Abstract
Aims
Establishing the dose–response relationship for clinically useful doses of aspirin, ibuprofen and paracetamol has been difficult. Indirect comparison from meta-analysis is compromised by too little information at some doses.
Methods
A systematic review of randomized, double-blind trials in acute pain comparing different doses of aspirin, ibuprofen and paracetamol was therefore undertaken.
Results
Fifty trials were found. Numerical superiority of higher over lower dose was found by the original authors in 37/50 trials (74%) and statistical superiority in 11/50 (22%). Twenty-eight trials had design, quality and data reporting characteristics to allow pooling of common doses; in 3/28 (11%) of the individual trials our calculations showed statistical superiority of higher over lower dose. Pooled comparison of 1000/1200 mg aspirin over 500/600 mg was statistically superior, with a number-needed-to-treat (NNT) for higher over lower dose of 16 (8 to < 100). Pooled comparison of 400 mg ibuprofen over 200 mg was statistically superior, with an NNT for higher over lower dose of 10 (6–23). Pooled comparison of 1000 mg paracetamol over 500 mg was statistically superior, with an NNT for higher over lower dose of 9 (6–20).
Conclusions
Use of trials making direct comparison of two different doses of target drugs revealed the underlying dose–response curve for clinical analgesia.
Keywords: aspirin, dose, ibuprofen, pain, paracetamol, response
Introduction
That there is a dose–response for analgesia with nonsteroidal anti-inflammatory drugs (NSAIDs) and paracetamol (acetaminophen) is not in doubt, from bench studies, studies in human experimental pain and common experience. Dose-related effects of paracetamol were seen using cold pressor pain [1] and on the R-III reflex [2]. The difficulty lies in revealing that analgesic dose–response in the setting of clinical pain.
For more than 50 years analgesic trial experts have commented on the flat dose–response curves which they observed in analgesic trials of these drugs in the clinical setting, and particularly with NSAIDs. Graded doses of NSAIDs showed flatter dose–response curves than those seen with opioids and led to the thinking that there was little perceptible difference between lower and higher doses of NSAIDs on the customary outcomes of single-dose studies. The impact of the higher dose was more likely to be observed on the duration of analgesic effect than on the peak effect.
These observations have stood the test of time and indeed the dose–response with these drugs has been hard to capture. Indirect approaches, with different doses of the same analgesic compared with the common comparator of placebo, usually fail to demonstrate dose–response (Table 1). This is a consequence of different studies, perhaps in slightly different conditions, and with large differences in numbers and random effects; very large numbers of patients may have been tested for one dose, but very small numbers for another (Table 1). Indirect methods probably have limited value in demonstrating relatively flat dose–response curves and small additions to datasets can make a difference. For paracetamol, for example, a significant dose–response derived at one time [3] by these indirect methods was no longer significant when further studies were added to the meta-analysis and different inclusion and exclusion criteria used [4].
Table 1.
Dose–response for aspirin, paracetamol and ibuprofen from systematic reviews of comparisons with placebo in single-dose oral studies
| Drug | Dose | Patients in comparison | NNT (95% CI) |
|---|---|---|---|
| Aspirin | 600/650 | 5061 | 4.4 (4.0, 4.9) |
| 1000 | 716 | 4.0 (3.2, 5.4) | |
| 1200 | 279 | 2.4 (1.9, 3.2) | |
| Ibuprofen | 200 | 1414 | 2.7 (2.4, 3.1) |
| 400 | 4703 | 2.4 (2.3, 2.6) | |
| 800 | 76 | 1.6 (1.3, 2.2) | |
| Paracetamol | 500 | 561 | 3.5 (2.7, 4.8) |
| 600/650 | 1207 | 5.4 (4.2, 4.7) | |
| 1000 | 2559 | 3.7 (3.3, 4.3) |
An alternative approach to the indirect method is the direct method, which studies only the trials that compare two or more different doses directly. The hope is that by combining such direct comparison studies a more robust view of the dose–response will emerge than has been the case with the indirect approach. The importance of having a better understanding of the dose–response for analgesia is that its slope may not necessarily be the same as the slope for adverse effects, and achieving maximal benefit at minimal risk is easier when you have a better idea of the efficacy of lower doses.
The aim of this study was to find reports of trials making direct comparisons of different doses of aspirin, ibuprofen and paracetamol in the same study, and to pool appropriate efficacy data from those studies to explore the dose–response relationship for analgesia with these drugs in the clinical setting.
Methods
We sought randomized, double-blind trials using at least two doses of aspirin, ibuprofen or paracetamol, with placebo or active comparator, in single-dose studies of analgesia in established pain, in any setting. Previous systematic reviews [4–6] were used to source trials, supplemented by electronic searches of PubMed (from January 2002 to June 2005) and the Cochrane Library (issue 1, 2005) using drug names and randomized trial. An in-house database [7] of hand-searched randomized trials with pain as an outcome was searched for active controlled trials that may not have included a placebo group. We excluded trials using multiple doses or systemic administration.
