Abstract
Aims
To assess the dose selection using population pharmacokinetics of Pegylated Intron-α2b (PEG-Intron) in patients with chronic myelogenous leukaemia (CML).
Methods
PEG-Intron 3–6 µg kg−1 was administered subcutaneously once a week and blood samples were collected up to 48 weeks of treatment. A total of 624 samples collected from 137 patients were included in the analysis. Nonlinear mixed-effects modelling was used to analyse the sparsely sampled concentration data from a clinical efficacy trial. Covariates in the analysis included weight, sex, age, race, serum creatinine and estimated creatinine clearance (CLcr).
Results
The apparent clearance of PEG-Intron decreased after repeated dosing. The clearance at treatment week 4 was 42.3 l day−1 (patients with CLcr 120 ml min−1) with interpatient variability 30%. At treatment week 48, the clearance value was reduced to 69% of its week 4 value. CLcr, a composite variable calculated from body weight, sex, age and serum creatinine, had a small but statistically significant influence on the clearance of PEG-Intron. The clearance of PEG-Intron in patients with CML was 40% higher than that of hepatitis C virus-infected patients.
Conclusion
The dose of PEG-Intron 6.0 µg kg−1 week−1 appeared appropriate in the treatment of patients with CML.
Keywords: Chronic myelogenous leukaemia, nonlinear mixed-effects models, PEG-Intron, population pharmacokinetics
Introduction
Chronic myelogenous leukaemia (CML) is a clonal myeloproliferative disorder, a neoplastic proliferation of the pluripotential stem cell. The antileukaemic effect of alpha interferon in CML is well documented by haematological response, cytogenetic response and significant survival benefit [1, 2]. In Philadelphia chromosome (Ph)-positive CML, single-arm and randomized studies have demonstrated the benefit of interferon-α[3, 4]. The recommended dosage of alpha interferon in CML is 4–5 million international units (MIU) m−2 daily by subcutaneous (s.c.) injection; the maximum tolerated dosage (4–10 MIU m−2 daily) is administered to maintain haematological remission [3, 4].
Pegylated interferon-α2b (PEG-Intron) is a polyethylene glycol (PEG; molecular weight 12 kDa) conjugated form of interferon-α2b (Intron A). It improves delivery by significantly prolonging the serum half-life of Intron A, thus allowing easier treatment schedules (weekly instead of daily dose). This has proved to be of great clinical use in reducing the frequency of administration and, potentially, increasing the efficacy [5]. The currently approved dosages of PEG-Intron, both alone and in combination with oral ribavirin, in the treatment of chronic hepatitis C (CHC) are 1.0 and 1.5 µg kg−1 once a week administered subcutaneously for 48 weeks, compared with three times a week for Intron A [6, 7]. A Phase I study in patients with CML has established 6.0 µg kg−1 week−1 as the maximum tolerated dose (MTD) [8]. Patients with chronic-phase CML who were intolerant of Intron A or had failed prior Intron A therapy received 0.75–9.0 µg kg−1 week−1 PEG-Intron. A complete haematological or improved cytogenetic response was observed in 48% of patients [8]. PEG-Intron is currently in the development of other clinical programmes, including the treatment of melanoma, multiple sclerosis and human immunodeficiency virus.
The population pharmacokinetics of PEG-Intron in the CHC patients has been reported [9]. Analysis has confirmed that the concentration of PEG-Intron increases after multiple dosing and the increase continues during the entire treatment period of 48 weeks. Body weight and estimated creatinine clearance had positive effects on the clearance of PEG-Intron. Other clinical and demographic variables showed little or no effects on the pharmacokinetic parameters. The pharmacokinetics of PEG-Intron at high doses was intriguing. As reported in a Phase I study among CML patients with PEG-Intron doses up to 9 µg kg−1 week−1, serum concentrations after 4 weeks of PEG-Intron administration did not show a dose-related increase at high doses, 7.5 and 9 µg kg−1 week−1[8]. However, interpretation of the observations was difficult due to the small number of patients (n = 3–6 per dose) and limited concentration data (up to 4 weeks of treatment). The objective of this study is to assess the pharmacokinetics of PEG-Intron in CML patients using sparsely sampled serum concentration. Population pharmacokinetics modelling was used to analyse the sparsely collected concentration data over 48 weeks of treatment from CML patients enrolled in a Phase II/III clinical trial. In this analysis, potential factors that might influence the pharmacokinetics of PEG-Intron were evaluated and the effects of important factors were quantified.
