Abstract
Aims
To evaluate the pharmacokinetics of R667, a novel emphysema agent, in patients with moderate to severe emphysema.
Methods
Multiple-dose pharmacokinetics of R667 and its metabolites in emphysematous patients were studied in a multicentre, randomized, single-blind, and placebo-controlled trial. Four groups of 10 patients per group received placebo, 0.2, 0.5, or 1 mg R667 once a day for 14–16 days. On day 14 (±1), blood samples were taken at predose and 1, 2, 3, 4, 6, 8, 10, 12, 16 and 24 h after dosing.
Results
Pharmacokinetic analysis of the data indicated that the mean steady-state Cmax and AUC(0,τ) of R667 appeared to be dose proportional over the dose range of 0.2–1 mg when administered to emphysematous patients. Mean metabolite to R667 ratios for Cmax or AUC(0,τ) were, in general, similar across the dose range of 0.2–1 mg.
Conclusions
The pharmacokinetics of R667 and its metabolites appeared to be similar for patients with emphysema and healthy volunteers. Multiple-dose administration of 0.2–1 mg of R667 for up to 16 days was well tolerated in patients with emphysema.
Keywords: emphysema, pharmacokinetics, R667
Introduction
The prevalence of chronic obstructive pulmonary disease (COPD) is estimated at between 2 and 10% in Europe and North America [1, 2]. COPD is also the fifth and fourth leading cause of death in Europeans and Americans, respectively [1, 3]. COPD may have several components including chronic bronchitis, chronic obstructive bronchitis, emphysema, or combinations of these conditions, and is characterized by a gradual loss of lung function over time. Emphysema represents one of the major pathophysiological abnormalities of COPD and is characterized by destruction of alveolar walls, enlargement of alveolar airspaces, and loss of lung elastic recoil, all resulting in a loss of the effective area for gas exchange. As a result, patients with emphysema increasingly lose their ability to breathe [4]. Emphysema occurs with a much higher prevalence in individuals ≥65 years old as compared with those between 45 and 64 years old. The prevalence is also higher in men than women, and in Caucasians than Blacks. Emphysema affects over two million Americans, costs more than $2.5 billion in annual health care expenses and contributes to 100 000 deaths in the U.S. each year [3].
Smoking is the leading cause of emphysema and COPD. Although smoking cessation slows the rate of lung damage and the decline in forced expiratory volume in 1 second (FEV1) toward levels observed in nonsmokers, existing damage to alveolar structures persists with no evidence that it can repair itself [5, 6]. Currently there are no medical treatments that assist in restoring lung structure and function. Instead, available drug therapies consist of the use of inhaled β2-adrenergic receptor agonists, anticholinergics, theophylline, and inhaled and systemic glucocorticoids, all of which are designed to reduce associated conditions such as bronchospasm and inflammation of the airways [5].
R667 is a novel retinoid compound being developed to address this unmet medical need for treatments that directly address the pathologic loss of alveolar structures in emphysema (Figure 1). It has been evaluated in animal models of emphysema, where it has been shown to stimulate the repair of alveolar damage caused by both chronic exposure to tobacco smoke and elastase [7]. In vitro studies with human microsomal preparations demonstrated the presence of five metabolites: 6, 7-dihydroxy (Ml), 6-dihydroxy (M2), 7-dihydroxy (M3), 6-oxo (M4a), and 7-oxo (M4b). This metabolic pattern was later confirmed following multiple oral dosing in healthy subjects. The metabolite Ml was present in low concentrations in human plasma and was not measured in this current study. Using an in vitro transactivation assay, the activity of metabolites M2, M3, M4a, and M4b represented 2%, 7%, 23%, and 12% of the parent drug R667, respectively.
Figure 1.
