Abstract
What is already known about this subject
In burn patients it has been shown ([2]), that there is a correlation between the creatinine clearance (CLCR) and the clearance of inulin.
The CLCR has never been studied in burn patients who have normal serum creatinine.
The Robert, Kirkpatrick and sMDRD formulae have never been evaluated in burn patients.
What this study adds
Despite burn patients having normal serum creatinine concentrations, the study showed that there are large variations in CLCR which cannot be detected by single serum creatinine measurements, and which have important implications for drug therapy.
It showed that the formulae currently used to calculate creatinine clearance on the basis of serum creatinine are inadequate for use in burn patients, and they should be abandoned in favour of direct measurement from a 24 h urine collection.
Aims
The aim of this study was to evaluate whether the renal function of burn patients could be correctly assessed using a single serum creatinine measurement, within normal limits, and three prediction equations of glomerular filtration taking into account, serum creatinine, age, weight and sex.
Methods
This was a prospective study comprising 36 adult burn patients with a serum creatinine <120 µmol l−1, within the second or third week following the burn injury. Renal function was assessed using serum creatinine, 24 h urinary CLCR, and the Cockcroft–Gault, Robert, Kirkpatrick and simplified MDRD equations.
Results
Despite normal serum creatinine concentrations in all patients, a significant number had a decreased CLCR. The urinary CLCR was <80 ml−1 min−1 1.73 m−2 in nine patients (25%), and <60 ml−1 min−1 1.73 m−2 in five patients (14%). Between the groups having a CLCR lower or greater than 80 ml−1 min−1 1.73 m−2 there were no differences in gender, burn indices, percentage of mechanically ventilated patients or length of hospital stay, but a difference in age. The highest CLCR (>140 ml−1 min−1 1.73 m−2) was found in 13 patients younger than 40 years. Regression analysis, residual and Bland–Altman plots revealed that neither the Cockcroft–Gault, Robert, Kirkpatrick nor sMDRD equations were specific enough for the assessment of renal function.
Conclusions
In burn patients with normal serum creatinine during the hypermetabolic phase, serum creatinine and creatine based predictive equations are imprecise in assessing renal function.
Keywords: burn patients, creatinine clearance, glomerular filtration rate, prediction equations, serum creatinine
Introduction
In a substantial number of burn patients, the early detection of renal dysfunction and subsequent adequate treatment can prevent progression to acute renal failure [1]. During the hypermetabolic phase, beginning 48 h after the thermal injury, an increased cardiac output is observed, with a concomitant increase in blood flow to the kidneys and liver. Thus, there is an increase in the glomerular filtration rate (GFR), as assessed by creatinine clearance [2, 3]. Many of the drugs given to burn patients need dose adjustment as a function of the GFR. With antibiotics eliminated mainly by renal glomerular filtration, a subpopulation of burn patients with a high GFR can eliminate drugs extremely rapidly, leading to poor efficacy. Individual therapeutic drug monitoring is required [4] as has been demonstrated in critically ill patients [5–8].
In clinical practice, the marker used daily for the evaluation of renal function in patients is serum creatinine [9–12]. However, serum creatinine may not be very suitable for this purpose as its concentration depends not only on renal elimination but also on creatinine generation and the volume of distribution. Muscle mass and the metabolic transformation of creatine have an impact on the serum creatinine concentration [13]. Many characteristics apart from renal function may influence the creatinine concentration, such as age, gender and race: younger patients, males and Blacks have higher serum creatinine concentrations for the same given GFR, compared with older patients, females and Caucasians [14, 15]. In addition, serum creatinine and GFR are not linearly related [16]. Direct measurement of the GFR, the gold standard for the assessment of renal function, with exogenous substances such as inulin, is not performed routinely in burn or intensive care units for practical and ethical reasons. Measured creatinine clearance (CLCR), however, can easily be determined in a burn patient, and it is the easiest method for evaluating renal function in clinical practice [17–19]. GFR is over-estimated by creatinine clearance in renal failure due to tubular creatinine secretion [17] but this overestimation is lower in patients with normal serum creatinine values. Moreover close correlations between iothalamate and creatinine clearances and between inulin and creatinine clearances were demonstrated by Loirat et al. [2] in burn patients.
However, the determination of clearance requires a steady-state situation.
Numerous equations have been developed to calculate the GFR using serum chemistry (usually serum creatinine concentration, SCR) with demographic data. The Cockcroft-Gault formula is the most commonly used for this purpose in adults [14], as recommended both by standard texts [20, 21] and by the recent FDA guidance document for dose adjustments in renal impairment [22]. In critically ill patients, Robert et al. showed that the incorporation of a corrected serum creatinine value and the use of an ideal body weight in the Cockcroft-Gault equation led to a better prediction of the GFR [23]. Kirkpatrick et al. [24–26] have demonstrated the usefulness of the adjustment of the Cockcroft & Gault method for therapeutic drug monitoring.
The new National Kidney Foundation Kidney/Disease Outcomes Quality Initiative (NKF K/DOQI) guidelines recommend estimating the GFR by the Modification of Renal Disease (MDRD) or Cockcroft-Gault (CG) equations [27]. In 2000, a simplified MDRD equation (sMDRD) was published in abstract form [28, 29]. These equations can be easily calculated at the bedside but they have not really been validated in burn patients. Given these considerations, the purpose of the present study was to evaluate the value of a single serum creatinine measurement, within normal limits, and the four predictive equations used for the assessment of glomerular function (Cockcroft-Gault, Robert, Kirkpatrick and the simplified MDRD formula), compared with creatinine clearance (CLCR) measured on a 24 h urine collection in burn patients during the hypermetabolic phase of the burn injury. The secondary aim was to quantify the GFR estimated by the CLCR in burn patients and to characterize the biological and clinical factors involved in any modifications.