Methods
Study subjects and treatments
This was a randomized, multicentre, open-label, parallel group, Phase II/III trial of safety and efficacy of PEG-Intron vs. INTRON A in adult subjects with newly diagnosed chronic-phase CML. One of its objectives was to evaluate pharmacokinetics of PEG-Intron in this subject population. Treatment length was dependent on response. Initially, a PEG-Intron dose of 6.0 µg kg−1 was administered subcutaneously once a week. During the treatment period, up to a maximum of 48 weeks, PEG-Intron doses could be held, reduced (to 4.5 or 3.0 µg kg−1 week−1) or discontinued based on individual tolerability of the dose. The actual dosing history for each patient was used in the analysis. Intron A 5 MIU m−2 day−1 was administered subcutaneously to a parallel group of patients. This study complied with ethical standards of the local institutional review boards. All patients provided written informed consent.
Blood collection and assay
PEG-Intron concentration data consisted of 624 PEG-Intron concentrations from 137 patients and no blood samples were collected from the Intron A-treated group.
Blood samples were drawn prior to PEG-Intron administration at treatment weeks 4, 12, 24, 36 and 48. Additional samples were drawn at the following specified times:
24 h post dosing on week 12
72 h post dosing on week 24
120 h post dosing on week 36.
Whole blood samples were collected into a plain tube and allowed to clot at room temperature for 15 min and then centrifuged at 4 °C and 1500 g for 15 min. The serum was removed and transferred into a cryogenic compatible tube, and the sample was stored frozen at ≤−20 °C and subsequently shipped frozen on dry ice to the analytical laboratory. Serum PEG-Intron concentrations were determined as previously described using a validated electrochemiluminescence (ECL) immunoassay [9]. This assay is linear and reproducible between 50 and 2000 pg ml−1. The assay limit of quantification was 50 pg ml−1 and variability was 12%.
Population pharmacokinetic model
A nonlinear mixed-effects modelling approach was used to analyse the data. The appropriate structural model was determined using intensive data from Phase I studies, where detailed concentration–time profiles were obtained up to 168 h during the week 4 of dosing [8–10]. These data were pooled with the sparsely sampled week 4 data from the present study to enable preliminary screening of the influence of covariates. The pharmacokinetic change after multiple dosing of PEG-Intron was modelled as the change in the apparent clearance, due to the accumulation following repeated dosing, is a function of increase in exposure (AUC) or decrease in clearance.
Several decreasing functions, including step function, exponential and Emax-type functions, were used in modelling [11]. An Emax-type function was selected to characterize the decline of PEG-Intron apparent clearance during this treatment period:
where t was the elapsed time in days relative to the treatment starting day, and CL0 (maximal or baseline apparent clearance), T50 (time for apparent clearance to reduce by 50% from Baseline CL0) and β (the slope parameter indicating the steepness of the decline) were the parameters in the clearance model. ηCL0 and ηT50 were random effects and were normally distributed with zero means and variances ω2CL0 and ω2T50, respectively. Effects of the covariates on CL0 and T50 were evaluated in a similar fashion, e.g. log-transformed CL0 is a linear function of log-transformed WT if body weight is a covariate:
where θCL0 and θWT are parameters and ηi,CL0 is the intersubject variability in CL0, which was assumed to be normally distributed with a mean of zero and a variance of ω2CL0. Other covariates in the analysis included age, sex, race (0 = White, 1 = other), baseline serum creatinine and estimated baseline creatinine clearance (CLcr) by the Cockcroft–Gault formula [12]. The CLcr estimate was stable over the course of the study. In the evaluation of covariate effects on clearance, continuous covariates were introduced into the parameter model in the fashion of linear additive terms of the log-transformed values. Different typical values of a pharmacokinetic variable were assigned to different subpopulations defined by a discrete covariate. The contribution of a covariate in a model was determined by the reduction in the minimum objective function value relative to the model without that covariate. The reduction was compared with a χ2 distribution for statistical significance at αENTRY = 0.01. For the covariates already in the model, when a new covariate was introduced, each of them had to maintain a significance level of αSTAY = 0.005 to remain in the model. The final model was then determined by both statistical and pharmacological rationales for incorporating these (and other) covariates into the model.