Molecular structure of R667
Several cytochrome P450 (CYP) enzymes (CYP3A4, CYP2C8, and CYP2C19) oxidized R667 to its metabolites. However, CYP3A4 was the major isoform responsible for in vitro transformation of R667 with a relatively high Km value of 59 µm. The IC50 values for R667 and its metabolites against human CYP3A4 were very high (>100 µm). The biotransformation of R667 was competitively inhibited by ketoconazole, midazolam, and erythromycin in human hepatic microsomes with inhibitory constants of 0.2 µm, 38 µm, and 65 µm, respectively. These in vitro findings suggest that the pharmacokinetics of R667 could potentially be influenced by strong inducers or inhibitors of CYP3A4. Based on the clinically efficacious concentrations of these drugs, R667 is unlikely to be inhibited by midazolam, but may be inhibited by ketoconazole and erythromycin [8].
R667 is predominantly metabolized by CYP3A4 in the in vitro setting (90%). Animal studies have been carried out using [14C]-labelled R667 to determine the excretion balance of total radioactivity and the metabolic pattern of R667 in various fluids. Cold assay techniques have established that the major metabolites and routes of excretion of R667 are qualitatively similar in humans and animals. The present study provided further quantitative information on mass balance and the routes of elimination of R667 in patients with emphysema, the target population for this drug development.
Methods
Study design
Multiple-dose pharmacokinetics of R667 and its metabolites were studied in a multicentre, randomized, single-blind, and placebo-controlled trial in emphysematous patients. Four groups of 10 patients per group received either placebo, 0.2, 0.5, or 1 mg of R667 once daily for 14–16 days. Study drug and the placebo were formulated as matching 0.1 mg and 0.5 mg soft-gelatin capsules and two capsules were taken orally within 30 min after the morning meal. On day 14 (±1), blood samples were taken at predose and 1, 2, 3, 4, 6, 8, 10, 12, 16 and 24 h after dosing. The total volume of blood loss during the study, for measurement of parent compound and metabolites, was approximately 40 ml.
The study was approved by the Institutional Review Board associated with each clinical site and conducted according to the principles outlined in the ‘Guideline for Good Clinical Practice’ ICH Tripartite Guideline (January 1997) which has its basis in the principles of the ‘Declaration of Helsinki’ (1996) and the principles of ‘Good Clinical Practice’ as outlined in the current version of 21 CFR, subchapter D, parts 312, 50, and 56.
Subjects
Patients aged 40 years or older, in a stable state of health with moderate to severe emphysema (FEV1≤70% predicted but ≥40% predicted; diffusing capacity of the lung for carbon monoxide (DLco) <80% predicted), at least grade 1 dyspnea on the Modified Medical Research Council Scale, and with emphysematous lung damage visually confirmed on either conventional or high resolution chest CT scan, were eligible for the study. Only subjects that provided written informed consent and were willing to comply with study procedures were included. Patients were excluded from the study if they were women of childbearing potential, men with partners of childbearing potential who would not use barrier contraception during the study, or had a history of psychiatric disorders. Patients with any solitary nodule in the lung requiring further medical intervention and any medical history or active conditions likely to compromise their safety were excluded. Patients on maintenance therapy with corticosteroids, those with concomitant medications that were CYP3A4 inhibitors or inducers, or with a history of alcohol, drug dependency or substance abuse, were also excluded from the study. Hypertriglyceridaemia ≥300 mg dl−1 was also an exclusion factor.
Bioanalytical methods
The concentrations of R667 and four major metabolites M2, M3, M4a, and M4b in plasma were determined by Advion BioSciences, Inc, Ithaca, New York, using a selective, accurate, and reproducible liquid chromatography tandem mass spectrometry (LC/MS/MS) analytical method [9]. Isotopic internal standards were used for all analytes. Analytes were extracted from plasma by solid phase extraction (SPE) with a Waters Oasis MAX 96-well block. Two different chromatographic separations were required to separate the isomeric hydroxy and keto metabolites from their respective isomer. The analytes were detected in the high performance liquid chromatography (HPLC) column effluents by turbo ion spray LC/MS/MS with selected reaction monitoring (SRM) in the positive ion mode. The lower limit of quantification was 0.01 ng ml−1 for all analytes using a 0.5 ml plasma aliquot for analysis.