Methods
Patients
During a prospective study concerning the various dosage regimens of ceftazidime used to treat burn patients [30], the CLCR was measured in most of the studied patients. Briefly, after approval by the local ethics committee, our study was carried out on 50 informed patients in the Burns Unit of the University Hospital of Toulouse-Rangueil over a period of 4 years. The patients were studied during the secondary phase of their burn injuries. The antibiotics were prescribed for local infections or for sepsis. The patients were randomly divided into two groups. One group initially received a dose of 6 g of ceftazidime per 24 h in three separate short perfusions of 2 g each, i.e. every 8 h. The second group was given six doses of 1 g each, i.e. every 4 h. Blood was sampled in dry tubes at the troughs and peaks 24 h and 48 h after the start of the treatment for monitoring.
Clinical and biological data
For each patient, the usual biological parameters and the specific burn indices were recorded:
Percentage of burn area (SB) [31],
Baux index equal to age + SB [32],
Unit Burn Standard (UBS) index (SB +3 fold percentage of 3rd degree of burn) [32, 33] and
Tobiasen index [34], calculated as a score involving sex, age, inhalation injury, third stage of burn and percentage of burn area.
Fifteen days, on average, after the burn injury, urine was sampled over 24 h and plasma just before drug administration, for the measurement of creatinine concentration. In some patients creatinine clearance was repeatedly measured but we only took into account the first measurement.
Among the whole population of our study [30], we only chose patients who were haemodymically stable, not being treated with diuretics, without renal failure or any change in serum creatinine concentrations (less than 10%) during the 48 h period of study. In all patients bilirubin was normal and the serum creatinine was lower than 120 µmol l−1. Neither cephalosporin nor cimetidine were given before the first creatinine measurement.
SCR measurement and calibration
All creatinine measurements were performed in the same laboratory. Blood samples were obtained simultaneously with the CLCR measurement. A modified kinetic Jaffe colorimetric method was used with a COBAS MIRA (ABX diagnostics) analyzer, and a two–point calibration applied to each assay. Before measurement, ultrafiltration of plasma through a 20 kDa cut-off membrane (MPS-1; Amicon, Beverly, MA) was carried out to remove chromogens that were linked to albumin such as bilirubin and other large proteins. In the absence of an international standard for creatinine assays, the linearity of the measurements was verified by using plasma samples from normal subjects to which increasing amounts of creatinine hydrochloride (Sigma Chemicals, Perth, Australia) had been added. Linear regression analysis showed that the relationship between measured and expected creatinine concentrations was 1.0008 ± 0.006 (95% confidence interval 0.997, 1.020) and that the y-intercept was 0.014 ± 0.013 (95% confidence interval 0.013, 0.041). The squared Spearman rank coefficient of correlation was 0.998. Internal quality controls showed a coefficient of variation of 2.3% during the period.
Assessment of glomerular filtration rate (GFR)
Creatinine clearance was calculated according to:
where urine creatinine (UCR) and serum creatinine (SCR) were expressed in µmol l−1 and V corresponded to the urinary rate (diuresis) in ml min−1. At the same time, the GFR was calculated using the Cockcroft formula [14]:
for men, with age in years and weight in kg. A correcting factor of 0.85 was used for women. The derivative formula proposed by Robert et al. [23] uses the ideal body weight and serum creatinine concentration corrected to 85 µmol l−1 when the actual value is lower than 85 µmol l−1. Ideal body weight was determined as 50 kg for men and 45.5 kg for women, plus 2.3 kg for each inch >5 feet [35]. Kirkpatrick et al. [24] proposed a correction for the serum creatinine lower than 60 µmol l−1 which is rounded to 60 µmol l−1 before the Cockcroft calculation.
The simplified formula of the Modification of Diet in Renal Disease index (sMDRD) [15, 29] was also calculated according to:
where serum creatinine (SCR) was expressed in mg dl−1.
The urinary creatinine loss per day was calculated as urine creatinine concentration (mg dl−1) × 24 h urine volume (ml)/100 × 1.73/body surface area (m2), expressed as (mg 24 h−1 1.73 m−2), and as urine creatinine concentration (mg dl−1) × 24 h urine volume (ml)/100/actual body weight (kg), expressed as (mg kg−1 24 h−1).
Statistical analysis
The statistical approach involved linear regression, and a multiple analysis of variance, correlation between measured and estimated CLCR and demographic characteristics of interest (i.e. burn area, Baux Index, UBS, Tobiasen, days postburn injury, and other biological parameters). To compare the different parameters according to the measured CLCR groups, parametric and nonparametric tests were applied as appropriate.
The predictability of the individual estimation of the GFR by the Cockcroft, Robert and the sMDRD formulae compared with the CLCR was evaluated by residual plots and the Bland & Altman concordance method [36]. This approach is based on the graphical drawing of the parameters being compared. Bland & Altman plots evaluate the mean difference ± 2 SDs between two methods of measurement over the average of the measurements by the two methods. The method leads to the determination of bias and concordance limits between one method and another. Data are presented as mean ± SD (interquartile range).
The precision and the bias of the individual estimation (CLCR estimated) of the GFR by the Cockcroft, Robert, Kirkpatrick and the sMDRD formulae compared with the CLCR was evaluated according to Sheiner & Beal [37] by the following equations:
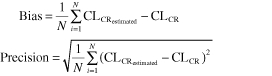 |
The results are expressed as a percentage of the mean measured CLCR.
The results were analyzed with the StatView statistical software package, version 5.0. A P value ≤0.05 was considered as statistically significant.
Results
The characteristics of the patients are shown in Table 1. The population studied included 36 patients (22 men and 14 women) aged 54 ± 22 years. The mean burn area was 21 ± 16% of the total body area and 33% of the patients were mechanically ventilated. The study was performed on day 15 ± 10 (4–44).
Table 1.
