Abstract
Aims
Although cysteamine was first used in the treatment of cystinosis in 1976 and approved by the FDA as cysteamine bitartrate (Cystagon™) in 1994, surprisingly little pharmacological data are available for this compound. Cysteamine and its related drugs are currently being evaluated for the treatment of Huntington’s and Parkinson’s disease. The aim of te study was to understand the pharmacokinetics of cysteamine bitartrate following gastrointestinal infusion.
Method
Cysteamine bitartrate was delivered through a naso-enteric catheter into the stomach (n = 8), small intestine (n = 8) and caecum (n = 4) of normal subjects. Plasma cysteamine concentrations were determined using LC-MS/MS.
Results
The rate and extent of drug absorption were assessed by comparing AUC(0, ∞), Cmax and tmax, among the gastrointestinal infusion sites. Total cysteamine exposure, expressed as area under the curve (AUC(0, ∞)) was greatest when the drug was infused into the small intestine (4331.3 ± 1907.6 min × µm) followed by stomach (3901.9 ± 1591.9 min × µm) and caecum (3141.4 ± 1627.6 min × µm). Cysteamine infusion into the small intestine resulted in the most rapid rise to maximal plasma concentrations (tmax = 21 ± 0.56 min); tmax was delayed to 50 ± 26 min and 64 ± 26 min after gastric and caecal infusion, respectively. The maximum cysteamine plasma concentration (Cmax) was reached after infusion of the drug into the small intestine (51 ± 21 µm), which was higher than plasma Cmax concentrations after gastric (39 ± 16 µm) and caecal infusion (23 ± 15 µm).
Conclusions
The pharmacokinetic data generated help extend our understanding of cysteamine.
Keywords: plasma cysteamine, tandem mass spectrometry
Introduction
Cysteamine (β-mercapto-ethylamine) bitartrate is currently the only drug approved for the treatment of cystinosis, a rare autosomal recessive disease caused by the abnormal intralysosomal accumulation of the amino-acid cystine within various tissues [1]. Cysteamine has been shown to lower intracellular cystine concentrations by reacting with intralysosomal cystine to form the mixed disulphide of cysteamine and cysteine, which then leaves the lysosome via the lysine transport system [2]. Regular treatment with cysteamine has been shown to reduce the rate of progression of renal and thyroid failure in children with cystinosis [3–5], and more recently animal studies have suggested a potential use for cystamine (which is converted to cysteamine) for the treatment of Huntington’s and Parkinson’s disease [6–11]. Although the mechanism of cysteamine action in cystinosis is understood, little is known about the pharmacokinetics of the drug.
From a recent study evaluating cysteamine-induced gastric acid secretion in children with cystinosis, it was determined that hypergastinaemia was commonly seen following oral cysteamine, particularly in those who also had regular gastrointestinal symptoms [12, 13]. One child was reported to have a peak serum gastrin concentration which was three times higher when cysteamine was delivered into the jejunum as compared with the stomach [12]. A possible reason for this would be that cysteamine is more efficiently absorbed from the small intestine. The present study was designed to evaluate how effectively cysteamine bitartrate is absorbed following delivery of the drug into various parts of the intestinal tract.
Methods
This prospective study was approved by the University of California at San Diego (UCSD), Human Research Protection Program, and informed consent was obtained from each participant. Study subjects were recruited locally and admitted to the UCSD General Clinical Research Center.
Patients
Eight healthy adult control patients (six male, two female, one African American, seven Caucasians) with a mean age of 23.2 years (range 19–28 years) and a mean weight of 80.7 kg (range 62.4–107.0 kg) were enrolled. All subjects were asked to discontinue use of acid suppressants, antibiotics, nonsteroidal anti-inflammatory drugs, pro-kinetic agents and antihistamines 2 weeks prior to admission. Baseline chemistry, Helicobacter pylori serology, complete blood count and urinalysis were performed upon admission. Urinary pregnancy tests were performed for women.
Cysteamine bitartrate delivery
Cysteamine was delivered through a silicone rubber naso-enteric tube (Dentsleeve Pty Ltd, Australia, now Dentsleeve International Ltd, Mississauga, Canada), 3 mm in diameter and 4.5 m in length. The tube, specifically made for this study, had a tungsten weighted tip and immediately proximal to this was an inflatable balloon (3 ml capacity). The infusion port (1 mm diameter) through which drug was delivered was 5 cm proximal to the balloon.