Both authors independently read each report that could possibly be described as a randomized controlled trial. Information on analgesic efficacy abstracted by one author was checked by the other. No adverse event information was abstracted. Inclusion of trials and analysis was performed at two levels.
At the first level we had a broad approach to trial inclusion, using those trials reporting results of pain intensity or pain relief over a 4–6-h period. From these we extracted information about dose–response in terms first of a numerical superiority of a higher dose over a lower dose (where there was a measure indicating greater pain relief with a higher than a lower dose of analgesic, even if that difference was not statistically significant), and second, any statistical significance of that numerical difference as described in the original report.
At the second level we used only trials in established pain with initial pain of at least moderate intensity and at least 10 patients per group. For each trial, mean total pain relief (TOTPAR), sum pain intensity difference (SPID), visual analogue total pain relief (VASTOTPAR) or visual analogue sum pain intensity difference (VASSPID) values for each drug group were converted to %maxTOTPAR by division into the calculated maximum value [8]. The proportion of patients in each treatment group who achieved at least 50%maxTOTPAR was calculated using valid equations [9–11]. The number of patients with at least 50%maxTOTPAR was then used to calculate relative benefit and number-needed-to-treat (NNT) for analgesic vs. placebo.
Statistical significance of individual trials as claimed by the original paper was noted. Using the calculated information on number of patients with at least 50%maxTOTPAR, we additionally calculated the relative benefit and NNT, with 95% confidence interval (CI). Relative risk was calculated using a fixed effects model [12], with no statistically significant difference between treatments assumed when the 95% CIs included unity. When there was a statistically significant difference of relative benefit or risk (the CI did not include 1), NNT was calculated [13] using the pooled number of observations.
Results
Fifty trials of at least two doses of aspirin, ibuprofen and paracetamol were found. Results for each of the drugs are presented separately, with results tabulated in Tables 2 and 3 and presented graphically in Figure 1. Individual trial results are in Appendix 1.
Table 2.
First-level analysis, numerical difference and statistical significance quoted in the original paper
| Analysis | Aspirin | Ibuprofen | Paracetamol |
|---|---|---|---|
| Number of trials with comparisons | 18 | 20 | 12 |
| Numerically better with higher dose | 12 | 16 | 9 |
| Statistical significance with higher dose | 2 | 5 | 4 |
Table 3.
Second-level analysis, using calculated numbers of patients with at least 50%maxTOTPAR
| Dose of drug (mg) | Number of | Percent with at least 50% pain relief | Higher vs. lower dose | |||||
|---|---|---|---|---|---|---|---|---|
| Drug | High | Low | Trials | Patients | High | Low | Relative benefit (95% CI) | NNT (95% CI) |
| Aspirin | 1000 | 500 | 2 | 301 | 62 | 52 | 1.2 (0.97, 1.4) | |
| Aspirin | 1200 | 600 | 6 | 608 | 39 | 34 | 1.2 (0.93, 1.4) | |
| Aspirin | 1000/1200 | 500/600 | 8 | 909 | 47 | 40 | 1.2 (1.01, 1.3) | 16 (8, >100) |
| Ibuprofen | 800 | 400 | 13 | 994 | 68 | 59 | 1.2 (1.1, 1.3) | 10 (6, 23) |
| Paracetamol | 1000 | 500/650 | 7 | 933 | 64 | 52 | 1.2 (1.1, 1.4) | 9 (6, 20) |
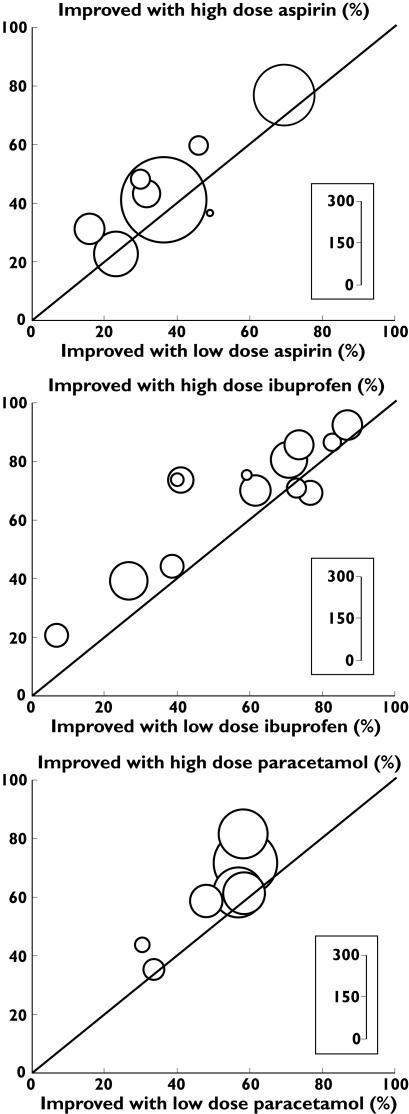
Figure 1.