Clinical characteristics of 36 burn patients
| Age (years) | Weight (kg) | Height (cm) | Body area (m2) | Burn area (%) | Baux index | UBS | Tobiasen index | |
|---|---|---|---|---|---|---|---|---|
| Mean | 54 | 71.6 | 168.3 | 1.81 | 21.3 | 75.4 | 56.3 | 7 |
| SD | 22 | 17.3 | 8.8 | 0.2 | 15.7 | 24.0 | 42.5 | 2 |
| Minimum | 21.0 | 48.0 | 155.0 | 1.5 | 10.0 | 31.0 | 10.0 | 4.0 |
| Maximum | 90.0 | 120.0 | 185.0 | 2.4 | 90.0 | 122.0 | 168.0 | 12.0 |
| Coefficient of variation (%) | 40.1 | 24.1 | 5.2 | 12.9 | 73.8 | 31.8 | 75.5 | 24.4 |
Serum creatinine was low, equal to 76 ± 23 µmol l−1, while serum urea was higher than the normal limits, being equal to 7.7 ± 5.0 mmol l −1 (3.1–23.8).
The GFR calculated according to the Cockcroft formula was 101 ± 52 (38–306) ml min−1 1.73 m−2, by the Robert formula it was 71 ± 23 (33–111) ml min−1 1.73 m−2, with the Kirkpatrick correction it was 92 ± 34 (36–164) ml min−1 1.73 m−2 and according to the sMDRD equation 98 ± 38 (48–186) ml min−1 1.73 m−2, while the CLCR was 119 ± 53 (33–224) ml−1 min−1 1.73 m−2. These results are detailed in Table 2.
Table 2.
Glomerular filtration rate in ml min−1 1.73 m−2 and in ml min−1 (n = 36) estimated by creatinine clearance (CLCR), and the Cockcroft & Gault, Robert, Kirpatrick and sMDRD formulae
| CLCR | Cockcroft formula | Robert formula | Kirkpatrick formula | sMDRD formula | |
|---|---|---|---|---|---|
| Mean ± SD (ml min−1 1.73 m−2) | 118.8 ± 53 | 101.6.0 ± 52.4 | 71.3 ± 22.8 | 91.9 ± 33.9 | 97.8 ± 37.5 |
| Range | 33.4–224.4 | 37.8–306.1 | 32.7–111.2 | 35.9–164.4 | 47.4–185.6 |
| Coefficient of variation (%) | 44.4 | 51.6 | 32 | 36.9 | 38.4 |
| Mean ± SD (ml min−1) | 123.4 ± 57.2 | 106.9 ± 60.9 | 74.3 ± 25.2 | 97.3 ± 43.2 | 101.6 ± 40.6 |
| Range | 33.2–275 | 33.5–353.8 | 30.2–124.7 | 33.5–230 | 46.4–219 |
| Bias (%) | – | −13.4 | −39.8 | −21.1 | −17.6 |
| Precision (%) | – | 46.7 | 52.6 | 39.6 | 44.8 |
Urinary creatinine excretion for women was 1175 ± 447 (664–1988) mg 24 h−1 1.73 m−2, and for men was 1528 ± 646 (451–2511) mg 24 h−1 1.73 m−2. As the urinary creatinine excretion in normal subjects has been established to be 1230 mg 24 h−1 1.73 m−2 for females, and 1600 mg 24 h−1 1.73 m−2 for males [38], the urinary creatinine excretion of these burn patients was therefore within the normal limits.
A negative linear relationship was demonstrated between the urinary creatinine excretion expressed in mg 24 h−1 1.73 m−2 or in mg kg−1 24 h−1 and age: r2 = 0.549 and P < 0.0001 and r2 = 0.5 with P < 0.0001. A positive linear relationship was shown between the urinary creatinine excretion (mg 24 h−1 1.73 m−2 and mg kg−1 24 h−1) and the volume of the maintaining liquids infused on the day of the CLCR measurement (respectively r2 = 0.143 with P = 0.0328 and r2 = 0.174 with P = 0.0175).
Assessment of serum creatinine as a marker of the GFR
An unexpected finding was that a significant number of patients had a decreased CLCR, despite having a normal serum creatinine. In nine patients (25%), the CLCR was <80 ml min−1 1.73 m−2 and in five patients (14%), the CLCR was <60 ml min−1 1.73 m−2. Burn patients with a CLCR lower than 80 ml min−1 1.73 m−2 had a lower 24 h creatinine excretion (Table 3). There were no differences in gender, specific burn indices, percentage of mechanically ventilated patients or length of hospital stay following the burn injury. The age was higher in patients with a CLCR lower than 80 ml min−1 1.73 m−2. The same data were found by comparing the nine patients with a CLCR <80 ml min−1 1.73 m−2 and the 15 patients who had a CLCR >120 ml min−1 1.73 m−2 (Table 3).
Table 3.