NONMEM (Nonlinear Mixed-Effects Model [13]) was used for model building and parameter estimation. In the PEG-Intron models with time-varying clearance, the observed concentrations and dosing history of PEG-Intron of each individual were included in the input data. SAS [14] (SAS Inc., Cary, NC, USA) was used for data management and analyses before and after modelling. S-Plus [15] (Insightful, Durham, NC, USA) was used for generating graphical displays.
Results
Model development
The demographics of the 137 patients are summarized in Table 1. Ages ranged from 20 to 75 years with a mean of 51 years. The male:female ratio was approximately 1.3 : 1. All serum creatinine values were within a normal range. Eighty-four percent of patients were White and 111 of the 137 patients were from centres outside the USA.
Table 1.
Summary of patient demographics
| Study | N (Nf, Nm)* | Age (years)† | Weight (kg)† | SrCr (mg dl−1)† | CLcr (ml h−1)† | Race (N1, N2, N3)‡ |
|---|---|---|---|---|---|---|
| ALL | 137 (59, 78) | 51 (20–75) | 74.3 (42–137) | 0.8 (0.4–1.1) | 113 (53–223) | 137 (115, 3, 19) |
| US | 26 (14, 12) | 51 (25–75) | 80.9 (53–137) | 0.8 (0.5–1.0) | 122 (74–223) | 26 (16, 2, 8) |
| International | 111 (45, 66) | 51 (20–75) | 72.7 (42–120) | 0.8 (0.4–1.1) | 110 (53–206) | 111 (99, 1, 11) |
Nf and Nm are the numbers of females and males, respectively.
Numbers are mean (minimum–maximum).
N1, White; N2, black; N3, other.
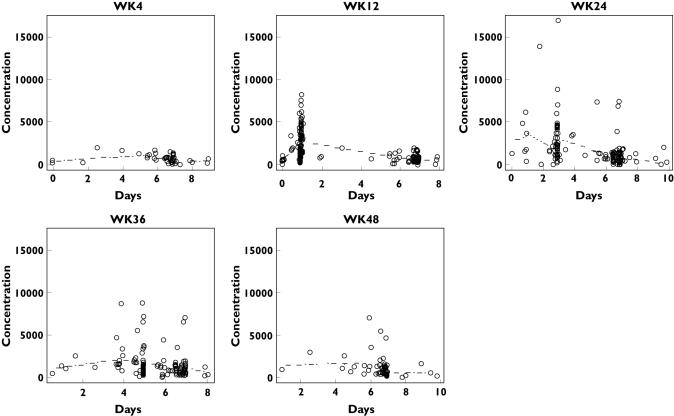
The structural model was determined by analysing the intensively sampled Phase I data for weeks 1 and 4 [8]. A one-compartment model with first-order absorption and elimination was found to be appropriate for Phase I data. The model was then used to characterize the Phase II/III data of 624 PEG-Intron concentrations from 137 patients. In this model, only the baseline clearance (CL0) was modelled as a function of covariates. The steepness parameter β was modelled as a fixed effect to avoid numerical difficulties. The absorption parameter, Ka, was fixed at the mean of 1.90 day−1 from the intensive Phase I data from hepatitis C patients [9, 10] since there was no liver dysfunction-related difference between the pharmacokinetics of PEG-Intron in healthy subjects [16] and hepatitis C patients [17] at the 1 µg kg−1 dose. The error was assumed to be normally distributed with mean zero and variance σ2, which was modelled with a multiplicative part and an additive part. All dosing history, including the actual total dose of PEG-Intron in µg, up to the last sampling time for each individual patient was included in the analysis. The plots of PEG-Intron concentration vs. elapsed time from the previous dose by treatment weeks are displayed in Figure 1.
