart transplant recipients. Pharmacogenetics. 2003;13:89–95. doi: 10.1097/00008571-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Yi SY, Hong KS, Lim HS, Chung JY, Oh DS, Kim JR, Jung HR, Cho JY, Yu KS, Jang IJ, Shin SG. A variant 2677A allele of the MDR1 gene affects fexofenadine disposition. Clin Pharmacol Ther. 2004;76:418–27. doi: 10.1016/j.clpt.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Chowbay B, Zhou S, Lee EJ. An interethnic comparison of polymorphisms of the genes encoding drug-metabolizing enzymes and drug transporters: experience in Singapore. Drug Metab Rev. 2005;37:327–78. doi: 10.1081/dmr-28805. [DOI] [PubMed] [Google Scholar]
- 23.Ozawa S, Soyama A, Saeki M, Fukushima-Uesaka H, Itoda M, Koyano S, Sai K, Ohno Y, Saito Y, Sawada J. Ethnic differences in genetic polymorphisms of CYP2D6, CYP2C19, CYP3As and MDR1/ABCB1. Drug Metab Pharmacokinet. 2004;19:83–95. doi: 10.2133/dmpk.19.83. [DOI] [PubMed] [Google Scholar]
- 24.Tang K, Ngoi SM, Gwee PC, Chua JM, Lee EJ, Chong SS, Lee CG. Distinct haplotype profiles and strong linkage disequilibrium at the MDR1 multidrug transporter gene locus in three ethnic Asian populations. Pharmacogenetics. 2002;12:437–50. doi: 10.1097/00008571-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Kroetz DL, Pauli-Magnus C, Hodges LM, Huang CC, Kawamoto M, Johns SJ, Stryke D, Ferrin TE, DeYoung J, Taylor T, Carlson EJ, Herskowitz I, Giacomini KM, Clark AG Pharmacogenetics of Membrane Transporters Investigators. Sequence diversity and haplotype structure in the human ABCB1 (MDR1, multidrug resistance transporter) gene. Pharmacogenetics. 2003;13:481–94. doi: 10.1097/00008571-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Kim RB, Leake BF, Choo EF, Dresser GK, Kubba SV, Schwarz UI, Taylor A, Xie HG, McKinsey J, Zhou S, Lan LB, Schuetz JD, Schuetz EG, Wilkinson GR. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther. 2001;70:189–99. doi: 10.1067/mcp.2001.117412. [DOI] [PubMed] [Google Scholar]
- 27.Sakaeda T, Nakamura T, Horinouchi M, Kakumoto M, Ohmoto N, Sakai T, Morita Y, Tamura T, Aoyama N, Hirai M, Kasuga M, Okumura K. MDR1 genotype-related pharmacokinetics of digoxin after single oral administration in healthy Japanese subjects. Pharm Res. 2001;18:1400–4. doi: 10.1023/a:1012244520615. [DOI] [PubMed] [Google Scholar]
- 28.Shon J, Chun H, Kim K, Kim E, Yoon Y, Jang I, Shin S, Shin J. The PK and PD of fexofenadine in relation to MDR1 genetic polymorphism in Korean healthy subjects. Clin Pharmacol Ther. 2002;71:P71. [Google Scholar]