Comparison of patients with a measured creatinine clearance (CLCR) greater than 80 or 120 ml min−1 1.73 m−2 and lower than 80 ml min−1 1.73 m−2
| CLCR (>120 ml min−1 1.73 m−2) | CLCR > (80 ml min−1 1.73 m−2) | CLCR (<80 ml min−1 1.73 m−2) | P# | P§ | |
|---|---|---|---|---|---|
| n | 15 (62.5%) | 27 (75%) | 9 (25%) | ||
| Age (years) | 34 ± 12 | 47 ± 20 | 74 ± 11 | 0.0025 | <0.0001 |
| Gender (male) | 9 (60%) | 17 (62.96%) | 5 (55.55%) | 0.73 | 0.7358 |
| Actual body weight (kg) | 67 ± 16 | 71 ± 18 | 72 ± 15 | 0.939 | 0.4259 |
| Ideal body weight (kg) | 64 ± 10 | 65 ± 11 | 66 ± 13 | 0.899 | 0.6441 |
| Burn area (%) | 26 ± 20 | 23 ± 17 | 18 ± 11 | 0.5206 | 0.3270 |
| Baux index | 60 ± 22 | 70 ± 24 | 92 ± 16 | 0.012 | 0.0008 |
| UBS | 67 ± 51 | 57 ± 47 | 54 ± 26 | 0.8332 | 0.4824 |
| Tobiasen index | 7 ± 2 | 7 ± 2 | 8 ± 1 | 0.2210 | 0.1356 |
| Days preceding burn injury | 11 ± 6 | 13 ± 7 | 19 ± 15 | 0.314 | 0.4016 |
| Mechanical ventilation | 4 (26.7%) | 7 (25.9%) | 3 (33.3%) | 0.735 | 0.7358 |
| Serum creatinine (µmol l−1) | 70.06 ± 23.92 | 71.8 ± 21.5 | 88.9 ± 24.1 | 0.1924 | 0.0758 |
| measured creatinine clearance (ml min−1) | 173.8 ± 47.65 | 144.7 ± 48.8 | 59.5 ± 21.9 | <0.0001 | <0.0001 |
| Urinary creatinine excretion (mg 24 h−1 1.73 m−2) | 1890 ± 490 | 1593 ± 537 | 786 ± 259 | 0.0003 | 0.0001 |
| Urinary creatinine excretion (mg kg−1 24 h−1) | 29.2 ± 7.5 | 24.0 ± 8.8 | 11.6 ± 3.8 | 0.0012 | 0.0001 |
| Cockcroft-Gault (ml min−1 1.73 m−2) | 124.9 ± 35.6 | 113.4 ± 52.8 | 66.3 ± 32.7 | 0.0171 | 0.0006 |
| Robert (ml min−1 1.73 m−2) | 88.8 ± 16.7 | 78.1 ± 20.7 | 50.8 ± 16.1 | 0.001 | <0.0001 |
| Kirkpatrick (ml min−1 1.73 m−2) | 113.4 ± 24.5 | 101.0 ± 30.4 | 64.7 ± 30.3 | 0.0038 | 0.0003 |
| Simplified MDRD (ml min−1 1.73 m−2) | 119 ± 36.6 | 106.4 ± 36.3 | 72.3 ± 30.1 | 0.0159 | 0.0039 |
: comparison between less and more than 80 ml min−1 1.73 m−2.
: comparison between less than 80 ml min−1 1.73 m−2 more than 120 ml min−1 1.73 m−2.
Comparison of the methods for calculating renal function
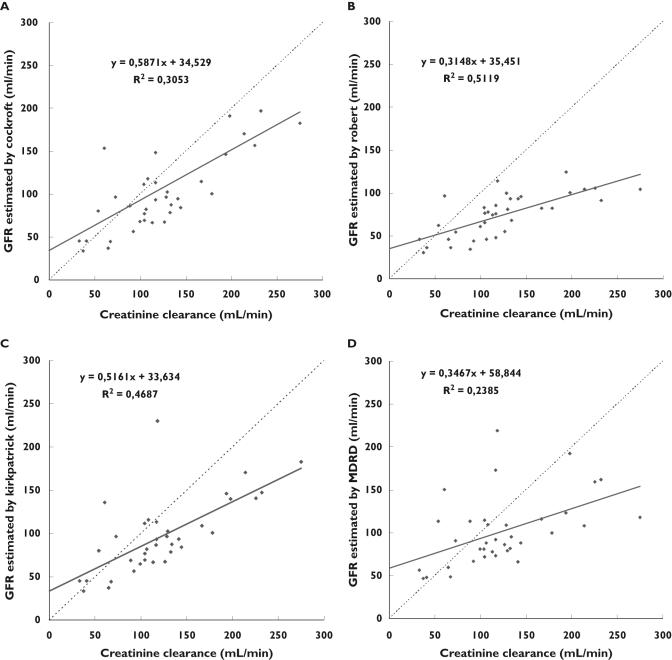
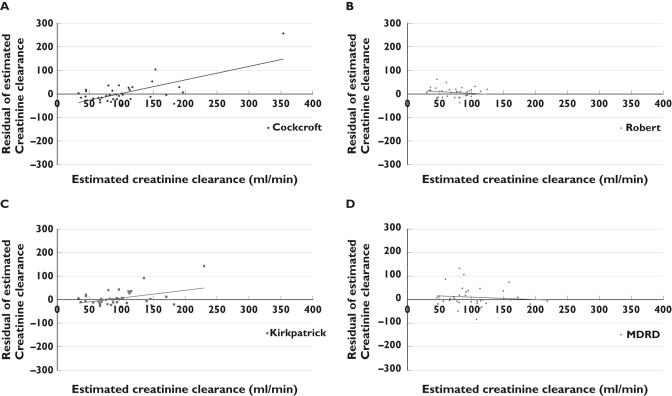
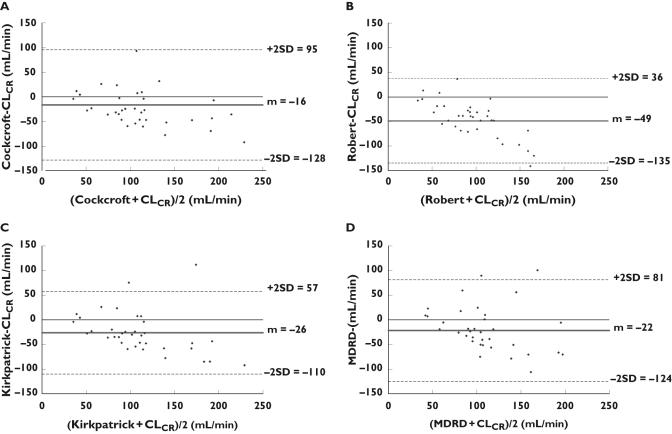
As shown in Table 3 and Figure 1A, a linear relationship was demonstrated between CLCR and the Cockcroft GFR estimation (r2 = 0.3053 and P < 0.0005) according to the equation ‘Cockcroft GFR = 0.59 × CLCR + 34.5’. The ordinate intercept was not different from 0 (P = 0.05) but the slope was statistically different from 1 (P < 0.0001) even if it was the highest one. In spite of this relationship, the individual predictability evaluated by the residual plot and Bland & Altman method was poor as shown in Figures 2 and 3. With the Cockcroft formula the mean bias (mean of the deviation between both methods) was −16 ml min−1, the precision was 46.7% and its confidence interval (±2 SD) ranged from 95 to 128 ml min−1. As also shown on Figure 2A by the residuals trend, these results indicate that for the lowest clearances (<60 ml min−1), the creatinine clearance is overestimated by the Cockcroft formula, and for the normal or high CLCR, it is an under-estimate.
