Abstract
What is already known about this subject
Among children, medication palatability is crucial for adherence to therapeutic regimen.
Several studies have measured the palatability of antimicrobial suspensions in paediatric patients by means of a visual analogue scale palatability score.
What this study adds
This is the first analysis comparing the taste and smell acceptability of angiotensin II receptor blockers among paediatric patients with kidney disease.
From the perspective of the child with kidney disease, the taste of pulverized candesartan is significantly superior to that of pulverized irbesartan, losartan, telmisartan or valsartan.
Aim
Angiotensin II receptor blockers are widely prescribed in kidney disease. Among children, medication palatability is crucial for adherence.
Methods
Taste and smell acceptability of five angiotensin II receptor blockers were compared among 21 nephropathic children using a visual analogue scale palatability score.
Results
The score assigned to pulverized tablets of candesartan cilexetil was significantly higher than that assigned to pulverized tablets of irbesartan, losartan, telmisartan and valsartan.
Conclusions
From the perspective of the nephropathic child, the taste of pulverized candesartan cilexetil is superior to that of irbesartan, losartan, telmisartan or valsartan.
Keywords: candesartan cilexetil, hypertension, irbesartan, losartan, palatability, telmisartan, valsartan
Introduction
Angiotensin II receptor blockers, known colloquially as angiotensin antagonists, are orally available drugs, which inhibit the renin–angiotensin II–aldosterone system by blocking the type 1 of angiotensin II receptor. Their use is largely established in patients with kidney disease because, like converting enzyme inhibitors, they are more effective than most other antihypertensive drugs in slowing the progression towards end-stage kidney disease. Although the different angiotensin antagonists differ in their pharmacokinetics and dosing, all demonstrate a similar clinical effectiveness in kidney disease [1, 2].
Extremely important to treatment success is patient adherence to therapeutic regimen. Among paediatric patients, medication palatability is crucial for adherence to therapeutic regimen. Consequently, given similar clinical effectiveness, taste considerations greatly affect drug selection of caregivers [3–5]. We compared the taste and smell acceptability of five angiotensin antagonists which had been pulverized among paediatric patients with kidney disease.
Subjects and methods
Eligible for the comparison, which had been approved by an Institutional Review Board, were children 4–11 years of age with acute or chronic kidney disease and arterial hypertension, overt proteinuria or both if they were willing to comply with appropriate instructions necessary to complete the comparison, which was not commercially sponsored. Subjects who had been previously treated with an angiotensin antagonist were not included.
Each study consisted of one session lasting 2–3 h. After randomization assignment, the principal investigator accompanied each child to a private test area and described procedures and rating scales. It was explained to the child that he would be asked ‘how much did you like the taste of this medication’ and encouraged to indicate his preference by pointing to the appropriate face on a gender-specific, 10-cm visual analogue scale that depicts five degrees of pleasure: ‘really good’, ‘good’, ‘not sure’, ‘bad’ and ‘really bad’. The explanation was repeated if the child did not understand. Each child received 1 mg of candesartan cilexetil, irbesartan, losartan, telmisartan and valsartan directly into the oral cavity from an opened capsule shell. The agents had been identified to the child by letter only. No attempt was made to disguise the colour of the different preparations. Between tastings of the different drugs, the children received a cracker to eat and rinsed their mouth several times to remove any residual taste from the previous drug. Immediately after each test dose, the child rated the taste pointing to the appropriate face. In addition, at the end of the session the child was asked which agent he thought tasted the best. After completion of the tasting phase, each child remained at the study site for approximately 1 h to monitor for any adverse events. Finally, a follow-up telephone call was made to the family 24–48 h after the taste test to evaluate possible adverse events [3].
A pharmacist had crushed and pulverized commercially available tablets of candesartan cilexetil, irbesartan, losartan, telmisartan and valsartan and prepared capsule shells containing 1 mg of active substance.
The taste scores from the visual analogue scales were analysed using nonparametric analysis of variance for repeated measures (Friedman test). The proportion of subjects who rated each angiotensin II antagonist as best tasting was compared using the χ2 goodness of fit test. Significance was assumed when P < 0.05 (two tailed).
Results
Between October 2005 and May 2006 a total of 21 paediatric patients (eight girls and 13 boys, ranging in age from 4.2 to 11, median 7.8 years) with an underlying chronic (N = 12) or acute (N = 9) kidney disease completed the taste test. There were nine subjects with arterial hypertension, four with pathological proteinuria and eight with both hypertension and proteinuria. Eight of the patients with chronic kidney disease were on medication with a variety of antihypertensive drugs.
