Abstract
• Background and Aims Lotus (Nelumbo nucifera) has been cultivated as an ornamental and food plant in Japan for more than 1000 years. As large areas are required for its cultivation (approximately 2 m2 per plant), physiological research, such as into the effect of environmental factors on dormancy, has not been well studied until recently. In this paper, seedlings were used to examine environmental factors affecting dormancy induction.
• Methods In a first experiment, seeds were sown from 6 April to 6 October at 2-month intervals, and cultivated for 2 months in an unheated greenhouse. In a second experiment, seeds were prepared for germination on 16 November and 16 May and the seedlings were grown at 25 or 30 °C under natural daylength in phytotron growth rooms. After 1 month, the seedlings were cultivated at 20, 25 or 30 °C for a further month. The number of leaves and rhizome branches on the main stem were counted, and growth of rhizomes on the main stem was calculated using a rhizome enlargement index (= maximum internode diameter/internode length) after 2 months of culture in both experiments.
• Key Results Rhizomes elongated without enlargement when the seeds were sown in April and June. Sowing the seeds in August and October resulted in rhizome enlargement from the tenth and fifth internodes, respectively. Rhizomes enlarged in the November-sowing but elongated in the May-sowing irrespective of temperature treatments under natural daylength in the phytotron rooms. The seedlings cultivated from May at 25–30 °C for 2 months had more leaves, and more rhizome branches and nodes than those cultivated from November.
• Conclusions Short days led to induced dormancy in lotus.
Keywords: Dormancy, leaf production, Nelumbo nucifera, photoperiod, rhizome branching, rhizome enlargement, temperature
INTRODUCTION
Lotus (genus Nelumbo), an aquatic plant, consists of two species, N. nucifera (Indian lotus) and N. pentapetara (American lotus). The former originated in the eastern part of Asia and the northern part of Australia, whereas the latter originated in the eastern part of North America and the northern part of South America (Sakamoto, 1977; Toyoda, 1981). These species show considerable variations in flower colour and shape, and have been cultivated as ornamental plants as a result. The rhizome and seeds of N. pentapetara were commonly eaten by Native Americans, and those of N. nucifera are still a part of the Oriental diet. Commonly called the edible lotus, the rhizomes of N. nucifera are grown, cooked and used very much like potatoes. The crop was introduced from China and has been cultivated for more than 1000 years in Japan (Komatsu et al., 1975; Sakamoto, 1977; Toyoda, 1981).
The enlarged rhizome sprouts in early spring and the rhizome elongates with emerging floating and upright leaves until late summer. Three to four distal rhizome internodes begin to enlarge in late summer and enlarged rhizomes are harvested from late summer to the following spring.
Among geophytes, underground organs modified from parts of the stem are classified into corms, tubers and rhizomes. It is recognized that these organs, being used for asexual propagation, are key to the survival strategy of plants during unfavourable periods for their growth. The formation of tubers has been studied with regard to photoperiod and temperature in Solanum tuberosum (potato), Helianthus tuberosus and Begonia evansiana (Hamner and Long, 1939; Esashi and Nagao, 1958; Esashi et al., 1964; Snyder and Ewing, 1989). There are also some studies on the environmental factors affecting formation of corms and cormels (Tsukamoto and Inaba, 1961; Asahira et al., 1968; Imanishi et al., 1970). However, there are few reports on whether rhizome enlargement is affected by environmental factors, e.g. temperature and/or photoperiod.
An enlarged rhizome is also found in N. nucifera, acting as a dormant organ to aid survival of the plant under unfavourable circumstances. Although N. nucifera is an important vegetable crop in Japan, the dynamics of its rhizome enlargement are relatively poorly known. Rhizomes enlarged in the previous year are usually used for commercial cultivation. The length of the main stem reaches to about 11 m when enlarged rhizomes are planted (Fig. 1), and each plant requires a large area (more than 2 m2 per plant) for cultivation. Detailed investigation of below-ground rhizomes is laborious and time-consuming, explaining why research into the physiology of these organs in N. nucifera has been limited. Small rhizomes with compact plants can be obtained when seedlings are cultivated (Fig. 1). The effect of temperature and photoperiod on induction of dormancy was investigated by using seedlings to provide an efficient physiological approach.
Fig. 1.
Schematic drawing illustrating growth in lotus.
MATERIALS AND METHODS
Plant materials
Open-pollinated seeds of Nelumbo nucifera Gaertn. ‘Chugoku’ were used in all experiments. The seeds were prepared for germination by soaking in concentrated H2SO4 for 3 h and then rinsing with distilled water. They were then soaked in distilled water for 1 d at 25 °C. After removing softened seed coats, the seeds were incubated in distilled water at 25 °C under continuous fluorescent light (39·1 µmol m−2 s−1) until germination (6 days).