Figure 1.
Correlation between glomerular filtration rate estimated by the Cockcroft & Gault (A), Robert (B), Kirkpatrick (C) and sMDRD (D) formulae and creatinine clearance measured over 24 h
Figure 2.
Plot of the residuals and their trend of the glomerular filtration rate estimated by the Cockcroft & Gault (A), Robert (B), Kirkpatrick (C) and sMDRD (D) formulae
Figure 3.
Concordance study (Bland & Altman method) between the glomerular filtration rate estimated by the Cockcroft & Gault (A), Robert (B), Kirkpatrick (C) and sMDRD (D) formulae and creatinine clearance measured over 24 h
As shown in Figures 1B, 2B and 3B, the results obtained by the Robert formula show that the best significant linear relationship was observed between CLCR and the Robert GFR estimation (r2 = 0.5119 and P < 0.0001) according to the equation ‘Robert GFR = 0.31 × CLCR + 34.4’ with an ordinate statistically different from 0 (P = 0.0001), a slope different from 1 (P < 0.0001), a higher bias (−49 ml min−1), a poor precision (52.6%) and a low predictability of GFR (confidence interval ranging from 36 to −135 ml min−1) especially for the lower clearances.
The Kirkpatrick correction provides the following relationship ‘Kirkpatrick GFR = 0.52 × CLCR 0 + 33.6’ (r2 = 0.4687 and P < 0.0001). The slope was significantly different from 1 (P = 0.0127) and the ordinate was different from 0 (P < 0.0001). Even if for the highest values, the predictability decreases, the bias was −26.1 ml min−1 and the precision was the best (39.6%). The confidence interval (±2 SD) ranged from 57 to −110 ml min−1.
The results obtained by the simplified MDRD formula show that a linear relationship was found between CLCR and the sMDRD GFR estimation (r2 = 0.28385 and P = 0.0025) according to the equation ‘sMDRD GFR = 0.33 × CLCR + 58.8’. The residual plot in Figure 2D evidenced a low individual predictability. The Bland & Altman method showed a poor concordance (Figure 3): the bias was −22 ml min−1 and the precision was 44.8% with a large confidence interval ranging from 81 to −124 ml min−1.
Relationships between the GFR and burn indices
No correlation was found between the measured CLCR and the severity of the burn indices, but an inverse linear correlation was seen between CLCR and the Baux index (r2 = 0.328 and P < 0.0003). The observed decrease in the GFR as a function of the Baux index was related to the age of the patients as shown by the multiple analysis of variance. Another correlation between CLCR was found with the Tobiasen index (r2 = 0.170 and P = 0.0191) but this relationship disappeared when age was taken into account (P = 0.09).
We found a negative relationship between measured CLCR and serum haemoglobin (r2 = 0.253 and P = 0.0034) and between measured CLCR and the haematocrit (r2 = 0.219 and P = 0.0069). Proteinaemia and the volume of the liquid infusion did not influence the CLCR whatever the method used and mechanical ventilation did not influence the measured or estimated CLCR.
Due to the low individual predictability of the Cockcroft, Robert, Kirkpatrick and sMDRD approaches, no other correlation was studied with burn indices or clinical and biological parameters.
Discussion
In this group of burn patients with normal serum creatinine, the measured CLCR varied over a wide range and revealed unexpectedly low values (<80 ml min−1 1.73 m−2) in 25% of the patients and very high values (>120 ml min−1 1.73 m−2) in 42% of the cases.
These data therefore suggest that in burn patients during the hypermetabolic phase, serum creatinine is a less reliable tool to detect moderate changes in renal function than currently accepted. Serum creatinine alone may not be sufficient for the assessment of renal function as it was used recently in a population pharmacokinetic approach of ceftazidime and imipenem treatment in burn patients [11, 12]. The most plausible explanation for the low sensitivity of serum creatinine for the detection of renal insufficiency in critically ill patients is the depressed production of creatinine, as was suggested by the approximately 50% decrease in 24 h urinary creatinine excretion in burn patients with a measured CLCR <80 ml min−1 1.73 m−2. The reduction in creatinine excretion in the urine can be related to a loss of muscle mass and to a decrease in creatinine secretion. The low levels of creatinine excretion in our patients who had a CLCR <80 ml min−1 1.73 m−2 suggested that muscle loss had already occurred to a considerable degree, possibly even caused by catabolism preceding the day of the creatinine assay. Two other factors may have contributed further to the relatively low serum creatinine concentrations in proportion to the assessed renal function. The dietary intake of creatine was negligible as all the patients received enteral or parenteral nutrition. In addition, creatine is also produced by the liver, and disturbed liver metabolism is often present in burn patients. In addition, in hypovolaemic and dehydrated patients, the tubular creatinine secretion can be decreased [39]. This phenomenon could explain the negative relationship between the measured CLCR and the serum haemoglobin and between the measured CLCR and the haematocrit, because the increase of these two parameters can reveal dehydration. It must be noted that our patients were euvolaemic and without any sign of dehydration. This phenomenon also explains the positive linear relationship between the urinary creatinine excretion and the volume of infused liquids. In addition, interference in the analysis of biological parameters may give falsely elevated creatinaemia [18]. In this study, our method of SCR measurement and calibration reduced this interference. Although interference due to cephalosporin has been described when the creatininaemia was measured by using the Jaffe method [40], no changes in this parameter were observed during the overall period of the study. Finally, the serum creatinine may be falsely low due the increase in the volume of distribution in oedematous patients.