On day 1 of the study, the naso-enteric tube was inserted into the stomach and 1475 mg cysteamine bitartrate (500 mg cysteamine-base) dissolved in 10 ml of water was infused into the stomach over 1–2 min. By day 3 of the study the tip of the tube had passed into the proximal small intestine just distal to the ligament of Treitz (confirmed fluoroscopically) and cysteamine bitartarte administration was repeated, with a dose equal to day 1. The balloon was then inflated with water and peristalsis propelled the tube distally. Tube position within the caecum was confirmed fluoroscopically on day 5, and cysteamine bitartrate was infused into the caecum, with equal dose to day 1. In three cases, drug infusion into the caecum was performed on day 7 due to slow tube progression through the intestine. If the tube had migrated too far it was retracted into the desired location. Subjects were fasted overnight before cysteamine bitartrate administration.
Cysteamine measurements
Following an overnight fast (except for water) blood samples were taken at baseline and measured at 5, 10, 20, 30, 45, 60, 75, 90, 105, 120, 150 min, and 3, 4, 6, 8, 10, 12 and 16 h after intraluminal infusion of cysteamine bitartrate. To measure plasma cysteamine, 1 ml blood samples were collected in heparinized vacutainers, centrifuged within 1 h and plasma was stored at −18 °C. Using a previously described method [12–14], the concentration of cysteamine was measured using LC-MS/MS (API 2000 LC/MS/MS, Applied Biosystems, Foster City, CA). Cysteamine concentrations were calculated using a calibration curve, which was prepared by spiking plasma with buffered cysteamine. Solutions and quality control samples were analyzed with each batch.
Pharmacokinetic analysis
The cysteamine noncompartmental plasma pharmacokinetics were determined using WinNonlin v4.0.1 (Pharsight, Mountain View, CA). The area under the curve plasma concentration-time profile was established by linear trapezoidal integration.
Statistical analysis
Means and standard deviations are reported for all variables. For each pharmacokinetic outcome measure, sample means were compared among the three sites of the intestinal tract where cysteamine was introduced. Mixed model REML repeated measures analysis of variance, with the injection site fixed and subjects as the random effect, was used. This method takes into account all observed data, even though some subjects have missing measurements for the caecum site. If an effect was detected at the 5% significance level, Tukey’s honestly significant difference (HSD) test was applied to determine which site(s) accounted for the difference(s). Mid-ileum data from two subjects were not included in the statistical analysis.
Results
In all subjects the naso-enteric tube passed successfully from the stomach into the upper small intestine. However, it did not progress any further in two subjects. In two of the subjects the tube only reached the mid-ileum, but it did progress to the caecum in four subjects. There were no reported adverse effects with the insertion or removal of the naso-enteric tube. None of the subjects reported any symptoms following cysteamine infusion at any site.
Cysteamine pharmacokinetics
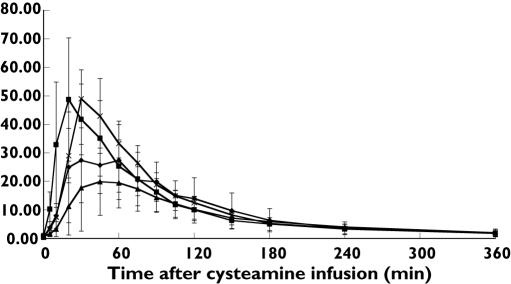
The mean plasma cysteamine concentration-time profiles following infusion of cysteamine bitartrate into various intestinal sites are displayed in Figure 1. The rate and extent of drug absorption were assessed by comparing AUC(0, ∞), Cmax and tmax, respectively, among the gastrointestinal infusion sites. Total cysteamine exposure, expressed as area under the curve (AUC(0, ∞)), was greatest when the drug was infused into the small intestine (4331.3 ± 1907.6 min × µm) followed by stomach (3901.9 ± 1591.9 min × µm) and caecum (3141.4 ± 1627.6min × µm). Based on area under the plasma concentration-time profile, the extent of cysteamine absorption did not differ significantly with the site of infusion (Table 1). However, significant differences in the rate of cysteamine absorption were observed based on infusion site. Cysteamine infusion into the small intestine resulted in the most rapid rise to maximal plasma concentrations (tmax = 21 ± 6 min); the time to maximal plasma concentration was delayed significantly to 50 ± 26 min and 64 ± 26 min after gastric and caecal infusion (P = 0.0076), respectively. The maximum cysteamine plasma concentration (Cmax) was reached after infusion of the drug into the small intestine (51 ± 21 µm), which was nonsignificantly higher than plasma Cmax concentrations after gastric (39 ± 16 µm) and significantly higher than after caecal infusion (23 ± 15 µm, P = 0.04). The terminal phase half-life (t/2) was not significantly different across infusion sites; the mean t1/2 was 94.5 min, 112.7 min, and 98.3 min following gastric, intestinal, and caecal infusion, respectively.