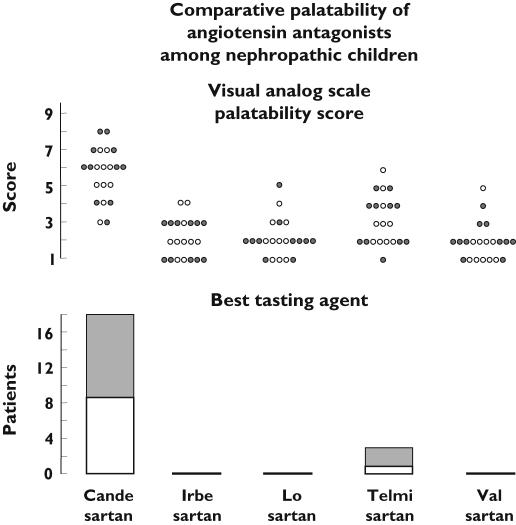
No adverse effects were noted during or after the taste tests. None of the 21 patients graded the palatability score of the different angiotensin antagonists as really good. The average visual analogue scale palatability score assigned by the patients to candesartan cilexetil was significantly higher (P = 0.000) than that assigned to the other agents, as shown in Figure 1. This result was confirmed when the children were asked which agent they preferred: 18 children selected candesartan cilexetil as best tasting, whereas three selected telmisartan. None of the children chose irbesartan, losartan or valsartan (Figure 1). The difference between candesartan cilexetil and the remaining drugs was statistically significant (P = 0.000). The results were similar in 11 patients aged ≥7.8 years and in 10 patients aged <7.8 years. There were no sex differences in the results. Furthermore, the order of presentation did not affect the selection of an agent as best tasting.
Figure 1.
Comparative palatability of five angiotensin II receptor blockers among 21 nephropathic children. The gender-specific visual analogue scale palatability score (upper panel) assigned to candesartan cilexetil was significantly higher (P = 0.000; Friedman test) than that assigned to the other agents. When the patients were asked which agent they preferred (lower panel), significantly (P = 0.000; χ2 goodness of fit test) more children selected candesartan cilexetil as best tasting. The results were similar in 11 children aged ≥7.8 years (grey symbols) and in 10 children aged <7.8 years (open symbols)
Discussion
A problem which often affects drugs originally designed for use in adults, such as angiotensin antagonists, is the lack of suspensions or other age-appropriate drug formulations [3–5]. Parents therefore crush available tablets and administer the medication mixed with solid food or a palatable drink. The present analysis indicates that, from the perspective of the paediatric patient with kidney disease, the taste of 1 mg of pulverized candesartan cilexetil is significantly superior to that of an identical amount of pulverized irbesartan, losartan, telmisartan or valsartan. This is probably related to the neutral taste of candesartan cilexetil.
We evaluated the taste of crushed angiotensin antagonists by means of a visual ‘smile-face’ scale, which has been widely used to evaluate the palatability of antimicrobials in children ≥4 years old [3].
Trials performed in adulthood suggest a similar efficacy within the class of angiotensin antagonists, candesartan cilexetil 1.0 mg once daily being as effective as telmisartan 2.5 mg once daily, losartan 6.2 mg once daily, irbesartan 9.4 mg once daily and valsartan 10 mg once daily [1, 2].
The taste and smell acceptability of angiotensin antagonists among children with kidney disease was not assessed in a dose-equivalent way, in view of the fact that in this clinical setting concurrent administration of candesartan cilexetil 1.0 mg and equivalent doses of telmisartan, losartan, irbesartan and valsartan would be expected to reduce blood pressure significantly and acutely. The use of equivalent doses, obviously a situation closer to reality, might have further advantaged candesartan.
Obviously, few angiotensin antagonists have been investigated in childhood and available information often relies on investigations without control, on retrospective analysis or on inadequate numbers of patients. Nevertheless, currently available data indicate that extrapolating adult doses of the angiotensin antagonists irbesartan [6–8], losartan [9–11] and, probably, even candesartan [12] is safe and effective in treating children with arterial hypertension or proteinuria.
The major drawback of the present taste test is that none of our patients graded the palatability of the different angiotensin antagonists as really good. The solution to this problem, development of an assortment of palatable suspensions containing angiotensin antagonists, is unlikely to be pursued because of the costs involved.