There is a certain analogy between our findings and those obtained by Hoste et al. in 28 critically ill patients [10]. The question of the validity of the equations for the assessment of renal function remains for these burn patients. To our knowledge, our study is the only one that has tested the Kirkpatrick and the sMDRD equation in burn patients without renal disease compared with the actual GFR obtained by measuring CLCR. In clinical practice, creatinine clearance calculated by the Cockcroft formula still remains the most useful measure of glomerular clearance, except in certain cases such as liver insufficiency [19]. Robert et al. showed that in critically ill patients, the incorporation of a corrected serum creatinine value and the use of ideal body weight in the Cockcroft-Gault equation led to a better prediction of the GFR [23]. Kirkpatrick et al. [24–26] have proposed another correction factor which provides a greater precision to the estimated clearance. However the different authors did not analyze burn patients. Lin et al. [41], on the basis of a comparison of their findings with those in the published literature, showed that the sMDRD equations perform much more poorly in subjects without kidney disease than in those with chronic kidney disease. This is not surprising since the sMDRD equation was derived from a study where the participants were selected as having moderate to severe renal failure (the measured I125 iothalamate mean GFR was 39.8 ml min−1 1.73 m−2) [15]. In spite of this, drug development and therapeutic drug monitoring is normally always based on these parameters. In burn patients, the drug monitoring must take into account the fact that the GFR is increased compared with other intensive care patients [2–4].
The validation of new methods of measurement or evaluation for a bio-clinical parameter is often based on a linear relationship. This is usually used to prove a strong correlation between the tested methods and the reference one. The very high CLCR reported in our burn patients probably skewed the linear regression between measured and estimated CLCR by over-weighting. Moreover, our study highlighted the limits of linear regression analysis. In spite of a significant relationship between the measured CLCR and the commonly used prediction equations for the assessment of glomerular function, these calculations did not provide an accurate individual predictability as shown by the residuals' plots, the calculated bias, precision and confidence intervals. These last indices are used to compare the population estimates with the individual measured values. Individual results are subject to interindividual variability which is not taken into account in population models whatever the covered field (pharmacokinetics, biology, anthropometry…).
The studied indices of predictability provide a more accurate conclusion about the validity of the population model, especially for extreme results. As the variability is very important in burn population due to the high proportion of patients with an increased GFR this approach is required. For example with the Cockcroft formula, since the slope (0.59) of the correlation between the Cockcroft calculation and actual creatinine clearance is significantly lower than 1, the higher the GFR, the more dramatically the Cockcroft clearance is an underestimate. These observations in burn patients with a high GFR complete data from the literature. For instance, in a study including 19 burnt men, Lott et al. [42] compared several GFR evaluation methods and concluded that the Cockcroft & Gault formula is useful in spite of a systematic overestimation of GFR when the CLCR is lower than 60 ml min−1. In that study, the statistical approach did not provide any bias and confidence intervals but our results are in agreement with the over-estimation for the lowest clearances. Loirat et al. [2] reported a significant relationship between the measured CLCR and the GFR evaluation by using inulin or iothalamate clearances.
The increase in the CLCR observed in our study corresponds to data in the literature [2, 43]. To explain this increasing GFR, several assumptions were successively invoked: increase in cardiac output contemporary with hyper-catabolism, hypoproteinaemia, and hypervolaemia [44]. In our population, no correlation was found between the CLCR and energy expenditure estimated according to Curreri et al. [45], or the volume of infused liquids. These data are not able to respond to the question of the impact of catabolic response to stress or hypervolaemia on the GFR. The influence of hypoproteinaemia cannot be seen as a factor since all the patients included in our study had a proteinaemia lower than or equal to 50 g l−1. In one case, Sosa et al. [46] have simulated the loss of creatinine by the burn injuries. However, the lack of a relationship between CLCR and the extent or the depth of the burn process seen in our study mitigates against this hypothesis. The lack of correlation between the CLCR and burn indices (burn areas, infused volume) except age was also demonstrated by Loirat et al. [2]. In their study, the highest CLCR was observed in younger patients (25 ± 10 years) and we report the same phenomenon. In our population, 13 patients were younger than 40 years and had a mean CLCR of 168.7± 34.9 ml min−1 1.73 m−2 (vs. 90.6 ± 38.1 ml min−1 1.73 m−2 in the older patients). The decrease in the rate of glomerular filtration, the involution of nephronic units and the reduction of renal blood flow explain the high frequency of renal function deficiency in elderly patients. It was observed that glomerular ageing was correlated with patient age in only two thirds of the patients, and this phenomenon accounted for the inaccuracy of the CLCR estimated by formulae [47]. This decrease in the clearance was also observed in 13 patients included in our study: they were older than 65 years and presented with a CLCR of 72.3 ± 25.6 ml min−1 1.73 m−2. In our burn population, the absence of an effect of mechanical ventilation with Positive End Expiratory Pressure (PEEP) on the CLCR is not consistent with the literature data [48] but could be explained by the euvolaemia of our patients. Moreover, our results highlighted that the classical indices used to assess the severity of burn injuries do not furnish any significant information on the GFR in these patients.
A highly predictive model for GFR calculation would be very useful for the clinician. In burn patients with normal GFR, a model with an acceptable predictability such as those of Kirkpatrick or Cockcroft may be used and results are very easily obtained. However, in clinical practice in burn units, it is not possible to determine a priori if a patient with normal serum creatinine has a high GFR. Therefore, the underestimation of GFR calculated by the studied equations could lead to an underestimated dosing requirement for many drugs. This point becomes critical for antibiotics used against resistant strains and for which therapeutic drug monitoring is not readily available [49]. In such circumstances, creatinine clearance should be measured formally. This is not very difficult in practice.