Figure 1.
Plots of PEG-Intron immunoassay concentration data (pg ml−1) vs. elapsed time from last dose by treatment week. The broken line is the fitted line generated using population mean parameters
Covariate analysis and final model
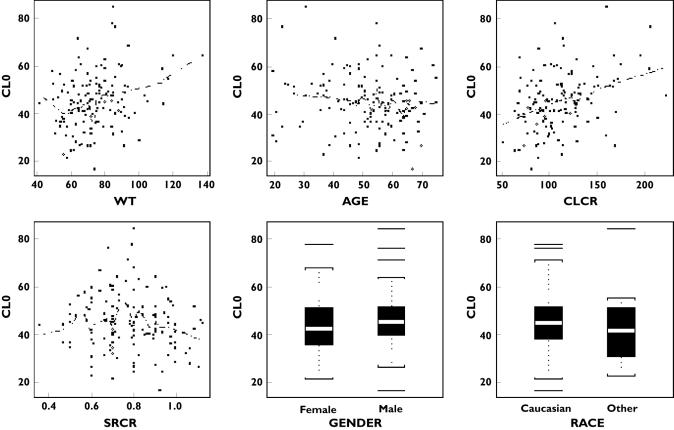
The models for covariate evaluation are summarized in Table 2. Among the single-covariate models, a significant reduction in the objective function from the no-covariate model (Model 1) occurred only for the CLcr model (Model 6). Among the four variables, body weight, age, sex and serum creatinine (SrCr) that were used in the estimation of CLcr, sex had the largest reduction (but did not reach the significance level of 0.01). Introducing Sex into the CLcr model (Model 11) did not result in a significant additional reduction. The CLcr model (Model 6) was selected for refinement. Without compromising the overall fit of the CLcr model (Model 6), in the final model the parameter β was fixed to 1 to avoid numerical difficulties.
Table 2.
Models for covariate evaluation
| Model no. | Covariate | Obj | Ref.Model | ΔObj. | DF | P-value |
|---|---|---|---|---|---|---|
| 1 | No covariate | 8615.563 | – | – | – | |
| 2 | WT | 8611.877 | 1 | 3.686 | 1 | 0.0549 |
| 3 | Sex | 8609.966 | 1 | 5.597 | 1 | 0.0180 |
| 4 | Age | 8612.237 | 1 | 3.326 | 1 | 0.0682 |
| 5 | SrCr | 8614.428 | 1 | 1.135 | 1 | 0.2867 |
| 6* | CLcr | 8598.603 | 1 | 16.960 | 1 | <0.0001 |
| 7 | Race | 8613.705 | 1 | 1.858 | 1 | 0.1729 |
| 8 | WT + Sex | 8609.404 | 3 | 0.562 | 1 | 0.4535 |
| 9 | Age + Sex | 8606.967 | 3 | 2.999 | 1 | 0.0833 |
| 10 | SrCr + Sex | 8601.732 | 3 | 8.234 | 1 | 0.0041 |
| 11 | CLcr + Sex | 8596.660 | 6 | 1.943 | 1 | 0.1633 |
Obj, Minimum objective function;ΔObj, difference in obj; DF, degrees of freedom; SrCr, serum creatinine; CLcr, estimated creatinine clearance; WT, body weight.
Final model.
This model run showed a remarkable reduction in the minimum objective function. Sensitivity analysis was carried out on the parameters with fixed values in the previous model run, i.e. Ka, the absorption rate, and β, the steepness parameter in the Emax function. The model runs with these parameters fixed at different values within ±20% of 1.9 day−1 (for Ka) and 1.0 (for β) were performed. All runs converged and there was little change in the parameter estimates, indicating the model fitted the data robustly in the neighbourhood of these fixed values. The resulting model with the refinement incorporated in the CLcr model (or Model 6) was determined as the final population pharmacokinetics model for PEG-Intron for the CML patients.