Effects of sowing date on induction of dormancy
Seeds were prepared for germination from 6 April to 6 October at 2-month intervals. After germination, four seedlings were transplanted to a plastic container (28 × 40 × 15 cm) containing sandy soil with 30 g slow-release fertilizer (N : P : K=16 : 5 : 10 %). Each container was filled with water, which was replaced each week. Twelve seedlings were used for each treatment and the containers were placed in line. The seedlings were grown under natural daylength conditions in an unheated greenhouse. The average ambient temperature in Fukuoka gradually increased from April (15 °C) to June (23 °C) to August (30 °C), whereas it decreased from August (30 °C) to October (20 °C) to December (10 °C). The latitude in Fukuoka is 33.35°N, and the maximum and minimum daylengths were, respectively, 14 h 20 min and 12 h 44 min from 6 April to 6 June, 14 h 26 min and 13 h 43 min from 6 June to 6 August, 13 h 43 min and 11 h 43 min from 6 August to 6 October, and 11 h 43 min and 10 h 4 min from 6 October to 6 December; i.e. daylength gradually increased from 6 April to 21 June, gradually decreasing from 21 June to 6 August thereafter. The rhizome elongates with emerging leaves of two types: floating and upright. Rhizome enlargement proceeds by enlarging of 3–4 distal internodes with a break in leaf production and in rhizome elongation and branching. The numbers of leaves and rhizome branches on the main stem were counted, and growth of rhizomes in the main stem was calculated using a rhizome enlargement index (=maximum internode diameter/internode length) after 2 months of culture.
Effects of temperature and photoperiod on induction of dormancy
Seeds were prepared for germination on 16 November, 2000 and 16 May, 2001. Four seedlings were transplanted to one plastic container as in the previous experiment, and one container was used for each treatment. The seedlings were grown at 25 or 30 °C under natural daylength in a phytotron glass room of the Biotron Institute, Kyushu University. After 1 month of cultivation, the seedlings were cultivated at 20, 25 and 30 °C as follows: (A) 30 °C for 2 months; (B) 25 °C for 1 month after 30 °C for 1 month; (C) 20 °C for 1 month after 30 °C for 1 month; (D) 30 °C for 1 month after 25 °C for 1 month; (E) 25 °C for 2 months; and (F) 20 °C for 1 month after 25 °C for 1 month. The containers were placed in racks without overlapping each other. Maximum and minimum daylengths during the second experiment were, respectively, 14 h 26 min and 13 h 58 min from 16 May to 16 July, and 10 h 26 min and 9 h 57 min from 16 November to 16 January. Growth of above- and below-ground parts was investigated in a similar manner to that in experiment 1. The effects of temperature and photoperiod on floating leaf, upright leaf, number of rhizome branches and number of rhizome nodes were analysed by two-way ANOVA to determine which factors influenced growth.
RESULTS
Effects of sowing date on induction of dormancy
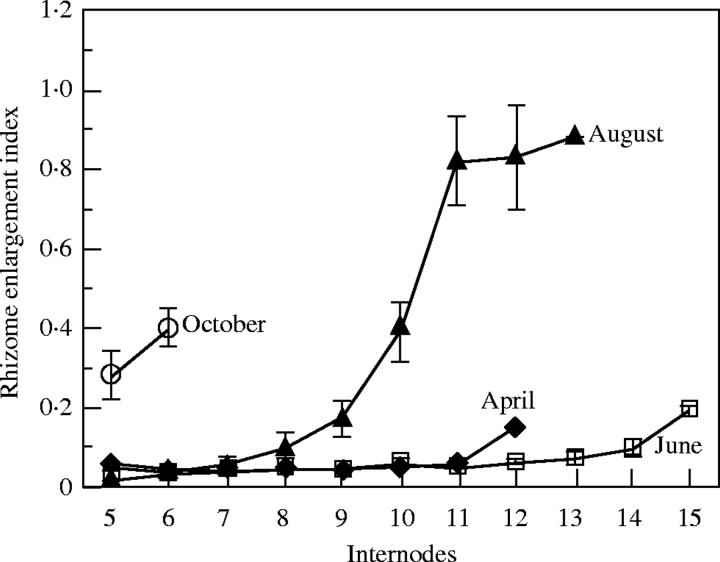
The plants sown in June had the greatest number of floating leaves, with lesser numbers in those sown in April and August (Fig. 2). The fewest number of floating leaves was seen in plants that had been sown in October. Upright leaves were also observed most numerously in the plants sown in June, but were not present in the plants sown in October. The number of rhizome branches was highest (9·2) in plants sown in June, and lowest (1·2) in plants sown in October (Fig. 3). The youngest internode of the rhizome enlarged in all plants when seeds were sown in August and October (Fig. 4). Rhizome elongation without enlargement was recognized in all the plants sown in April and June. Rhizome enlargement indices from the first to fourth internodes were not measured for any of the treatments because these internodes were always extremely short regardless of when the seeds were sown. June-sowing produced the highest number of nodes, with April- and August-sowing producing lower numbers. Fewest nodes were produced in the plants sown in October (Fig. 5). When the seeds were sown in April and June, rhizome enlargement indices were lower than 0·2. Although the indices were lower than 0·2 from the fifth to ninth internodes in the plants sown in August, they gradually increased from the 10th internodes and reached 0·88 in the 13th internodes. When seeds were sown in October, the indices were higher than 0·2 in both the fifth and the sixth internodes.