In conclusion, while creatinaemia is an easy marker to use, which may explain the frequent reference to it, in a cohort of burn patients studied during the hypermetabolic phase of burn injury, and with normal serum creatinine values, serum creatinine proved a very insensitive screening test for the early detection of renal dysfunction. The most probable explanation is that a decreased creatinine load resulted in a lower serum creatinine for a given GFR. The Cockcroft–Gault, the Robert, the Kirkpatrick and the sMDRD equations calculate the CLCR on the basis of parameters such as sex, weight and age. For the different values of CLCR, only the age varies. Thus, significant over- and under-estimations of real values by mathematical calculation may occur. Therefore, a creatinine clearance through 24 h urine collection should be used to detect modifications of the renal function in burn patients with normal serum creatinine concentrations. A new formula must be defined to accurately estimate glomerular filtration in the burn population.
References
- 1.Ronco C, Bellomo R. Prevention of acute renal failure in the critically ill. Nephron Clin Pract. 2003;93:C13–20. doi: 10.1159/000066646. [DOI] [PubMed] [Google Scholar]
- 2.Loirat P, Rohan J, Baillet A, Beaufils F, David R, Chapman A. Increased glomerular filtration rate in patients with major burns and its effect on the pharmacokinetics of tobramycin. N Engl J Med. 1978;299:915–9. doi: 10.1056/NEJM197810262991703. [DOI] [PubMed] [Google Scholar]
- 3.Ecklund J, Grandberg P, Liljedahl S. Studies on renal function in burns. Acta Chirurgica Scand. 1979;136:627–40. [PubMed] [Google Scholar]
- 4.Weinbren MJ. Pharmacokinetics of antibiotics in burns patients. J Antimicrob Chemother. 2001;47:720. doi: 10.1093/oxfordjournals.jac.a002690. [DOI] [PubMed] [Google Scholar]
- 5.Lipman J, Wallis SC, Boots RJ. Cefepime vs. cefpirome: the importance of creatinine clearance. Anesth Analg. 2003;97:1149–54. doi: 10.1213/01.ANE.0000077077.54084.B0. [DOI] [PubMed] [Google Scholar]
- 6.Highet VS, Forrest A, Ballow CH, Schentag JJ. Antibiotic dosing issues in lower respiratory tract infection: population-derived area under inhibitory curve is predictive of efficacy. J Antimicrob Chemother. 1999;43(Suppl A):55–63. doi: 10.1093/jac/43.suppl_1.55. [DOI] [PubMed] [Google Scholar]
- 7.Kashuba AD, Ballow CH, Forrest A. Development and evaluation of a Bayesian pharmacokinetic estimator and optimal, sparse sampling strategies for ceftazidime. Antimicrob Agents Chemother. 1996;40:1860–5. doi: 10.1128/aac.40.8.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinks AA, Mouton JW, Touw DJ, Heijerman HG, Danhof M, Bakker W. Population pharmacokinetics of ceftazidime in cystic fibrosis patients analyzed by using a nonparametric algorithm and optimal sampling strategy. Antimicrob Agents Chemother. 1996;40:1091–7. doi: 10.1128/aac.40.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kellum JA, Levin N, Bouman C, Lameire N. Developing a consensus classification system for acute renal failure. Curr Opin Crit Care. 2002;8:509–14. doi: 10.1097/00075198-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Hoste EA, Damen J, Vanholder RC, Lameire NH, Delanghe JR, Van den Hauwe K, Colardyn FA. Assessment of renal function in recently admitted critically ill patients with normal serum creatinine. Nephrol Dial Transplant. 2005;20:747–53. doi: 10.1093/ndt/gfh707. [DOI] [PubMed] [Google Scholar]
- 11.Dailly E, Pannier M, Jolliet P, Bourin M. Population pharmacokinetics of ceftazidime in burn patients. Br J Clin Pharmacol. 2003;56:629–34. doi: 10.1046/j.1365-2125.2003.01915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dailly E, Kergueris MF, Pannier M, Jolliet P, Bourin M. Population pharmacokinetics of imipenem in burn patients. Fundam Clin Pharmacol. 2003;17:645–50. doi: 10.1046/j.1472-8206.2003.00190.x. [DOI] [PubMed] [Google Scholar]
- 13.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–53. [PubMed] [Google Scholar]
- 14.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 16.Shemesh O, Golbetz H, Kriss JP, Myers BD. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985;28:830–8. doi: 10.1038/ki.1985.205. [DOI] [PubMed] [Google Scholar]
- 17.Bauer JH, Brooks CS, Burch RN. Clinical appraisal of creatinine clearance as a measurement of glomerular filtration rate. Am J Kidney Dis. 1982;2:337–46. doi: 10.1016/s0272-6386(82)80091-7. [DOI] [PubMed] [Google Scholar]
- 18.Kellen M, Aronson S, Roizen MF, Barnard J, Thisted RA. Predictive and diagnostic tests of renal failure: a review. Anesth Analg. 1994;78:134–42. doi: 10.1213/00000539-199401000-00022. [DOI] [PubMed] [Google Scholar]
- 19.Papadakis MA, Arieff AI. Unpredictability of clinical evaluation of renal function in cirrhosis. Prospective study. Am J Med. 1987;82:945–52. doi: 10.1016/0002-9343(87)90156-2. [DOI] [PubMed] [Google Scholar]
- 20.Aronoff GR, Berns JS, Brier ME. Dosing guidelines for adults. 4. Philadelphia: American College of Physicians-American Society of Internal Medicine; 1999. Drug prescribing in renal failure. [Google Scholar]
- 21.Kasiske BL, Keane WF. Laboratory assessment of renal disease: Clearance, urinalysis, and renal biopsy. In: Saunders J, editor. The Kidney. Philadelphia: Brenner and Rector; 2000. pp. 1129–70. [Google Scholar]
- 22.Drug Information Branch CfDEaR. Guidance for Industry: Pharmacokinetics in patients with impaired renal functionStudy design, data analysis, and impact on dosing and labeling. 1998.