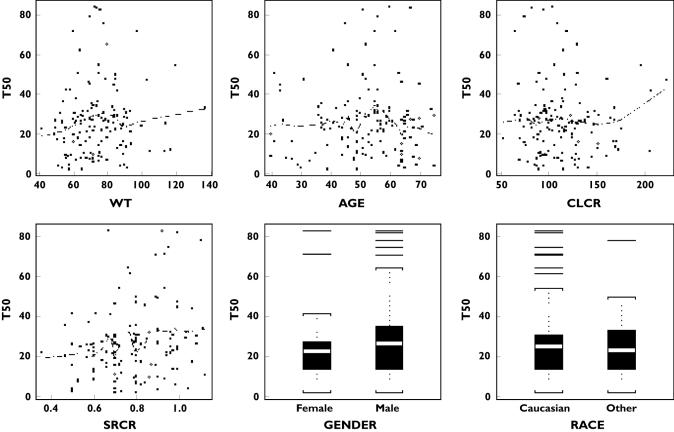
The parameter estimates and their standard errors of the final model are given in Table 3. For patients with CLcr 120 ml min−1, the population mean baseline clearance was estimated at 44.1 l day−1 with 35% interpatient variability. The estimated mean time for clearance decline to its half value was almost 96 weeks (or 23.8 × 28 days), twice the treatment period of 48 weeks. The interpatient variability in T50 was 109%. Based on the model equation and the mean parameter estimates, the mean clearances at treatment weeks 4 and 48 were calculated at 42.3 l day−1 and 29.3 l day−1, respectively. The reduction in clearance from week 4 to week 48 was 30.8%. The individual estimates of parameters CL0 and T50 vs. covariates and other variables are plotted in Figures 2 and 3 (based on final model), respectively. The individual CL estimates at different treatment days were calculated based on the individual parameter estimates. The smooth line of these clearances for all patients, using a local linear regression method (‘loess’ function in S-plus), is given in Figure 4 (the solid line for this trial and broken lines from CHC trials).
Table 3.
Parameter estimates in the final model
| parameter | Estimate | SE | ω (CV) |
|---|---|---|---|
| θCL0 (l day−1) | 44.1 | 2.3 | 35% |
| θT50 (28 days) | 23.8 | 4.4 | 109% |
| β | 1.00 | Fixed | – |
| θCLcr | 0.21 | 0.090 | – |
| Ka (day−1) | 1.90 | Fixed | – |
| V (l) | 149.0 | 12.1 | 59% |
| σɛ (l day−1) | 41.4 ± 84.2% | – | – |
ω, Intersubject variability; SE, standard error; CV, coefficient of variation;θCL0, incept term in baseline;θT50, time for baseline clearance decline to 1/2 ofθ CL0;β, steepness parameter; Ka, absorption rate constant;σɛ residual variability.
Figure 2.
Individual CL0 estimates vs. covariates and some demographic and clinical variables
Figure 3.
Individual T50 estimates vs. covariates and some clinical and demographic variables
Figure 4.