Pharmacokinetic analysis
Pharmacokinetic parameters for R667 and its metabolites were estimated by the noncompartmental method using WinNonlin Professional 4.1.a. The actual sampling times recorded on the CRF were used to calculate individual pharmacokinetic parameters (Cmax, tmax, AUC(0,τ), and CL/F). Nominal times were used for graphical presentation and descriptive summation (numbers of observation (n), arithmetic mean, standard deviation (SD), minimum (min), maximum (max), and CV%). Prior to estimation of pharmacokinetic parameters, plasma concentrations that were below the limit of quantification for the assay (BLQ) were assigned a value of zero if they occurred prior to the first measurable concentration in the profile and were treated as missing and eliminated from the analysis if they occurred during the terminal phase
Results
Subjects
Across the four treatment groups (40 patients) the majority of patients were Caucasian (80–90%). For the four treatment groups, the mean age ranged from 62 to 65 years, the mean body mass index (BMI) from 25 to 29 kg m−2, and the mean weight from 72 to 82 kg (Table 1). Half of the patients were current smokers and 48% of the patients were male. On average, pulmonary function tests demonstrated the presence of moderate to severe obstructive lung disease and a moderate impairment in gas exchange, consistent with moderate to severe emphysema. Their history of tobacco use and pulmonary function characteristics are summarized in Table 1.
Table 1.
Smoking and pulmonary function characteristics of the 40 emphysematous patients enrolled into the study
| Placebo (n = 10) | R667 0.2 mg (n = 10) | |||||
|---|---|---|---|---|---|---|
| Parameter (Unit) | Mean ± SD | Min | Max | Mean ± SD | Min | Max |
| Current smoker/Past smoker | 30%/70% | 70%/30% | ||||
| Age (years) | 62 ± 8 | 46 | 73 | 64 ± 10 | 52 | 83 |
| Weight (kg) | 82 ± 24 | 51 | 121 | 73 ± 19 | 48 | 116 |
| BMI (kg m−2) | 29 ± 7 | 20 | 39 | 25 ± 5 | 19 | 37 |
| Smoking (pack-years)a | 51 ± 36 | 15 | 133 | 78 ± 46 | 15 | 160 |
| FVC (l) | 2.5 ± 0.4 | 1.7 | 3.3 | 2.8 ± 0.6 | 1.6 | 3.8 |
| FEV1 (l) | 1.5 ± 0.4 | 0.9 | 2.2 | 1.6 ± 0.5 | 0.9 | 2.3 |
| FEV1 (% predicted)b | 54 ± 9 | 40 | 70 | 58 ± 11 | 43 | 80 |
| DLco (% predicted)b | 67 ± 14 | 47 | 76 | 48 ± 19 | 34 | 83 |
| R667 0.5 mg (n = 10) | R667 1.0 mg (n = 10) | |||||
| Current smoker/Past smoker | 50%/50% | 50%/50% | ||||
| Age (years) | 65 ± 11 | 48 | 85 | 62 ± 8 | 50 | 78 |
| Weight (kg) | 72 ± 14 | 54 | 96 | 75 ± 13 | 56 | 98 |
| BMI (kg m−2) | 25 ± 3 | 20 | 29 | 25 ± 4 | 21 | 35 |
| Smoking (pack-years)a | 55 ± 22 | 19 | 92 | 51 ± 23 | 20 | 86 |
| FVC (l) | 3.0 ± 1.0 | 1.9 | 5.2 | 3.5 ± 0.8 | 2.4 | 4.5 |
| FEV1 (l) | 1.5 ± 0.3 | 1.2 | 2.0 | 1.7 ± 0.5 | 1.0 | 2.5 |
| FEV1 (% predicted)b | 57 ± 18 | 40 | 97 | 53 ± 12 | 38 | 73 |
| DLco (% predicted)b | 41 ± 12 | 28 | 53 | 50 ± 15 | 24 | 69 |
pack-years = number of cigarette packs smoked per day × number of years of smoking.
percentage predicted = measured value as a percentage of the normal predicted value for each individual based on their sex, age and height. FVC = postbronchodilator forced vital capacity. FEV1 = postbronchodilator forced expiratory volume in 1 second. DLco = postbronchodilator diffusing capacity of the lung for carbon monoxide.