Individual studies with extractable data. L’Abbé plots of the proportions of patients improved on high and low doses for the individual direct comparisons with aspirin, ibuprofen and acetaminophen. The size of the circle representing a trial is proportional to the number of patients studied in the trial
Aspirin
There were 17 citations [14–30] with relevant direct comparisons (18 trials) of more than one dose of aspirin. There were four comparisons of 500 and 1000 mg and 10 of 600 or 650 g and 1000 or 1200 mg. Other comparisons included doses of 200, 300, 325, 625, 650, 750 and 900 mg. The numbers of patients studied were 232 at 500 mg, 344 at 600 mg, 332 at 1000 mg and 314 at 1200 mg.
Of the four 500 vs. 1000 mg comparisons, three showed numerically greater efficacy with the higher dose and one showed the same efficacy (Table 2). Of the eight 600 vs. 1200 mg comparisons, six showed numerically greater efficacy with the higher dose. Of the six other dose comparisons, three showed numerically greater efficacy with the higher dose. Only two of 18 comparisons reported a statistically significant difference between two doses of aspirin, one [20] showing more effect with 650 mg than 325 mg, while another [23] demonstrated a difference only between 1200 mg and 300 mg, but not between 1200 and 600 mg.
At the second level of analysis eight trials [14, 17, 18, 21, 22(two studies), 23, 28] had extractable data. The size of the comparisons varied from 21 to 250 patients, with an average of 114 patients and median of 89 patients. Neither comparisons between 500 mg and 1000 mg, nor 600 mg and 1200 mg produced a significant dose–response (Table 3). Pooling extractable data from 500 or 600 mg as the lower dose and 1000 or 1200 mg as the higher dose, there were extractable data from 909 patients. At least 50% pain relief occurred more frequently (47% of patients) at the higher than at the lower dose (40%; Figure 1). The relative benefit was 1.2 (95% CI 1.01, 1.3) and the NNT for one additional patient to obtain more than 50% pain relief at higher rather than lower dose was 16 (8 to < 100).
In this second level (NNT) analysis, no single trial had a significant dose–response, greater effect with higher than with lower dose, in our calculations.
Ibuprofen
Eighteen citations [16, 31–47] had relevant direct comparisons (20 trials) of more than one dose of ibuprofen. There were 14 comparisons of 200 and 400 mg and three of 400 and 800 mg. The numbers of patients studied were 159 at 50 mg, 192 at 100 mg, 821 at 200 mg, 20 at 300 mg, 741 at 400 mg, 73 at 600 mg, 119 at 800 mg and 20 at 900 mg.
Of the 14 200 vs. 400 mg comparisons, 12 showed numerically greater efficacy with the higher dose, two showed the same efficacy and two showed lower efficacy with the higher dose. Overall, 16 of 20 comparisons had numerically greater efficacy for higher than lower dose (Table 2). Five comparisons reported statistical significance for higher over lower dose, four for 400 mg over 200 mg [32, 38, 39, 44] and one for 200 mg over 100 mg [37].
At the second level of analysis, 12 reports had extractable data from 13 trials [16, 32, 33 (study 4), 34 (studies 4 and 5), 35, 38, 39, 41, 43–45]. The size of the comparisons varied from 33 to 120 patients, with an average of 76 patients and median of 79 patients. Using data from 200 mg as the lower dose and 400 mg as the higher dose, there were extractable data from 994 patients (Table 3). At least 50% pain relief occurred more frequently (68% of patients) at the higher than at the lower dose (59%) (Figure 1). The relative benefit was 1.2 (95% CI 1.1, 1.3) and the NNT for one additional patient to obtain more than 50% pain relief at the higher rather than the lower dose was 10 (6, 23).
In this second level (NNT) analysis, one trial [32] had a significant dose–response of higher over lower dose in our calculations.
Paracetamol
Nine citations [28, 48–55] had relevant direct comparisons (12 trials) of more than one dose of paracetamol. One comparison of 650 and 1000 mg, four citations (six studies) of 500, 1000 and 1500 mg, two of 1000 and 2000 mg, five of 500 and 1000 mg. The number of patients studied was 450 at 500 mg, 87 at 625 mg, 622 at 1000 mg, 207 at 1500 mg and 79 at 2000 mg.
Of the nine 500 vs. 1000 mg comparisons, eight had numerically greater efficacy with the higher dose, with nine of 12 overall having numerically greater efficacy with the higher dose (Table 2). Four comparisons found statistical significance for higher over lower dose, two for 1000 mg over 500 mg [50, 51], one for 2000 over 1000 [54] and one for 1000 mg over 650 mg [48].
At the second level of analysis seven studies [28, 48, 49 (studies 1, 2 and 3), 50, 52] had extractable data. The size of the comparisons varied from 60 to 216 patients, with an average of 133 patients and median of 136 patients. Pooling extractable data from 500 mg and 650 mg as the lower dose and 1000 mg as the higher dose, there were extractable data from 933 patients (Table 3). At least 50% pain relief occurred more frequently (64% of patients) at the higher than at the lower dose (52%; Figure 1). The relative benefit was 1.2 (95% CI 1.1, 1.4) and the NNT for one additional patient to obtain more than 50% pain relief at the higher rather than the lower dose was 9 (6, 20).