Mean PEG-Intron apparent clearance relative to duration of treatment. The solid line is for the current chronic myelogenous leukaemia trial and the three broken lines are for previous chronic hepatitis C trial [9, 10]. The smooth lines (solid and broken) were obtained using local linear regression (‘loess’ function in S-plus). 6.0 mcg/kg (——–), 0.5 mcg/kg (—), 1.0 mcg/kg (—), 1.5 mcg/kg (—)
Discussion
The main findings of this analysis were: (i) apparent clearance of PEG-Intron declined (or concentrations increased) during multiple dosing to the CML patients with 3.0–6.0 µg kg−1 week−1 of PEG-Intron; and (ii) the baseline clearance in this trial was 40% higher than that from previous hepatitis C trials. The reduction in clearance from treatment week 4 to the end of treatment (week 48) was 30.8% in this trial. This is comparable to a 33.7% reduction in the hepatitis C patients with PEG-Intron administered at 0.5–1.5 µg kg−1 week−1[9, 10]. The reason for the clearance reduction could be due to accumulation associated with long t1/2 of PEG-Intron following repeated dosing. and could also be due to a change in the number of cellular interferon receptors in response to repeated administration of PEG-Intron [9]. The mean baseline clearance in this trial with CML patients was 40% higher at 44.1 l day−1, compared with 31.5 l day−1 for the hepatitis C patients in previous studies [9]. This suggests an under-dose-proportional increase in the exposure as the PEG-Intron dose increased. For example, quadrupling of the PEG-Intron dose from 1.5 µg kg−1 to 6.0 µg kg−1 causes only a 2.9-fold increase in exposure if there is a 40% increase in clearance. This finding appeared to substantiate the observation in a Phase I study, in which the AUC at treatment week 4 was not dose-related as PEG-Intron was administered at high doses [8]. It is plausible that the decreasing or saturation in bioavailability as PEG-Intron administration at high doses had contributed to the elevated clearance.
The mean PEG-Intron clearances from this trial and previous CHC trials during treatment are plotted in Figure 4. The solid line is for the current CML trial and the three broken lines are for previous CHC trials. The clearance in the CML patients was systematically higher throughout the entire treatment period of 48 weeks. The decline profiles were similar across all doses from 0.5 to 6.0 µg kg−1 week−1.
Covariates had little or no effect on PEG-Intron clearance. Creatinine clearance was the only covariate with a statistically significant influence on the clearance of PEG-Intron, probably because 30% of total clearance was renally mediated [17]. Body weight had an effect (P= 0.0549), but this did not reach the assigned significance level. This led to the selection of CLcr as a covariate in the final model of this analysis. In contrast, in the hepatitis C patients, both body weight and CLcr showed similar effects, and body weight was selected and included in the final model.
The clinical implications of these findings remain to be evaluated. The PEG-Intron dosage selected for this Phase III study was based on the tolerance of PEG-Intron and the exposure of Intron A from a Phase I study in the CML patients. The maximum tolerated dose over a 4-week dosing period is defined at 7.5–9.0 µg kg−1 week−1[8]. However, due to the observations of the dose-related and mild to moderate adverse events, the proposed PEG-Intron dose for future studies was selected to be 6.0 µg kg−1 week−1. In the clinical assessment of this study, this PEG-Intron dose had demonstrated an efficacy result which was clinically comparable to that of the active control, in which patients received Intron A at a 5 MIU m−2 daily [18]. The safety profiles of PEG-Intron and Intron A in the CML patients in this study were also comparable. A prospectively developed guideline for dose modification/discontinuation was effective in the management of adverse events during treatment [18]. As covariates had little or no influence on the pharmacokinetic parameters, these findings do not support any further dose adjustment of PEG-Intron based on demographic characteristics.
In summary, the pharmacokinetic behaviour of PEG-Intron at high doses in CML patients was described. CLcr had a small influence on the clearance of PEG-Intron. The dose of PEG-Intron 6 µg kg−1 week−1 appeared appropriate in the treatment of patients with CML and no further dose adjustment was required from the pharmacokinetic point of view.
Acknowledgments
Funded by a grant from Schering-Plough Research Institute, Kenilworth, NJ, USA.