Bioanalytical analysis
The precision of the assay for plasma samples as measured by the coefficient of variation (CV) from the analysis of the quality control (QC) samples was <11.1% for M4b and <7.2% for R667 and other metabolites. The accuracy as measured by relative error (RE) from the analysis of the QC samples was within 94–105% for all analytes. The analytes were stable in plasma under all analytical and storage conditions as well as after processing.
Pharmacokinetics
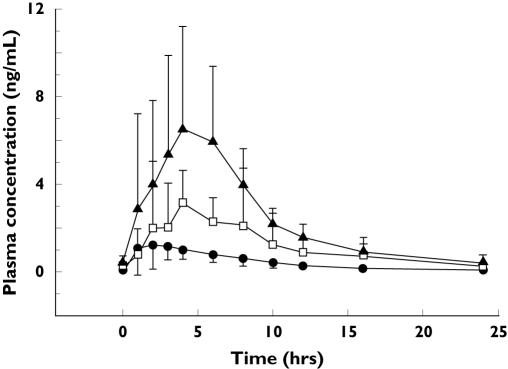
The plasma R667 concentration-time profile and the principal pharmacokinetic parameters after multiple-dose of R667 (0.2–1 mg) once daily are summarized in Figure 2 and Table 2.
Figure 2.
Mean plasma concentration ± SD vs. time profiles for R667 on day 14 (±1) following once a day oral dosing with 0.2 (•), 0.5 (□) or 1 mg (▴) of R667
Table 2.
Mean pharmacokinetics of R667 following multiple-dose oral administration of 0.2, 0.5, or 1 mg of R667 to emphysematous patients
| Parameter (units) | Dose (mg) | n | Mean ± SD | Min | Max | CV (%) |
|---|---|---|---|---|---|---|
| Cmax (ng ml−1) | 0.2 | 9 | 1.80 ± 1.10 | 0.81 | 4.04 | 61.1 |
| 0.5 | 10 | 4.84 ± 2.57 | 2.91 | 9.72 | 53.1 | |
| 1 | 10 | 10.3 ± 3.59 | 5.34 | 15.6 | 34.9 | |
| tmax (h) | 0.2 | 9 | 3.92 ± 2.34 | 1.07 | 8.03 | 59.6 |
| 0.5 | 10 | 4.09 ± 2.05 | 1.80 | 8.00 | 50.2 | |
| 1 | 10 | 4.02 ± 2.41 | 1.00 | 8.00 | 59.9 | |
| AUC(0,τ) (ng ml−1 h) | 0.2 | 9 | 10.3 ± 3.23 | 5.81 | 15.0 | 31.3 |
| 0.5 | 10 | 29.0 ± 12.4 | 15.8 | 51.6 | 42.7 | |
| 1 | 10 | 57.7 ± 12.6 | 35.4 | 78.7 | 21.8 | |
| CL/F (l h−1) | 0.2 | 9 | 21.3 ± 7.46 | 13.3 | 34.4 | 35.0 |
| 0.5 | 10 | 20.4 ± 8.40 | 9.70 | 31.7 | 41.3 | |
| 1 | 10 | 18.2 ± 4.40 | 12.7 | 28.3 | 24.2 |
SD. = standard deviation. CV = coefficient of variation.
Pharmacokinetic analysis of the data indicated that the mean steady-state Cmax and AUC(0,τ) of R667 appeared to be dose proportional over the dose range of 0.2–1 mg when administered to emphysematous patients (Table 2).
Four major metabolites, M2, M3, M4a, and M4b, were observed in plasma after administration of multiple doses of R667 to 29 patients with emphysema. Mean metabolite to R667 ratios for Cmax and AUC(0,τ) are presented in Tables 3 and 4. In general, mean metabolite to R667 ratios for Cmax or AUC(0,τ) appeared to be similar across the dose range of 0.2–1 mg. The rank order for R667 and its metabolites after steady state administration of 0.2–1 mg R667 for 14–16 days was as follows:
Table 3.