In this second level (NNT) analysis, two trials [28, 48] had a significant dose–response for higher over lower dose in our calculations.
Discussion
Using trials making a direct comparison of two different doses of the target drugs, the underlying dose–response curve for clinical analgesia could be teased out of the data, but for only a limited part of the dose–response relationship. For all three drugs, aspirin, ibuprofen and paracetamol, the meta-analysis produced the expected result, statistically significant relative benefit for higher dose over lower dose, with NNT values of 16, 10 and 9, respectively. Although results of 37/50 (74%) individual trials showed numerical superiority of a higher over a lower dose, only 11/50 (22%) achieved statistical separation of the effect of the two doses according to the original authors. In those trials where data could be pooled, only 3/28 (11%) were individually significant in our calculation of relative benefit using numbers of patients with at least 50%max TOTPAR.
For the pooled analysis of higher vs. lower doses of the three drugs we had extractable data from about 500 patients on each of the two doses (aspirin 453 and 456, ibuprofen 529 and 517, paracetamol 467 and 466). The quantity of data may be vitally important to this question. Given that the difference in efficacy is about 10% more patients having at least 50% pain relief with the higher dose, it is likely that about 500 patients on each dose are required for reliable determination of the dose–response with the direct method, although many more would be required to have confidence in the magnitude of the difference [56]. Only three of the 28 trials in the pooled analysis had patient numbers >200 in the comparison and 17 had <100. That few were able individually to demonstrate a statistical difference between higher and lower doses of analgesic was therefore predictable. The three trials that did have a significant difference had 80, 175 and 216 patients in the comparison of higher and lower dose.
The number of patients required to demonstrate a difference using indirect comparison of each dose with placebo would probably be greater than that for direct comparison. Indirect comparisons would be more open to variables other than random chance, like the setting or cultural differences that might adversely effect sensitivity.
The slope of the analgesic dose–response with aspirin, ibuprofen and paracetamol was not steep, with doubling of dose from a lower to a higher level in the clinically useful range producing an absolute increase of 10% in the number of patients with at least 50% pain relief and an NNT of 10 for the difference. This is flatter than the slope of the dose–response curve seen with opioids, although there is not a large amount of dose–response information for opioids. For example, comparing a single dose of intramuscular morphine of 10 mg with 20 mg in 74 patients in a single trial [57], 31% and 62% achieved at least 50% pain relief over 4 h. The absolute difference of 31% with a doubling of dose (an NNT for higher vs. lower dose of 3) represents a dose–response curve slope about three times steeper than for aspirin, ibuprofen or paracetamol.
There are important limitations, over and above the limited range of doses for which trials were available, and the limited number of trials and patients. First is the fact that we used the only outcome available for meta-analysis, the number of patients with at least half pain relief, derived from typically reported pain relief and intensity outcomes [9–11]. Other outcomes can be derived from acute pain studies [58], e.g. the number of patients with different levels of pain relief, but this cannot be done using published average values. Nor is it possible from average values of pain intensity or pain relief measures to know, for example, how many patients individually had a particular reduction in pain, say by half. We can, however, be reasonably sure that different pain models contribute relatively little to differences between trials [59]. Finally, how different doses affect adverse events cannot be addressed by the information available. Adverse events in acute pain studies are influenced by a variety of factors, making analysis unreliable except in exceptional circumstances [60].
There are practical implications of the actual but limited dose–response with aspirin, ibuprofen and paracetamol. Obviously, where it is safe to do so and where higher rates of good analgesia are required, a higher dose will deliver adequate analgesia for more patients than a lower dose (within the clinically useful range of doses). Where safety is paramount, e.g. in long-term use of analgesics in older patients with gastrointestinal, renal or cardiac risk factors, lower doses might often deliver adequate analgesia with lower risk of rare but serious adverse events, especially with NSAIDs.
Conflict of interest
R.A.M. and H.J.M. have received lecture fees from pharmaceutical companies and research support from charities and government sources at various times. Neither author has any direct stock holding in any pharmaceutical company.
Acknowledgments
The study was supported by Pain Research funds, the Oxford Pain Relief Trust, and by an unrestricted educational grant from GlaxoSmithKline. The terms of the financial support from GlaxoSmithKline included freedom for authors to reach their own conclusions, and an absolute right to publish the results of their research, irrespective of any conclusions reached. GlaxoSmithKline did have the right to view the final manuscript before publication, and did so. GlaxoSmithKline had no involvement in the design or conduct of the study, writing the manuscript or decision to publish.
Appendix
Appendix 1.