Figure 1.
Mean plasma cysteamine levels, µM (± SD), following infusion of cysteamine bitartrate into various intestinal sites. Stomach (n = 8) (✦); proximal SI (n = 8) (▪); cecum (n = 4) (▴); mid-ileum (n = 2) ( )
)
Table 1.
Pharmacokinetic parameters following single-dose administration of cysteamine to different gastrointestinal sites in healthy volunteers
| Parameter | Stomach (n = 8) | Small Intestine (n = 8) | Caecum (n = 4) | Mid-ileum (n = 2) |
|---|---|---|---|---|
| λZ (h-1) | 0.0086 (0.0030) | 0.0070 (0.0028) | 0.0259 (0.0350) | 0.0057 (0.0011) |
| t1/2 (min) | 94.5 (48.0) | 112.7 (42.5) | 98.3 (46.5) | 124.6 (23.3) |
| AUC(0,tlast) (min × µm) | 3613.3 (1383.5) | 3987.8 (1659.0) | 2804.2 (1323.0) | 4215.1 (1048.5) |
| AUC(0, ∞) (min × µm) | 3901.9 (1591.9) | 4331.3 (1907.6) | 3141.4 (1627.6) | 4556.4 (947.7) |
| %AUCextrap | 6.4 (5.2) | 6.8 (5.0) | 8.6 (5.6) | 7.9 (3.8) |
| tmax (min) | 50a (26) | 21b (6) | 64a (26) | 30 0 |
| Cmax (µm) | 39a,b (16) | 51a (21) | 23b (15) | 48.9 (14.4) |
Standard deviations listed in parentheses.
Within-group mean values with non-matching superscripts are significantly different (P < 0.05). Data from mid-ileum cysteamine infusion were not included in the statistical analysis.
In two subjects, cysteamine bitartrate was infused into the mid-ileum and the absorption pattern seemed comparable with the absorption from the small intestine (Table 1).
The range of values by the analytical method used was 1–100 µm. Three quality control samples at 2, 30 and 55 µm were run with each batch. Repeatability expressed as the relative standard deviations (n = 5) at each level, were 9.0%; 5.3% and 5.0%, respectively. The intermediate precision over 10 months was 12.7%, 4.5% and 5.3%, respectively,
Discussion
In 1994 cysteamine bitartrate (Cystagon™) was approved by the FDA for the treatment of cystinosis, a rare disease of intralysosomal cystine accumulation [15]. Prior to the availability of cysteamine most patients would succumb to renal ‘death’ by 9 years of age, as well as thyroid and growth failure in their early teens [3–5, 16]. However, clinical studies, which were initiated as early as 1978, have shown that regular treatment with cysteamine delays the progression of renal failure and the need for transplantation as well as improving growth potential in patients with cystinosis [3, 4]. Cysteamine works as a treatment for cystinosis by forming a mixed disulphide with intralysosomal cystine, which then leaves the lysosome through the lysine transport system [2, 17].
More recently there has been considerable interest in the use of cystamine (which is reduced to cysteamine) and cysteamine for the treatment of Huntington’s disease (HD) and Parkinson’s disease. Excessive transglutaminase activity, which is detected within the brains of HD patients, is thought to contribute to cell death and striated neuronal loss. Cystamine is a competitive inhibitor of transglutaminase and murine studies have suggested that cystamine can prevent the development of striatal neuropathology in the YAC128 mouse model of HD [7, 18]. Although most studies have been performed in mice, enough interest has been generated to start studies in humans with HD. A short-term dose finding and tolerability study of cysteamine bitartrate in HD patients was undertaken and reported that adult patients were able to tolerate cysteamine (20 mg kg−1 day−1 in four divided doses). Patients were unable to tolerate higher doses of cysteamine because of side-effects including nausea, vomiting, difficulty walking and abnormal odour [19]. Other studies have now also suggested a neuroprotective effect of cystamine in aged parkinsonian mice [20].