Cmax ratios of metabolites to R667 following multiple-dose oral administration of 0.2, 0.5, or 1 mg of R667 to emphysematous patients
| Cmax ratio | R667 (mg) | n | Mean ± SD | Min | Max | CV (%) |
|---|---|---|---|---|---|---|
| M2:R667a | 0.2 | 9 | 0.206 ± 0.0699 | 0.108 | 0.322 | 33.9 |
| 0.5 | 10 | 0.239 ± 0.0769 | 0.143 | 0.345 | 32.2 | |
| 1 | 10 | 0.212 ± 0.0632 | 0.124 | 0.338 | 29.9 | |
| M3:R667b | 0.2 | 9 | 0.551 ± 0.278 | 0.217 | 0.992 | 50.4 |
| 0.5 | 10 | 0.562 ± 0.254 | 0.213 | 1.00 | 45.2 | |
| 1 | 10 | 0.475 ± 0.256 | 0.271 | 1.06 | 53.8 | |
| M4a:R667c | 0.2 | 9 | 0.167 ± 0.135 | 0.0600 | 0.469 | 80.7 |
| 0.5 | 10 | 0.218 ± 0.111 | 0.0590 | 0.413 | 50.8 | |
| 1 | 10 | 0.184 ± 0.0822 | 0.107 | 0.306 | 44.6 | |
| M4b:R667d | 0.2 | 9 | 0.241 ± 0.135 | 0.0810 | 0.42 | 56.1 |
| 0.5 | 10 | 0.337 ± 0.162 | 0.0470 | 0.559 | 48.0 | |
| 1 | 10 | 0.303 ± 0.184 | 0.131 | 0.610 | 60.7 |
Calculated metabolite M2:R667 ratio.
Calculated metabolite M3:R667 ratio.
Calculated metabolite M4a:R667 ratio.
Calculated metabolite M4b:R667 ratio.
Table 4.
AUC(0,τ) ratios of metabolites to R667 following multiple-dose oral administration of 0.2, 0.5, or 1 mg of R667 to emphysematous patients
| AUC(0,τ) ratio | R667 (mg) | n | Mean ± SD | Min | Max | CV (%) |
|---|---|---|---|---|---|---|
| M2:R667a | 0.2 | 9 | 0.262 ± 0.106 | 0.139 | 0.412 | 40.7 |
| 0.5 | 10 | 0.279 ± 0.0959 | 0.154 | 0.418 | 34.4 | |
| 1 | 10 | 0.265 ± 0.0678 | 0.160 | 0.372 | 25.6 | |
| M3:R667b | 0.2 | 9 | 0.717 ± 0.329 | 0.321 | 1.23 | 45.9 |
| 0.5 | 10 | 0.769 ± 0.333 | 0.283 | 1.35 | 43.4 | |
| 1 | 10 | 0.656 ± 0.320 | 0.335 | 1.19 | 48.8 | |
| M4a:R667c | 0.2 | 9 | 0.317 ± 0.243 | 0.100 | 0.822 | 76.6 |
| 0.5 | 10 | 0.419 ± 0.217 | 0.0890 | 0.719 | 51.8 | |
| 1 | 10 | 0.391 ± 0.188 | 0.234 | 0.763 | 48.0 | |
| M4b:R667d | 0.2 | 9 | 0.543 ± 0.308 | 0.164 | 1.02 | 56.8 |
| 0.5 | 10 | 0.783 ± 0.366 | 0.0850 | 1.26 | 46.8 | |
| 1 | 10 | 0.724 ± 0.416 | 0.277 | 1.50 | 57.4 |
Calculated metabolite M2 to R667 ratio.
Calculated metabolite M3 to R667 ratio.
Calculated metabolite M4a to R667 ratio.
Calculated metabolite M4b to R667 ratio.
Cmax: R667 > M3 > M4b > M2 > M4a
AUC(0,τ): R667 > M3 > M4b > M4a > M2.
