Abstract
Aims
Inhaled corticosteroids (ICS) are the cornerstone of asthma treatment. At high doses they can give rise to systemic side-effects such as hypothalamic-pituitary-adrenal (HPA) axis suppression. This effect may depend on the delivery system, which in turn alters drug deposition and adsorption. We hypothesized that adrenal suppression depends on the rate of steroid absorption rather than the total steroid dose received.
Methods
Eight healthy adult males were recruited for a randomized double-blind placebo controlled trial. Adrenocortical suppression ability was demonstrated by a 30% suppression of early morning cortisol following 1 mg dexamethasone. Subjects then attended in the evening on two occasions receiving 500 µg of intravenous beclomethasone monopropionate (17-BMP) for either 15 min or 2 h. Overnight urinary cortisol : creatinine (C : C) ratio was measured before and after the infusion and an 08.00 h serum cortisol was measured following the infusion.
Results
Mean C : C pre and post 15 min infusion was 5.97 and 3.22 (P= 0.005). Mean C : C pre and post 2 h infusion was 6.31 and 4.15 (P= 0.004). Delta C : C and mean 08.00 h cortisol for 15 min and 2 h infusion was 2.74 and 2.16 and 425 nmol l−1 and 400 nmol l−1, respectively (P= NS).
Conclusions
The rate of infusion of 17-BMP seemed to have little effect on the degree of adrenal suppression. Individual C : C ratios were reproducible. Differences in absorption of ICS are unlikely to explain observed differences in HPA axis suppression.
Keywords: adrenal axis suppression, asthma, beclomethasone
Introduction
Inhaled corticosteroids (ICS) are the cornerstone of asthma treatment. It is well known that long-term oral steroids have systemic side-effects such hypothalamic-pituitary-adrenal (HPA) axis suppression, cataracts and osteoporosis [1, 2]. ICS have local activity and high first pass metabolism, which minimizes systemic side-effects but at high doses ICS can also give rise to systemic complications because of absorption from the lung and partial clearance at first pass [3, 4].
Although the side-effects of ICS appear to be dose related the delivery system also has a contributory effect. In an editorial Dekhuijzen & Honour [5] speculated on the mechanisms behind the apparent lack of HPA suppression caused by chlorofluorocarbon (CFC)-free beclomethasone dipropionate (BDP). This method of drug delivery produces particles with a smaller median mass aerodynamic diameter, which not only results in greater lung deposition but also in a more rapid absorption of drug. Thus peak blood concentrations occur more rapidly compared with dose equivalent CFC BDP. One hypothesis was that a rapid achievement of peak circulating exogenous corticosteroid concentrations, as occurs with the rapid absorption of CFC free BDP, led to less HPA axis suppression. The aim of our study was to establish whether a rapid rate of change in exogenous steroid caused less HPA axis suppression than a slower elevation in plasma steroid concentration. The steroid we used was intravenous 17-beclomethasone monopropionate (17-BMP) the active metabolite of beclomethasone dipropionate. If the mechanism of suppression of the HPA axis caused by the different pharmacokinetic profiles of ICS could be elucidated then it would allow drug delivery that minimized side-effects without compromising the beneficial effects of inhaled steroids.
We hypothesized that the rate of steroid absorption is an important determinant of the degree of adrenal suppression.
Methods
Eight healthy adult males, mean age 34 years, were recruited to a randomized double-blind placebo controlled trial. Ethical approval was obtained from the local ethics committee and patient consent taken prior to screening. Sensitivity to adrenocortical suppression was demonstrated by a 30% suppression of early morning cortisol following 1 mg dexamethasone. Randomized subjects then attended in the evening on two occasions receiving 500 µg of intravenous 17-BMP supplied as a 5 ml ampoule containing 500 µg of 17-BMP in a vehicle of propylene glycol and ethanol (BCM Specials, Nottingham). The infusion was given for either 15 min or 2 h alongside appropriate saline placebo. Overnight urinary cortisol : creatinine (C : C) ratio was measured before and after the infusion and a 08.00 h serum cortisol was measured following the infusion. Cortisol was measured using the DPC (Llanberis, UK) Immulite 2000 immunoassay analyzer. Creatinine was measured using the Beckman Coulter (High Wycombe, UK) LX20 PRO analyzer. The study was powered (80%) at 0.05 significance for a 20% difference in C : C ratio.