Our present study provides useful data regarding the pharmacokinetics of cysteamine bitartrate and the results show that cysteamine absorption varies slightly between intestinal infusion sites in normal healthy adults. The total AUC (of the concentration-time curve) was similar following gastric and small intestinal delivery of cysteamine and this may have been related to the small sample sizes. However, the fact that Cmax was higher and was reached more rapidly following small intestinal infusion of cysteamine would suggest the cysteamine is most readily absorbed from this site. Further, the results from two patients with mid-ileum profiles indicate that cysteamine absorption does not vary greatly throughout the small intestine. This information may be useful not only to patients with cystinosis but also to individuals in whom the potential neuroprotective properties of cysteamine or cystamine may be beneficial. In fact, a recent study in children with cystinosis suggested that cysteamine delivered directly into the small intestine, by causing a significantly higher Cmax, caused prolonged intracellular depletion of leucocyte cystine [21]. This would suggest that use of an enteric-coated cysteamine preparation could result in less frequent daily dosing of the drug. In addition, patients receiving enteric-released cysteamine may suffer fewer gastrointestinal side-effects.
In this study we used a naso-enteric catheter, which enabled the infusion of cysteamine into specific gastrointestinal sites. The catheter was devised for long-term placement and following insertion its presence was well tolerated without any complaints of significant discomfort. In general, catheter removal was judged as the most objectionable aspect of the study. All patients were admitted to the General Clinical Research Center for the duration of the entire study, but were allowed to leave the hospital on the study-free days. We believe this device may have uses in future pharmacokinetic studies, simulation of slow-release preparations and possibly, with some manipulation, may also allow aspiration from different intestinal sites.
Although cysteamine was first used in cystinosis in 1976 [17] and approved by the FDA for use in cystinosis as cysteamine bitartrate (Cystagon™) in 1994, much pharmacologic and pharmacodynamic data remain to be defined for this compound. The pharmacokinetic data generated in the present study will hopefully be of use for the development of a controlled release form of cysteamine, and may benefit Huntington’s disease and Parkinson’s disease research as well as the treatment of cystinosis.
Acknowledgments
Support was provided by The Elizabeth Huth Coates Charitable Foundation, The Cystinosis Research Foundation and a National Institute of Health Grant MO1RR00827. In particular, we are indebted to the staff of the General Clinical Research Center, UCSD.
References
- 1.Gahl WA, Thoene JG, Schneider JA. Cystinosis. N Engl J Med. 2002;347:111–21. doi: 10.1056/NEJMra020552. [DOI] [PubMed] [Google Scholar]
- 2.Pisoni RL, Thoene JG, Christensen HN. Detection and characterization of carrier-mediated cationic amino acid transport in lysosomes of normal and cystinotic human fibroblasts. Role in therapeutic cystine removal? J Biol Chem. 1985;260:4791–8. [PubMed] [Google Scholar]
- 3.Kimonis VE, Troendle J, Rose SR, Yang ML, Markello TC, Gahl WA. Effects of early cysteamine therapy on thyroid function and growth in nephropathic cystinosis. J Clin Endocrinol Metab. 1995;80:3257–61. doi: 10.1210/jcem.80.11.7593434. [DOI] [PubMed] [Google Scholar]
- 4.Markello TC, Bernardini IM, Gahl WA. Improved renal function in children with cystinosis treated with cysteamine. N Engl J Med. 1993;328:1157–62. doi: 10.1056/NEJM199304223281604. [DOI] [PubMed] [Google Scholar]
- 5.Gahl WA, Reed GF, Thoene JG, Schulman JD, Rizzo WB, Jonas AJ, Denman DW, Schlesselman JJ, Corden BJ, Schneider JA. Cysteamine therapy for children with nephropathic cystinosis. N Engl J Med. 1987;316:971–7. doi: 10.1056/NEJM198704163161602. [DOI] [PubMed] [Google Scholar]