References
- 1.Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia. Biol Ther Ann Intern Med. 1999;131:207. doi: 10.7326/0003-4819-131-3-199908030-00008. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian HM, O’Brien S, Anderlini P, Talpaz M. Treatment of chronic myelogenous leukemia: current status and investigational options. Blood. 1996;87:3069–81. [PubMed] [Google Scholar]
- 3.Talpaz M, Kantarjian H, Kurzrock R. Interferon-alpha produces sustained cytogenetic responses in chronic myelogenous leukemia. Ann Intern Med. 1991;114:532. doi: 10.7326/0003-4819-114-7-532. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian HM, Smith TL, O’Brien S. Prolonged survival in chronic myelogenous leukemia after cytogenetic response to interferon-α therapy. Ann Intern Med. 1995;122:254–61. doi: 10.7326/0003-4819-122-4-199502150-00003. [DOI] [PubMed] [Google Scholar]
- 5.Glue P, Fang JW, Rouzier-Panis R, Raffanel C, Sabo R, Gupta SK, Salfi M, Jacobs S and the Hepatitis C Study Group. PEG-interferon alfa-2b: pharmacokinetics, pharmacodynamics, safety and preliminary efficacy data. Clin Pharmacol Ther. 2000;68:556–67. doi: 10.1067/mcp.2000.110973. [DOI] [PubMed] [Google Scholar]
- 6.Lindsay KL, Trepo C, Heintges T, Shiffman ML, Gordon SC, Hoefs JC, Schiff ER, Goodman ZD, Laughlin M, Yao R, Albrecht JK. A randomized, double-blind trial comparing pegylated interferon alfa-2b to interferon alfa-2b as initial treatment for chronic hepatitis C. Hepatology. 2001;34:395–403. doi: 10.1053/jhep.2001.26371. [DOI] [PubMed] [Google Scholar]
- 7.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 8.Talpaz M, O’Brien S, Rose E, Gupta S, Shan J, Cortes J, Giles FJ, Faderl S, Kantarjian HM. Phase 1 study of polyethylene glycol formulation of interferon alpha-2B (Schering 54031) in Philadelphia chromosome-positive chronic myelogenous leukemia. Blood. 2001;98:1708–13. doi: 10.1182/blood.v98.6.1708. [DOI] [PubMed] [Google Scholar]
- 9.Jen JF, Glue P, Ezzet F, Chung C, Gupta SK, Jacobs S, Hajian G. Population pharmacokinetic analysis of pegylated interferon Alfa-2b and interferon alfa-2b in patients with chronic hepatitis C. Clin Pharmacol Ther. 2001;69:407–21. doi: 10.1067/mcp.2001.115872. [DOI] [PubMed] [Google Scholar]
- 10.(Data on file) Population Pharmacokinetic Analysis of Pegylated Interferon Alfa-3b (Peg-Intron), Interferon Alfa-3b (Intron A) in Patients with Chronic Hepatitis C Enrolled in Study C/I97a-01000068050SPRI Report no. 2000.
- 11.Jen JF, Chung C, Glue P, Ezzet F, Gupta S, Hajian G. Modeling the accumulation of drug concentration during treatment. AAPS Pharmsci. 2000;2:4. [Google Scholar]
- 12.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 13.Beal SL, Sheiner LB. NONMEM Users Guides. UCSF: NONMEM Project Group; 1992. [Google Scholar]
- 14.SAS/STAT User’s Guide. Cary, NC: SAS Institute; 1990. Version 6. [Google Scholar]
- 15.S-PLUS User’s Manual. Seattle, WA: MathSoft Inc.; 1999. Version 2000. [Google Scholar]
- 16.Gupta SK, Pittenger AL, Swan SK, Marbury TC, Tobillo E, Batra V, Sack M, Glue P, Jacobs S, Affrime M. Single dose pharmacokinetics and safety of pegylated interferon-α2b in patients with chronic renal dysfunction. J Clin Pharmacol. 2002;42:1109–13. doi: 10.1177/009127002401382713. [DOI] [PubMed] [Google Scholar]
- 17.Glue P, Fang JWS, Rouzier-Panis R, Raffanel C, Sabo R, Gupta SK, Salfi M, Jacobs S and the Hapatitis C Intervention Therapy Group. Pegylated interferon-alpha2b: pharmacokinetics, pharmacodynamics, safety, and preliminary efficacy data. Clin Pharmacol Ther. 2000;68:556–67. doi: 10.1067/mcp.2000.110973. [DOI] [PubMed] [Google Scholar]
- 18.(Data on file) C98-026: A Randomized Phase II/III Trial of SCH 54031 PEG12000 Interferon alfa-2b (PEG Intron) vsINTRON® A in Subjects with Newly Diagnosed CML. Schering-Plough Research Institute; 2003. [Google Scholar]