Individual trial results
| High dose of analgesic | Low dose of analgesic | |||||
|---|---|---|---|---|---|---|
| Number of patients >50% | Number of patients >50% | |||||
| Reference [number] | maxTOTPAR | Total | percent | maxTOTPAR | Total | percent |
| Aspirin 1000 mg vs 500 mg | ||||||
| Ahlstrom 1974 [14] | 14 | 46 | 30 | 8 | 51 | 16 |
| Steiner 2003 [28] | 78 | 103 | 76 | 71 | 101 | 70 |
| Aspirin 1200 mg vs 600 mg | ||||||
| Fucella 1977 [18] | 16 | 27 | 59 | 14 | 31 | 45 |
| Mahler 1976 [22] | 14 | 30 | 47 | 9 | 29 | 31 |
| Mahler 1976 [22] | 4 | 11 | 36 | 5 | 10 | 50 |
| Parkhouse 1968 [23] | 51 | 125 | 41 | 46 | 125 | 37 |
| Forbes 1990 [17] | 17 | 71 | 24 | 17 | 68 | 25 |
| London 1983 [21] | 17 | 40 | 43 | 13 | 41 | 32 |
| Ibuprofen 800 mg vs 400 mg | ||||||
| Cooper 1977 [16] | 18 | 40 | 45 | 15 | 38 | 39 |
| Jain 1986 [43] | 45 | 49 | 92 | 41 | 47 | 87 |
| Cooper 1984 study4 [33] | 24 | 28 | 86 | 20 | 24 | 83 |
| Hersh 2000 [39] | 47 | 59 | 80 | 43 | 61 | 70 |
| Hersh 1993 [38] | 19 | 49 | 39 | 14 | 51 | 27 |
| McQuay 1996 [43] | 6 | 30 | 20 | 2 | 31 | 6 |
| Dionne and McCullagh 1998 [35] | 35 | 50 | 70 | 31 | 51 | 61 |
| Schou 1998 [44] | 41 | 49 | 84 | 36 | 49 | 73 |
| Cooper 86 s4 [34] | 31 | 45 | 69 | 26 | 34 | 76 |
| Cooper 86 S5 [34] | 22 | 31 | 71 | 23 | 32 | 72 |
| Bostrom 94 [32] | 29 | 40 | 73 | 16 | 40 | 40 |
| Seymour 96 [45] | 11 | 15 | 73 | 7 | 18 | 39 |
| Serymour 96 [45] | 12 | 16 | 75 | 10 | 17 | 59 |
| Paracetamol 1000 vs 500/650 | ||||||
| Laska 1983 study 1 [49] | 29 | 50 | 58 | 25 | 54 | 47 |
| Laska study 2 [49] | 41 | 68 | 61 | 39 | 68 | 58 |
| Laska study 3 [49] | 49 | 81 | 61 | 46 | 81 | 56 |
| McQuay 1986 [50] | 13 | 30 | 44 | 9 | 30 | 30 |
| Ragot 1991 [52] | 14 | 40 | 35 | 13 | 40 | 33 |
| Steiner 2003 [28] | 79 | 111 | 71 | 61 | 105 | 58 |
| Hopkinson 1974 [48] | 71 | 87 | 82 | 51 | 88 | 58 |
References
- 1.Yuan CS, Karrison T, Wu JA, Lowell TK, Lynch JP, Foss JF. Dose-related effects of oral acetaminophen on cold-induced pain: a double-blind, randomized, placebo-controlled trial. Clin Pharmacol Ther. 1998;63:379–83. doi: 10.1016/S0009-9236(98)90169-2. [DOI] [PubMed] [Google Scholar]
- 2.Piguet V, Desmeules J, Dayer P. Lack of acetaminophen ceiling effect on R-III nociceptive flexion reflex. Eur J Clin Pharmacol. 1998;53:321–4. doi: 10.1007/s002280050386. [DOI] [PubMed] [Google Scholar]
- 3.McQuay HJ, Edwards JE, Moore RA. Evaluating analgesia: the challenges. Am J Ther. 2002;9:179–87. doi: 10.1097/00045391-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Barden J, Edwards J, Moore A, McQuay H. Single dose oral paracetamol (acetaminophen) for postoperative pain. Cochrane Database Syst Rev. 2004;1:CD004602. doi: 10.1002/14651858.CD004602. [DOI] [PubMed] [Google Scholar]
- 5.Edwards JE, Oldman A, Smith L, Collins SL, Carroll D, Wiffen PJ, McQuay HJ, Moore RA. Single dose oral aspirin for acute pain. Cochrane Database Syst Rev. 2000;2:CD002067. doi: 10.1002/14651858.CD002067. [DOI] [PubMed] [Google Scholar]
- 6.Collins SL, Moore RA, McQuay HJ, Wiffen PJ, Edwards JE. Single dose oral ibuprofen and diclofenac for postoperative pain. Cochrane Database Syst Rev. 2000;2:CD001548. doi: 10.1002/14651858.CD001548. [DOI] [PubMed] [Google Scholar]
- 7.Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain. 1996;66:239–46. doi: 10.1016/0304-3959(96)03033-3. [DOI] [PubMed] [Google Scholar]
- 8.Cooper SA. Single-dose analgesic studies: the upside and downside of assay sensitivity. In: Max MB, Portenoy RK, Laska EM, editors. The Design of Analgesic Clinical Trials (Advances in Pain Research and Therapy. Vol. 18. New York: Raven Press; 1991. pp. 117–24. [Google Scholar]
- 9.Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics. Pain. 1996;66:229–37. doi: 10.1016/0304-3959(96)03032-1. [DOI] [PubMed] [Google Scholar]