Results
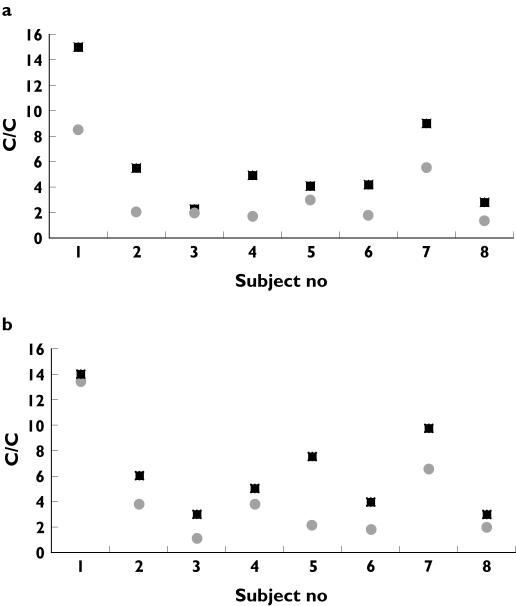
Mean C : C pre and post 15 min infusion was 5.97 and 3.22 (P= 0.005) (Figure 1a). Mean C : C pre and post 2 h infusion was 6.31 and 4.15 (P= 0.004) (Figure 1b). Thus the difference in C : C associated with the 15 min and 2 h infusion was 2.74 (SD 1.9) and 2.16 (SD 1.5) (P= NS). The coefficient of variance for the pre-infusion C : C was 21%. Mean 08.00 h cortisol for the 15 min and 2 h infusion was 425 nmol l−1 and 400 nmol l−1, respectively (P= NS).
Figure 1.
Pre (▪) and post (•) infusion overnight urinary cortisol : creatinine ratios following a) a 15 min infusion of 17-BMP and b) a 2 h infusion of 17-BMP
Discussion
To our knowledge this is the first use of intravenous 17-BMP to mimic the pharmacokinetics of inhaled BDP. BDP is metabolized in the lung to 17- BMP, 21-BMP and beclomethasone. 17-BMP has the highest affinity for glucocorticoid receptors and it is known to circulate at greater concentrations in the serum compared with other metabolic breakdown products [6–8]. Daley-Yates and colleagues [9] showed that the main active metabolite of inhaled BDP is 17-BMP and that following an inhaled dose of BDP the bioavailability 17-BMP is high (mean percentage 62%). Hence by infusing 17-BMP at two different rates we sought to mimic rapid and slow systemic bioavailability of 17-BMP seen with different inhalers. The use of the active metabolite allowed us to avoid any individual variation in the conversion of parent drug to 17-BMP.
By using different rates of infusion of intravenous 17-BMP we sought to mimic the pharmacokinetics of systemic corticosteroid bioavailability when inhaled from CFC free and conventional inhalers. The infusion times in our study were chosen to reflect the peak blood concentrations achieved after inhalation of CFC and hydroflurocarbon (HFA) BDP. They are based on the work of Seale et al. [10] who showed that the serum concentrations of BDP plus metabolites peaked at 15 min and 2 h following single inhaled doses of HFA-BDP and CFC-BDP, respectively.
The dose of 17-BMP was chosen to reflect the concentration achieved following high dose inhaled steroids and the level of urinary C : C suppression achieved was similar to that shown following 2000 µg of inhaled beclomethasone [11].
Because our study was small, an accurate measurement of adrenal suppression was required. There is controversy regarding the best method of assessing adrenal suppression. Assessing HPA axis suppression in heathly adults is a different scenario from the assessment of pathological, long-term, adrenal suppression when stimulation tests are used.
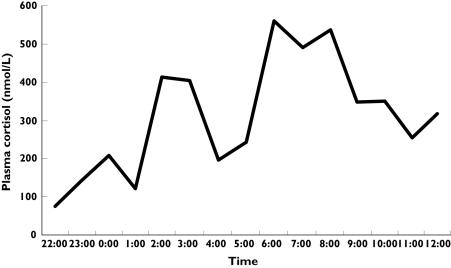
Methods of assessment of basal adrenocortical secretory activity commonly used include 24 h urine collection, which is associated with a risk of poor compliance, and overnight assessment of plasma cortisol. In studies using plasma cortisol the AUC is generally assessed to establish the degree of suppression [12, 13]. This method however, requires frequent blood sampling because of the pulsatile secretion of the adrenal hormones. Figure 2 shows the short-term variability of overnight cortisol on a study subject during the pilot study. Fifty-three blood samples were taken. Overnight plasma cortisol estimation is therefore time consuming, expensive and labour intensive.
Figure 2.
Overnight plasma cortisol (—) profile in a subject during the pilot study, with 15 min sampling
Since our study was of a crossover design and we were investigating the relative degree of adrenal suppression, the assessment of a population normal range of cortisol secretion at baseline was not required. We therefore took as our primary endpoint overnight urinary C : C ratios as it is claimed that within individuals it is a sensitive and reproducible index of change in cortisol secretion [4, 11, 14, 15]. Others have previously demonstrated that overnight and timed morning urinary C : C ratios are as sensitive as 24 h urinary free cortisol excretion [11, 14].
In this study we showed that individual C : C ratios were reproducible and a highly significant degree of adrenal suppression could be demonstrated at dosing consistent with clinical usage. Thus we suggest that in correctly designed studies this highly practical method of assessing adrenal suppression has advantages over other methods for gauging the relative potential of inhaled steroids to cause systemic side-effects. We demonstrated that differences in rates of infusion of 17-BMP had little effect on the degree of adrenal suppression in normal male subjects. However the coefficient of variance of suppression was large and this relates to the relatively small numbers. C : C ratios are an established methodology and previous studies with larger numbers have demonstrated much smaller CVs [11, 14]. We acknowledge that the large CV seen in our study does considerably reduce the degree of certainty with which we can suggest that changes in absorption rates do not cause different degrees of adrenal suppression.