- 6.Gentile V, Cooper AJ. Transglutaminases – possible drug targets in human diseases. Curr Drug Targets CNS Neurol Disord. 2004;3:99–104. doi: 10.2174/1568007043482552. [DOI] [PubMed] [Google Scholar]
- 7.Van Raamsdonk JM, Pearson J, Bailey CD, Bailey CDC, Rogers, Johnson GVW, Hayden MR, Leavitt BR. Cystamine treatment is neuroprotective in the YAC128 mouse model of Huntington disease. J Neurochem. 2005;95:210–20. doi: 10.1111/j.1471-4159.2005.03357.x. [DOI] [PubMed] [Google Scholar]
- 8.Fox JH, Barber DS, Singh B, Zucker B, Swindell MK, Norflus F, Buzescu R, Chopra R, Ferrante RJ, Kazantsev A, Hersch SM. Cystamine increases 1-cysteine levels in Huntington’s disease transgenic mouse brain and in a PC12 model of polyglutamine aggregation. J Neurochem. 2004;91:413–22. doi: 10.1111/j.1471-4159.2004.02726.x. [DOI] [PubMed] [Google Scholar]
- 9.Lesort M, Lee M, Tucholski J, Johnson GV. Cystamine inhibits caspase activity. Implications for the treatment of polyglutamine disorders. J Biol Chem. 2003;278:3825–30. doi: 10.1074/jbc.M205812200. [DOI] [PubMed] [Google Scholar]
- 10.Dedeoglu A, Kubilus JK, Jeitner TM, Matson SA, Bogdanov M, Kowall NW, Matson WR, Cooper AJ, Ratan RR, Beal MF, Hersch SM, Ferrante RJ. Therapeutic effects of cystamine in a murine model of Huntington’s disease. J Neurosci. 2002;22:8942–50. doi: 10.1523/JNEUROSCI.22-20-08942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Junn E, Ronchetti RD, Quezado MM, Kim SY, Mouradian MM. Tissue transglutaminase-induced aggregation of alpha-synuclein: Implications for Lewy body formation in Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci U S A. 2003;100:2047–52. doi: 10.1073/pnas.0438021100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dohil R, Newbury RO, Sellers ZM, Deutsch R, Schneider JA. The evaluation and treatment of gastrointestinal disease in children with cystinosis receiving cysteamine. J Pediatr. 2003;143:224–30. doi: 10.1067/S0022-3476(03)00281-6. [DOI] [PubMed] [Google Scholar]
- 13.Dohil R, Fidler M, Barshop B, Newbury RO, Sellers ZM, Deutsch R, Schneider JA. Esomeprazole therapy for gastric acid hypersecretion in children with cystinosis. Pediatr Nephrol. 2005;20:1786–93. doi: 10.1007/s00467-005-2027-1. [DOI] [PubMed] [Google Scholar]
- 14.Guan XHG, Dwivedi C, Matthees DP. A simultaneous liquid chromatography/mass spectrometric assay of glutathione, cysteine, homecysteine and their disulfides in biological samples. J Pharmaceut Biomed Anal. 2003;31:251–61. doi: 10.1016/s0731-7085(02)00594-0. [DOI] [PubMed] [Google Scholar]
- 15.Schneider JA. Approval of cysteamine for patients with cystinosis. Pediatr Nephrol. 1995;9:254. doi: 10.1007/BF00860767. [DOI] [PubMed] [Google Scholar]
- 16.Gretz NMF, Augustin R, Barrat TM, Bender-Gotze C, Brandis M, Bremer HJ. Survival time in cystinosis. A collaborative study. Proc Eur Dial Transplant Assoc. 1983;19:582–9. [PubMed] [Google Scholar]
- 17.Thoene JG, Oshima RG, Crawhall JC, Olson DL, Schneider JA. Cystinosis. Intracellular cystine depletion by aminothiols in vitro and in vivo. J Clin Invest. 1976;58:180–9. doi: 10.1172/JCI108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinto JT, Van Raamsdonk JM, Leavitt BR, Hayden MR, Jeitner TM, Thaler HT, Krasnikov BF, Cooper AJ. Treatment of YAC128 mice and their wild-type littermates with cystamine does not lead to its accumulation in plasma or brain: implications for the treatment of Huntington disease. J Neurochem. 2005;94:1087–101. doi: 10.1111/j.1471-4159.2005.03255.x. [DOI] [PubMed] [Google Scholar]
- 19.Dubinsky R, Gray CCYTE-I-HD. Phase I dose finding and tolerability study of cysteamine (Cystagon) in Huntington’s disease. Mov Disord. 2005 October 28 (Epub ahead of print) [DOI] [PubMed]
- 20.Tremblay ME, Saint-Pierre M, Bourhis E, Levesque D, Rouillard C, Cicchetti F. Neuroprotective effects of cystamine in aged parkinsonian mice. Neurobiol Aging. 2005 May 20 (Epub ahead of print) [DOI] [PubMed]
- 21.Dohil R, Fidler M, Barshop BA, Martin M, Gangoiti J, Deutsch R, Schneider JA. Understanding intestinal cysteamine absorption. J Pediatr. 148:764–9. doi: 10.1016/j.jpeds.2006.01.050. [DOI] [PubMed] [Google Scholar]