- 23.Robert S, Zarowitz BJ, Peterson EL, Dumler F. Predictability of creatinine clearance estimates in critically ill patients. Crit Care Med. 1993;21:1487–95. doi: 10.1097/00003246-199310000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Kirkpatrick CM, Duffull SB, Begg EJ. Pharmacokinetics of gentamicin in 957 patients with varying renal function dosed once daily. Br J Clin Pharmacol. 1999;47:637–43. doi: 10.1046/j.1365-2125.1999.00938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duffull SB, Dooley MJ, Green B, Poole SG, Kirkpatrick CM. A standard weight descriptor for dose adjustment in the obese patient. Clin Pharmacokinet. 2004;43:1167–78. doi: 10.2165/00003088-200443150-00007. [DOI] [PubMed] [Google Scholar]
- 26.Matthews I, Kirkpatrick C, Holford N. Quantitative justification for target concentration intervention – parameter variability and predictive performance using population pharmacokinetic models for aminoglycosides. Br J Clin Pharmacol. 2004;58:8–19. doi: 10.1111/j.1365-2125.2004.02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kidney Disease Outcome Quality Initiative. K/DOQI clinical practice guidelines for chronic kidney disease. Evaluation, Classification, and Stratification. Am J Kidney Dis. 2002;2(2):S1–S246. [PubMed] [Google Scholar]
- 28.Agarwal R, Vasavada N, Chase SD. Liquid chromatography for iothalamate in biological samples. J Chromatogr B Anal Technol Biomed Life Sci. 2003;785:345–52. doi: 10.1016/s1570-0232(02)00960-1. [DOI] [PubMed] [Google Scholar]
- 29.Levey AS, Greene T, Kusek JW, Beck GJA. Simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11:155A. [Google Scholar]
- 30.Conil J, Saivin S, Georges B, Samii K, Houin G. Anesthesiologists ASo Annual Meeting. San Francisco, CA: 2003. Ceftazidime drug monitoring by population pharmacokinetic approach in burn patients. [Google Scholar]
- 31.Lund CC, Browder NC. The estimation of areas of burns. Surg Gynecol Obstet. 1944;79:352–8. [Google Scholar]
- 32.Stern M, Waisbren BA. Comparison of methods of predicting burn mortality. Scand J Plast Reconstr Surg. 1979;13:201–4. doi: 10.3109/02844317909013057. [DOI] [PubMed] [Google Scholar]
- 33.Sachs A, Watson J. Four years' experience at a specialised burns centre. The Mcindoe Burns Centre 1965–68. Lancet. 1969;1:718–21. doi: 10.1016/s0140-6736(69)92663-4. [DOI] [PubMed] [Google Scholar]
- 34.Tobiasen J, Hiebert JH, Edlich RF. Prediction of burn mortality. Surg Gynecol Obstet. 1982;154:711–4. [PubMed] [Google Scholar]
- 35.Devine B. Gentamicin therapy. Drug Intell Clin Pharm. 1974;8:650–5. [Google Scholar]
- 36.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 37.Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9:503–12. doi: 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]
- 38.Delanghe J, De Slypere JP, De Buyzere M, Robbrecht J, Wieme R, Vermeulen A. Normal reference values for creatine, creatinine, and carnitine are lower in vegetarians. Clin Chem. 1989;35:1802–3. [PubMed] [Google Scholar]
- 39.Sjostrom PA, Odlind BG, Wolgast M. Extensive tubular secretion and reabsorption of creatinine in humans. Scand J Urol Nephrol. 1988;22:129–31. doi: 10.1080/00365599.1988.11690398. [DOI] [PubMed] [Google Scholar]
- 40.Grotsch H, Hajdu P. Interference by the new antibiotic cefpirome and other cephalosporins in clinical laboratory tests, with special regard to the ‘Jaffe’ reaction. J Clin Chem Clin Biochem. 1987;25:49–52. [PubMed] [Google Scholar]
- 41.Lin J, Knight EL, Hogan ML, Singh AK. A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. J Am Soc Nephrol. 2003;14:2573–80. doi: 10.1097/01.asn.0000088721.98173.4b. [DOI] [PubMed] [Google Scholar]
- 42.Lott RS, Uden DL, Wargin WA, Strate RG, Zaske DE. Correlation of predicted versus measured creatinine clearance values in burn patients. Am J Hosp Pharm. 1978;35:717–20. [PubMed] [Google Scholar]
- 43.Bonate PL. Pathophysiology and pharmacokinetics following burn injury. Clin Pharmacokinet. 1990;18:118–30. doi: 10.2165/00003088-199018020-00003. [DOI] [PubMed] [Google Scholar]
- 44.Martyn J. Clinical pharmacology and drug therapy in the burned patient. Anesthesiology. 1986;65:67–75. doi: 10.1097/00000542-198607000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Curreri PW, Richmond D, Marvin J, Baxter CR. Dietary requirements of patients with major burns. J Am Diet Assoc. 1974;65:415–7. [PubMed] [Google Scholar]
- 46.Sosa JL, Ward CG, Hammond JS. The relationship of burn wound fluid to serum creatinine and creatinine clearance. J Burn Care Rehabil. 1992;13:437–42. doi: 10.1097/00004630-199207000-00009. [DOI] [PubMed] [Google Scholar]
- 47.O'Connell MB, Dwinell AM, Bannick-Mohrland SD. Predictive performance of equations to estimate creatinine clearance in hospitalized elderly patients. Ann Pharmacother. 1992;26:627–35. doi: 10.1177/106002809202600503. [DOI] [PubMed] [Google Scholar]
- 48.Annat G, Viale JP, Bui Xuan B, Hadj Aissa O, Benzoni D, Vincent M, Gharib C, Motin J. Effect of PEEP ventilation on renal function, plasma renin, aldosterone, neurophysins and urinary ADH, and prostaglandins. Anesthesiology. 1983;58:136–41. doi: 10.1097/00000542-198302000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Berkhout J, Visser LG, van den Broek PJ, van de Klundert JA, Mattie H. Clinical pharmacokinetics of cefamandole and ceftazidime administered by continuous intravenous infusion. Antimicrob Agents Chemother. 2003;47:1862–6. doi: 10.1128/AAC.47.6.1862-1866.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]