Safety and tolerability
Safety parameters including adverse events, laboratory abnormalities, and vital signs were monitored during study treatment and at the post-treatment follow-up 24–34 days after the last dose. The enrolled patients tolerated the study treatment well and no significant safety concerns were observed. The incidence of adverse events in the 1 mg R667 arm (3/10) was not higher than that in the placebo arm (5/10). Each adverse event was seen in a single patient in any treatment arm. All adverse events were mild or moderate in intensity, and were considered unrelated to study treatment by the investigator, except for an event of constipation that occurred in the 0.5 mg R667 arm, which was considered remotely related to study drug. No treatment withdrawals for adverse events and no deaths occurred in the trial. No serious adverse events were reported in R667-treated patients. Some marked laboratory abnormalities were reported. Most of these marked laboratory abnormalities occurred on a single occasion in a single patient except for low lymphocyte count (0.2 mg R667 group) and the partial thromboplastin time (1.0 mg R667 group) each of which occurred in two patients. No abnormal vital signs were reported.
Discussion
Until recently, the pathologic destruction of lung tissue that occurs in emphysema was considered a progressive and irreversible process. However, this concept has been challenged by several animal models demonstrating that all-trans retinoic acid can promote alveolar septation and reverse elastase-induced lung damage in adult rats and mice [10–12]. Unfortunately, the long-term administration of all-trans retinoic to humans is complicated by the autoinduction of metabolizing enzymes that lead to rapid clearance of the drug and a 60–70% reduction in the average AUC(0,τ) over time [13]. Similar effects were observed when all-trans retinoic acid was used in a pilot study to treat emphysematous patients, significantly limiting drug exposure [14]. R667 is a novel retinoid derivative that also demonstrates the capacity torepair emphysematous lung damage in animal models [7]. This is the first report of its metabolism and pharmacokinetics in moderate to severe emphysematous patients.
The mean steady-state Cmax and AUC(0,τ) obtained after multiple oral dosing with 1 mg of R667 in our study population with moderate to severe emphysema (10.3 ng ml−1 and 57.7 ng ml−1 h, respectively) were comparable with those observed in healthy volunteers (8.32–10.5 ng ml−1 and 41.1–52.7 ng ml−1 h) [15–17]. Therefore, preliminary evidence would suggest that the pharmacokinetics of R667 in emphysematous patients and healthy volunteers appears to be similar. The mean metabolite to R667 ratios for Cmax and AUC(0,τ) in healthy volunteers were 0.23–0.31, 0.49–0.68, 0.22–0.30, and 0.36–0.47 for metabolite M2, M3, M4a, and M4b, respectively [15–17]. Compared with the values in Table 4, the mean metabolite to R667 ratios for AUC(0,τ) in emphysematous patients were also similar to those in healthy volunteers. The mean steady-state Cmax and AUC(0,τ) for R667 appeared to be dose proportional over the range of 0.2–1 mg and there has been no evidence of the self-induction of metabolism with repeated administration in either animals or humans.
In conclusion, these studies suggest that the pharmacokinetics of R667 and its metabolites appears to be similar for patients with emphysema and healthy volunteers. Multiple-dose administration of 0.2–1 mg of R667 for up to 16 days was well tolerated in patients with emphysema.
Acknowledgments
We thank the following investigators and their staff for recruitment and study support: Dr Charles Fogarty (Spartanburg, SC), Dr Henry D. Covelli (Coeur d Alene, ID), Dr Mark Buchfuhrer (Downey, CA), Dr Steven Kelsen (Philadelphia, PA), Dr Henry Gong Jr. (Downey, CA), Dr Joe Ramsdell (San Diego, CA), and Dr Luis Angles (Shawnee Mission, KS).