- 10.Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: verification from independent data. Pain. 1997;69:127–30. doi: 10.1016/s0304-3959(96)03251-4. [DOI] [PubMed] [Google Scholar]
- 11.Moore A, Moore O, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: use of pain intensity and visual analogue scales. Pain. 1997;69:311–5. doi: 10.1016/S0304-3959(96)03306-4. [DOI] [PubMed] [Google Scholar]
- 12.Gardner MJ, Altman DG. Statistics with ConfidenceConfidence Intervals and Statistical Guidelines. London: British Medical Association; 1989. [Google Scholar]
- 13.Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ. 1995;310:452–4. doi: 10.1136/bmj.310.6977.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahlstrom U, Kahnberg KE, Roos BE. Pentazocine and aspirin for pain following oral surgery. Acta Pharmacol Toxicol Copenh. 1974;35:325–36. doi: 10.1111/j.1600-0773.1974.tb00753.x. [DOI] [PubMed] [Google Scholar]
- 15.Bloomfield SS, Barden TP, Mitchell J. Aspirin and codeine in two postpartum pain models. Clin Pharmacol Ther. 1976;20:499–503. doi: 10.1002/cpt1976204499. [DOI] [PubMed] [Google Scholar]
- 16.Cooper SA, Needle SE, Kruger GO. Comparative analgesic potency of aspirin and ibuprofen. J Oral Surg. 1977;35:898–903. [PubMed] [Google Scholar]
- 17.Forbes JA, Jones KF, Kehm CJ, Smith WK, Gongloff CM, Zeleznock JR, Smith JW, Beaver WT, Kroesen M. Evaluation of aspirin, caffeine, and their combination in postoperative oral surgery pain. Pharmacotherapy. 1990;10:387–93. [PubMed] [Google Scholar]
- 18.Fuccella LM, Corvi G, Gorini F, Mandelli V, Mascellani G, Nobili F, Pedronetto S, Ragni N, Vandelli I. Application of nonparametric procedure for bioassay data to the evaluation of analgesics in man. J Clin Pharmacol. 1977;17:177–84. doi: 10.1177/009127007701700401. [DOI] [PubMed] [Google Scholar]
- 19.Holland IS, Seymour RA, Ward Booth RP, Ord RA, Lim KL, Hoare RC. An evaluation of different doses of soluble aspirin and aspirin tablets in postoperative dental pain. Br J Clin Pharmacol. 1988;26:463–8. doi: 10.1111/j.1365-2125.1988.tb03406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lasagna L, Calimlim JF. Oral ciramadol in the treatment of postoperative pain. Clin Pharmacol Ther. 1985;38:176–82. doi: 10.1038/clpt.1985.155. [DOI] [PubMed] [Google Scholar]
- 21.London RS, Sundaram GS, Feldman S, Goldstein PJ. Aspirin in the treatment of episiotomy pain. South Med J. 1983;76:844–5. doi: 10.1097/00007611-198307000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Mahler DL, Forrest WHJ, Brown CR, Shroff PF, Gordon HE, Brown BW, Jr, James KE. Assay of aspirin and naproxen analgesia. Clin Pharmacol Ther. 1976;19:18–23. doi: 10.1002/cpt197619118. [DOI] [PubMed] [Google Scholar]
- 23.Parkhouse JMR-L, Skolinik M, Peters H. The clinical dose–response to aspirin. Br J Anaesth. 1968;40:433–41. doi: 10.1093/bja/40.6.433. [DOI] [PubMed] [Google Scholar]
- 24.Seymour RA. Analgesic efficacy and plasma concentration of three analgesics in pain after lower third molar removal. SAAD Dig. 1983;5:172–88. [PubMed] [Google Scholar]
- 25.Seymour RA, Rawlins MD. Efficacy and pharmacokinetics of aspirin in post operative dental pain. Br J Clin Pharmacol. 1982;13:807–10. doi: 10.1111/j.1365-2125.1982.tb01870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seymour RA, Weldon M, Kelly P, Nicholson E, Hawkesford JE. An evaluation of buffered aspirin and aspirin tablets in postoperative pain after third molar surgery. Br J Clin Pharmacol. 1992;33:395–9. doi: 10.1111/j.1365-2125.1992.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skjelbred P. The effects of acetylsalicylic acid on swelling, pain and other events after surgery. Br J Clin Pharmacol. 1984;17:379–84. doi: 10.1111/j.1365-2125.1984.tb02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steiner TJ, Lange R, Voelker M. Aspirin in episodic tension-type headache: placebo-controlled dose-ranging comparison with paracetamol. Cephalalgia. 2003;23:59–66. doi: 10.1046/j.1468-2982.2003.00470.x. [DOI] [PubMed] [Google Scholar]