We did not assess the taste acceptability of angiotensin antagonists using pure chemicals. The approach applied in the present analysis, which does not discriminate the effect of the pure chemicals from that of the excipients, is practically relevant, considering that in clinical routine children are not given pure chemicals but broken tablets that contain both the active agent and the excipients.
In conclusion, the results of this analysis have the potential to influence prescribing habits of caregivers and adherence to the therapeutic regimen.
References
- 1.Conlin PR. Angiotensin II antagonists in the treatment of hypertension: more similarities than differences. J Clin Hypertens (Greenwich) 2000;2:253–7. [PubMed] [Google Scholar]
- 2.Israili ZH. Clinical pharmacokinetics of angiotensin II (AT1) receptor blockers in hypertension. J Hum Hypertens. 2000;14(Suppl. 1):S73–86. doi: 10.1038/sj.jhh.1000991. [DOI] [PubMed] [Google Scholar]
- 3.Nahata MC, Holas C, Chiu YL, Notario G, Kapral D. A pooled analysis of seven randomized crossover studies of the palatability of cefdinir oral suspension versus amoxicillin-clavulanate potassium, cefprozil, azithromycin, and amoxicillin in children aged 4–8 years. Clin Ther. 2005;27:1950–60. doi: 10.1016/j.clinthera.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Nevins TE. ‘Why do they do that?’ The compliance conundrum. Pediatr Nephrol. 2005;20:845–8. doi: 10.1007/s00467-005-1926-5. [DOI] [PubMed] [Google Scholar]
- 5.Nunn T, Williams J. Formulation of medicines for children. Br J Clin Pharmacol. 2005;59:674–6. doi: 10.1111/j.1365-2125.2005.02410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakarcan A, Tenney F, Wilson JT, Stewart JJ, Adcock KG, Wells TG, Vachharajani NN, Hadjilambris OW, Slugg P, Ford NF, Marino MR. The pharmacokinetics of irbesartan in hypertensive children and adolescents. J Clin Pharmacol. 2001;41:742–9. doi: 10.1177/00912700122010645. [DOI] [PubMed] [Google Scholar]
- 7.Franscini LM, Von Vigier RO, Pfister R, Casaulta-Aebischer C, Fossali E, Bianchetti MG. Effectiveness and safety of the angiotensin II antagonist irbesartan in children with chronic kidney diseases. Am J Hypertens. 2002;15:1057–63. doi: 10.1016/s0895-7061(02)03083-2. [DOI] [PubMed] [Google Scholar]
- 8.Gartenmann AC, Fossali E, von Vigier RO, Simonetti GD, Schmidtko J, Edefonti A, Bianchetti MG. Better renoprotective effect of angiotensin II antagonist compared to dihydropyridine calcium-channel blocker in childhood. Kidney Int. 2003;64:1450–4. doi: 10.1046/j.1523-1755.2003.00238.x. [DOI] [PubMed] [Google Scholar]
- 9.Ellis D, Vats A, Moritz ML, Reitz S, Grosso MJ, Janosky JE. Long-term antiproteinuric and renoprotective efficacy and safety of losartan in children with proteinuria. J Pediatr. 2003;143:89–97. doi: 10.1016/S0022-3476(03)00279-8. [DOI] [PubMed] [Google Scholar]
- 10.Ellis D, Moritz ML, Vats A, Janosky JE. Antihypertensive and renoprotective efficacy and safety of losartan. A long-term study in children with renal disorders. Am J Hypertens. 2004;17:928–35. doi: 10.1016/j.amjhyper.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Shahinfar S, Cano F, Soffer BA, Ahmed T, Santoro EP, Zhang Z, Gleim G, Miller K, Vogt B, Blumer J, Briazgounov I. A double-blind, dose–response study of losartan in hypertensive children. Am J Hypertens. 2005;18:183–90. doi: 10.1016/j.amjhyper.2004.09.009. (Erratum in: Am J Hypertens 2006; 19: 658) [DOI] [PubMed] [Google Scholar]
- 12.Simonetti GD, von Vigier RO, Konrad M, Rizzi M, Fossali E, Bianchetti MG CHIld Project. Candesartan cilexetil in children with hypertension or proteinuria: preliminary data. Pediatr Nephrol. 2006;21:1480–2. doi: 10.1007/s00467-006-0144-0. [DOI] [PubMed] [Google Scholar]