References
- 1.Halbert RJ, Isonaka 5 George D, Iqbal A. Interpreting COPD prevalence estimates: What is the true burden of disease? Chest. 2003;123:1684–92. doi: 10.1378/chest.123.5.1684. [DOI] [PubMed] [Google Scholar]
- 2.LoddenKemper R, Gibson GJ, Sibille Y. Chronic Obstructive Pulmonary Disease in European Lung White Book, the First Comprehensive Survey on Respiratory Health in Europe. 1. European Respiratory Society, European Lung Foundation; 2003. pp. 34–43. [Google Scholar]
- 3.Chronic Obstructive Pulmonary Disease Data Fact Sheet. NIH Publication No03–5228. U.S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute; March 2003. [Google Scholar]
- 4.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. GOLD Scientific Committee. Global strategy for the diagnosis, management, & prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 5.Celli BR, MacNee W. ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD. a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 6.Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166:675–9. doi: 10.1164/rccm.2112096. [DOI] [PubMed] [Google Scholar]
- 7.Bailey-Healy I, Ofulue AF, Belloni PN. Tissue repair in a rat emphysema model with a RARã agonist treatment. Am J Respir Crit Care Med. 2005;171:A99. [Google Scholar]
- 8.Liu HF, Cheng PS, Mau C-I, Weller PE, Tarnowski T. In vitro biotransformation of R667 in humans: identification of isoforms of cytochrome P-450 responsible for metabolism and inhibition kinetics of ketoconazole, midazolam, and erythromycin in human hepatic microsomes. Drug Metab Rev. 2002;34(Suppl 1):206. [Google Scholar]
- 9.Hoffmann BT, Kolis SJ, Kiang H, Mann M, Mulvana D, Bansal S. Quantification of a new drug candidate and four metabolites in human plasma by Turbo Ion Spray LC/MS/MS. AAPS J. 2004;6:W5027. [Google Scholar]
- 10.Massaro GD, Massaro D. Retinoic acid treatment abrogates elastase-induced pulmonary emphysema in rats. Nature Med. 1997;3:675–7. doi: 10.1038/nm0697-675. [DOI] [PubMed] [Google Scholar]
- 11.Belloni PN, Garvin L, Mao CP, Bailey-Healy I, Leaffer D. Effects of all-trans-retinoic acid in promoting alveolar repair. Chest. 2000;117:235S–241S. doi: 10.1378/chest.117.5_suppl_1.235s. [DOI] [PubMed] [Google Scholar]
- 12.Ishizawa K, Kubo H, Yamada M, Ishizawa K, Numasaki M, Ueda S, Suzuki T, Sasaki H. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett. 2004;556:249–52. doi: 10.1016/s0014-5793(03)01399-1. [DOI] [PubMed] [Google Scholar]
- 13.Muindi J, Frankel SR, Miller WH, Jr, Jakubowski A, Scheinberg DA, Young CW, Dmitrovsky E, Warrell RP., Jr Continuous treatment with all-trans retinoic acid causes a progressive reduction in plasma drug concentrations: implications for relapse and retinoid ‘resistance’ in patients with acute promyelocytic leukemia. Blood. 1992;79:299–303. [PubMed] [Google Scholar]
- 14.Mao JT, Goldin JG, Dermand J, Ibrahim G, Brown MS, Emerick A, McNitt-Gray MF, Gjertson DW, Estrada F, Tashkin DP, Roth MD. A pilot study of all-trans-retinoic acid for the treatment of human emphysema. Am J Respir Crit Care Med. 2002;165:718–23. doi: 10.1164/ajrccm.165.5.2106123. [DOI] [PubMed] [Google Scholar]
- 15.Chiu Y-Y, Gerber M, Kolis SJ, Rutman O, Davies B. Effect of CYP3A4 enzyme inhibitor ketoconazole on pharmacokinetics (PK) of a novel emphysema agent, R667, in healthy men. Drug Metab Rev. 2004;36(Suppl 1):296. [Google Scholar]
- 16.Chiu Y-Y, Gerber M, Kolis SJ, Rutman O, Gooden C, Davies B. Effect of rifampicin on the pharmacokinetics of a novel emphysema agent, R667, in healthy subjects. AAPS J. 2004;6:R6220. [Google Scholar]
- 17.Brennan B, Brown A, Kolis SJ, Rutman O, Gooden C, Davies B. Effect of R667, a novel emphysema agent, on the pharmacokinetics (PK) of midazolam, in healthy men. J Clin Pharmacol. 2006;46:222. doi: 10.1177/0091270005283836. [DOI] [PubMed] [Google Scholar]