- 29.Sunshine A, Laska E, Slafta J. Oral nefopam and aspirin. Clin Pharmacol Ther. 1978;24:555–9. doi: 10.1002/cpt1978245555. [DOI] [PubMed] [Google Scholar]
- 30.Trop D, Nucci C, Elie R, Gareau J. Double-blind comparative evaluation of tiaprofenic acid (Surgam registered) versus acetylsalicylic acid (ASA) in relieving pain following episiotomy. Curr Ther Res. 1983;34:274–9. [Google Scholar]
- 31.Bloomfield SS, Barden TP, Mitchell J. Comparative efficacy of ibuprofen and aspirin in episiotomy pain. Clin Pharmacol Ther. 1974;15:565–70. doi: 10.1002/cpt1974156565. [DOI] [PubMed] [Google Scholar]
- 32.Bostrom AA, Forbes JA, Adolfsson C, Beaver WT, Bell WE. Evaluation of bromfenac and ibuprofen for pain after orthopedic surgery. Pharmacotherapy. 1994;14:305–13. [PubMed] [Google Scholar]
- 33.Cooper SA. Five studies on ibuprofen for postsurgical dental pain. Am J Med. 1984;(Suppl.):70–7. doi: 10.1016/s0002-9343(84)80022-4. [DOI] [PubMed] [Google Scholar]
- 34.Cooper SA. The relative efficacy of ibuprofen in dental pain. Compend Contin Educ Dent. 1986;7:578–97. [PubMed] [Google Scholar]
- 35.Dionne RA, McCullagh L. Enhanced analgesia and suppression of plasma β-endorphin by the S(+)-isomer ibuprofen. Clin Pharmacol Ther. 1998;63:694–701. doi: 10.1016/S0009-9236(98)90094-7. [DOI] [PubMed] [Google Scholar]
- 36.Fleiss JL, Chilton NW, Wallenstein SL. Ridit analysis in dental clinical studies. J Dent Res. 1979;58:2080–4. doi: 10.1177/00220345790580110701. [DOI] [PubMed] [Google Scholar]
- 37.Forbes JA, Beaver WT, Jones KF, Kehm CJ, Smith WK, Gongloff CM, Zeleznock JR, Smith JW. Effect of caffeine on ibuprofen analgesia in postoperative oral surgery pain. Clin Pharmacol Therapeutics. 1991;49:674–84. doi: 10.1038/clpt.1991.85. [DOI] [PubMed] [Google Scholar]
- 38.Hersh EV, Cooper SA, Betts N, Wedell D, MacAfee K, Quinn P, Lamp C, Gaston G, Bergman S, Henry E. Single dose and multidose analgesic study of ibuprofen and meclofenamate sodium after third molar surgery. Oral Surg Oral Med Oral Pathol. 1993;76:680–7. doi: 10.1016/0030-4220(93)90034-2. [DOI] [PubMed] [Google Scholar]
- 39.Hersh EV, Levin LM, Cooper SA. Ibuprofen liquigel for oral surgery pain. Clin Therapeutics. 2000;22:1306–18. doi: 10.1016/s0149-2918(00)83027-1. [DOI] [PubMed] [Google Scholar]
- 40.Hopkinson JH. Ibuprofen versus propoxyphene hydrochloride and placebo in the relief of postepisiotomy pain. Curr Ther Res. 1980;27:55–63. [Google Scholar]
- 41.Jain AK, Ryan JR, McMahon FG, Kuebel JO, Walters PJ, Noveck C. Analgesic efficacy of low dose ibuprofen in dental extraction pain. Pharmacotherapy. 1986;6:318–22. doi: 10.1002/j.1875-9114.1986.tb03494.x. [DOI] [PubMed] [Google Scholar]
- 42.Laska EM, Sunshine A, Marrero I, Olson N, Siegel C, McCormick N. The correlation between blood levels of ibuprofen and clinical analgesic response. Clin Pharmacol Ther. 1986;40:1–7. doi: 10.1038/clpt.1986.129. [DOI] [PubMed] [Google Scholar]
- 43.McQuay HJ, Angell K, Carroll D, Moore RA, Juniper RP. Ibuprofen compared with ibuprofen plus caffeine after third molar surgery. Pain. 1996;66:247–51. doi: 10.1016/0304-3959(96)03043-6. [DOI] [PubMed] [Google Scholar]
- 44.Schou S, Nielsen H, Nattestad A, Hillerup S, Ritzau M, Branebjerg PE, Bugge C, Skoglund LA. Analgesic dose–response relationship of ibuprofen 50, 100, 200, and 400 mg after surgical removal of third molars: a single-dose, randomized, placebo-controlled, and double blind study of 304 patients. J Clin Pharmacol. 1998;38:447–54. doi: 10.1002/j.1552-4604.1998.tb04452.x. [DOI] [PubMed] [Google Scholar]