The half-life of 17-BMP formed following an intravenous dose of BDP is 2.7 h and it has extensive tissue distribution [9]. Thus although these results suggest that time to peak concentrations is not the determinant of adrenal suppression, we cannot exclude the suggestion that an infusion time greater than 2 h may be required to show a significant difference in HPA axis suppression. In addition a study in the acute setting such as ours does not provide information about chronic dosing where, for example, partial drug clearance between dosing may lead to accumulation. We conclude that the differences in absorption rate of ICS seem unlikely to explain any observed differences in HPA axis suppression.
References
- 1.Kwong FK, Sue MA, Klaustermeyer WB. Corticosteroid complications in respiratory disease. Ann Allergy. 1987;58:326–30. [PubMed] [Google Scholar]
- 2.Urban RCJ, Cotlier E. Corticosteroid-induced cataracts. Surv Ophthalmol. 1988;31:102–10. doi: 10.1016/0039-6257(86)90077-9. [DOI] [PubMed] [Google Scholar]
- 3.Pederson S O’Byrne. A comparison of the efficacy and safety of inhaled corticosteroids in asthma. Allergy. 1997;52:1–34. doi: 10.1111/j.1398-9995.1997.tb05047.x. [DOI] [PubMed] [Google Scholar]
- 4.Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy. Arch Intern Med. 1999;159:941–55. doi: 10.1001/archinte.159.9.941. [DOI] [PubMed] [Google Scholar]
- 5.Dekhuijzen PN, Honour JW. Inhaled corticosteroids and the hypothalamic-pituitary-adrenal (HPA) axis: do we understand their interaction? Resp Med. 2000;94:1–5. doi: 10.1053/rmed.2000.0791. [DOI] [PubMed] [Google Scholar]
- 6.Rohdewald P, Mollmann HW, Hochhaus G. Affinities of glucocorticoids for glucocorticoid receptors in human lung. Agents Actions. 1985;17:3–4. doi: 10.1007/BF01982622. [DOI] [PubMed] [Google Scholar]
- 7.Wurthwein G, Rohdewald P. Activation of beclomethasone dipropionate by hydrolysis to beclomethasone-17-monproprionate. Biopharm Drug Dispos. 1990;11:381–94. doi: 10.1002/bdd.2510110503. [DOI] [PubMed] [Google Scholar]
- 8.Anderson P, Ryrfeldt A. Biotransformation of topical glucocorticoids, budesonide and beclomethasone 17, 21-diproprionate in human liver and lung homogenate. J Pharm Pharmacol. 1984;36:763–5. doi: 10.1111/j.2042-7158.1984.tb04868.x. [DOI] [PubMed] [Google Scholar]
- 9.Daley-Yates PT, Price AC, Sisson JC, Pereira A, Dallow N. Beclomethasone dipropionate. absolute bioavailability, pharmacokinetics and metabolism following intravenous, oral, intranasal and inhaled administration in man. Br J Clin Pharmacol. 2001;51:400–9. doi: 10.1046/j.0306-5251.2001.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seale JP, Harrison LI. Effect of changing the fine particle mass of inhaled beclomethasone dipropionate on intrapulmonary deposition and pharmacokinetics. Resp Med. 1998;92:9–15. doi: 10.1016/s0954-6111(98)90212-8. [DOI] [PubMed] [Google Scholar]
- 11.McIntyre HD, Mitchell CA, Bowler S, Armstrong JG, Wooler JA, Cowley DM. Measuring the systemic effects of inhaled beclomethasone: timed morning urine collections compared with 24 hour specimens. Thorax. 1995;50:1280–4. doi: 10.1136/thx.50.12.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson HS, Stricker W, Casale TB, Raff H, Fourre JA, Aron DC, Newman KB. A comparison of methods for assessing hypothalamic-pituitary-adrenal (HPA) axis activity in asthma patients treated with inhaled corticosteroids. J Clin Pharmacol. 2002;42:319–26. doi: 10.1177/00912700222011355. [DOI] [PubMed] [Google Scholar]
- 13.Grahnen A, Eckernas SA, Brundin RM, Ling-Andersson A. An assessment of the systemic activity of single doses of inhaled fluticasone proprionate in healthy volunteers. Br J Clin Pharmacol. 1994;38:521–5. doi: 10.1111/j.1365-2125.1994.tb04393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong WM, Alaghband-Zadeh J, Jones J, Carter G, O’Shea D. The midnight to morning urinary cortisol increment is an accurate, noninvasive method for assessment of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab. 1999;84:3093–8. doi: 10.1210/jcem.84.9.5998. [DOI] [PubMed] [Google Scholar]
- 15.Lipworth BJ, Wilson AM. Dose response to inhaled corticosteroids: Benefits and risks. Sem Resp Crit Care Med. 1998;19:625–46. [Google Scholar]