- 45.Seymour RA, WardBooth P, Kelly PJ. Evaluation of different doses of soluble ibuprofen and ibuprofen tablets in postoperative dental pain. Br J Oral Maxillofac Surgery. 1996;34:110–4. doi: 10.1016/s0266-4356(96)90147-3. [DOI] [PubMed] [Google Scholar]
- 46.Sunshine A, Zighelboim I, Bartizek RD. A double-blind, placebo-controlled, single-dose comparison study of ibuprofen, and ibuprofen in combination with caffeine, in the treatment of postepisiotomy pain. Royal Soc Med Int Cong Symp Series. 1996;218:105–88. [Google Scholar]
- 47.Winter LJ, Bass E, Recant B, Cahaly JF. Analgesic activity of ibuprofen (Motrin) in postoperative oral surgical pain. Oral Surg Oral Med Oral Pathol. 1978;45:159–66. doi: 10.1016/0030-4220(78)90079-8. [DOI] [PubMed] [Google Scholar]
- 48.Hopkinson JH, Smith MT, Bare WW, Levin HM, Posatko RJ. Acetaminophen (500 mg) versus acetaminophen (325 mg) for the relief of pain in episiotomy patients. Current Therapeutic Res. 1974;16:194–200. [Google Scholar]
- 49.Laska EM, Sunshine A, Zighelboim I, Roure C, Marrero I, Wanderling J, Olson N. Effect of caffeine on acetaminophen analgesia. Clin Pharmacol Therapeutics. 1983;33:498–509. doi: 10.1038/clpt.1983.68. [DOI] [PubMed] [Google Scholar]
- 50.McQuay HJ, Poppleton P, Carroll D, Summerfield RJ, Bullingham RE, Moore RA. Ketorolac and acetaminophen for orthopedic postoperative pain. Clin Pharmacol Therapeutics. 1986;39:89–93. doi: 10.1038/clpt.1986.15. [DOI] [PubMed] [Google Scholar]
- 51.Quiding H, Oikarinen V, Sane J, Sjoblad A. Analgesic efficacy after single and repeated doses of codeine and acetaminophen. J Clin Pharmacol. 1984;24:27–34. doi: 10.1002/j.1552-4604.1984.tb01810.x. [DOI] [PubMed] [Google Scholar]
- 52.Ragot JP. Comparaison de l’activite antalgique de l’acide mefenamique et du paracetamol dans le traitement de la douleur apres extraction d’une 3eme molaire inferieure incluse. Inf Dent. 1991;73:1659–64. [PubMed] [Google Scholar]
- 53.Seymour RA, Kelly PJ, Hawesford JE. The efficacy of ketoprofen and paracetamol (acetaminophen) in postoperative pain after third molar surgery. Br J Clin Pharmacol. 1996;41:581–5. doi: 10.1046/j.1365-2125.1996.34015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skoglund LA, Skjelbred P, Fyllingen G. Analgesic efficacy of acetaminophen 1000 mg, acetaminophen 2000 mg, and the combination of acetaminophen 1000 mg and codeine phosphate 60 mg versus placebo in acute postoperative pain. Pharmacotherapy. 1991;11:364–9. [PubMed] [Google Scholar]
- 55.Strom C, Forsberg O, Quiding H, Engevall S, Larsson O. Analgesic efficacy of acetaminophen sustained release. J Clin Pharmacol. 1990;30:654–9. doi: 10.1002/j.1552-4604.1990.tb01869.x. [DOI] [PubMed] [Google Scholar]
- 56.Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything – large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain. 1998;78:209–16. doi: 10.1016/S0304-3959(98)00140-7. [DOI] [PubMed] [Google Scholar]
- 57.Norholt SE, Sindet-Pedersen S, Larsen U, Bang U, Ingerslev J, Nielsen O, Hansen HJ, Ersboll AK. Pain control after dental surgery: a double-blind, randomised trial of lornoxicam versus morphine. Pain. 1996;67:335–43. doi: 10.1016/0304-3959(96)03126-0. [DOI] [PubMed] [Google Scholar]
- 58.Moore RA, Edwards JE, McQuay HJ. Acute pain: individual patient meta-analysis shows the impact of different ways of analysing and presenting results. Pain. 2005;116:322–31. doi: 10.1016/j.pain.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Barden J, Edwards JE, McQuay HJ, Moore RA. Pain and analgesic response after third molar extraction and other postsurgical pain. Pain. 2004;107:86–90. doi: 10.1016/j.pain.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 60.Edwards JE, McQuay HJ, Moore RA, Collins SL. Reporting of adverse effects in clinical trials should be improved. Lessons from acute postoperative pain. J Pain Symptom Manage. 1999;18:427–37. doi: 10.1016/s0885-3924(99)00093-7. [DOI] [PubMed] [Google Scholar]