Fig. 2.

Effect of time of sowing on the number of upright and floating leaves of plants grown in an unheated greenhouse for 2 months. Vertical bars represent ±s.e. Statistically significant differences at P < 0.05 (Tukey's test) are indicated by different lower-case letters.
Fig. 3.
Effect of time of sowing on the number of rhizome branches of plants grown in an unheated greenhouse for 2 months. Vertical bars represent ±s.e. Statistically significant differences at P<0.05 (Tukey's test) are indicated by different lower-case letters.
Fig. 4.
Distal parts of rhizomes of plants grown in an unheated greenhouse for 2 months after seed sowing in April (A), June (B), August (C) and October (D). Scale bars = 1 cm.
Fig. 5.
Changes in enlargement index of main rhizomes of plants grown in an unheated greenhouse for 2 months after seed sowing in April, June, August and October. Vertical bars represent ±s.e.
Effects of temperature and photoperiod on induction of dormancy
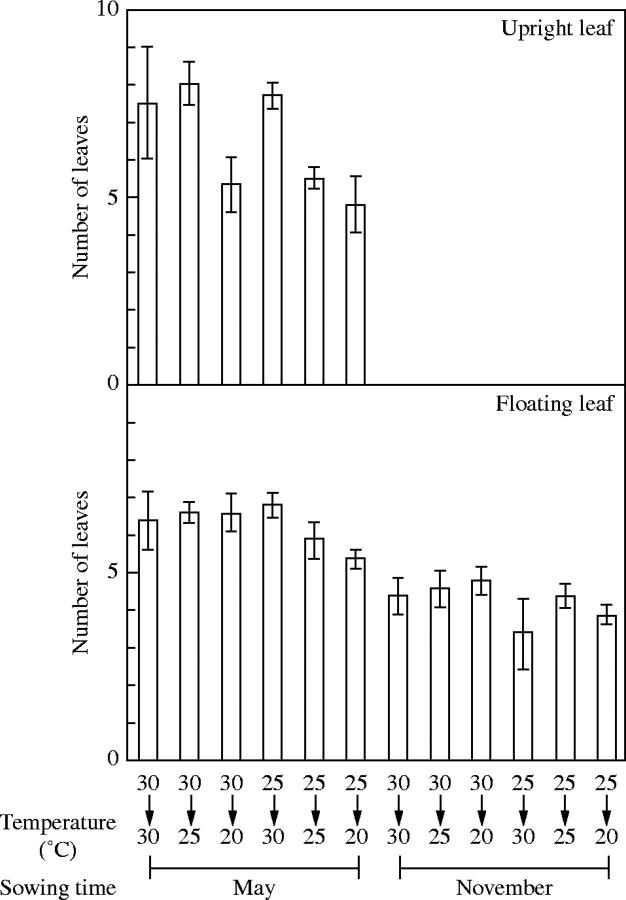
A two-way ANOVA of the number of floating leaves indicated a significant (P < 0·001) main effect of photoperiod (Table 1). There was a statistically significant difference in the number of floating leaves between the plants of the May- and November-sowings (Fig. 6). There was no significant difference in the number of floating leaves between the treatments when seeds were sown in May or November. A two-way ANOVA revealed that photoperiod and temperature also affected the number of upright leaves (Table 1). No upright leaves appeared in the plants sown in November (Fig. 6). The number of upright leaves was relatively high in May-sown plants when the cultivation temperature was high. A two-way ANOVA indicated a significant effect of photoperiod (P < 0·001) and temperature (P < 0·05) on the number of rhizome branches (Table 1). The number of rhizome branches in the plants sown in May was higher than in those sown in November, and the plants sown in May tended to produce more rhizome branches when cultivation temperature was high (Fig. 7).
Table 1.
Factors influencing numbers of floating leaves, upright leaves, rhizome branches and rhizome nodes, expressed as F-values
| Effect | No. of floating leaves | No. of upright leaves | No. of rhizome branches | No. of rhizome nodes |
|---|---|---|---|---|
| Temperature | 1·121ns | 2·755* | 3·079* | 12·998*** |
| Photoperiod | 42·339*** | 328·833*** | 90·256*** | 529·817*** |
| Temperature × Photoperiod | 0·689ns | 2·755* | 1·964ns | 4·193** |
P < 0·05;
P < 0·01;
P < 0·001;
ns, not significant.
Fig. 6.
Effect of temperature and seed sowing time (May and November) on the number of upright and floating leaves of plants grown under controlled temperature with natural daylength in a phytotron room for 2 months. Vertical bars represent ±s.e.
Fig. 7.
Effect of temperature and seed sowing time (May and November) on the number of rhizome branches of plants grown under controlled temperature with natural daylength in a phytotron room for 2 months. Vertical bars represent ±s.e.
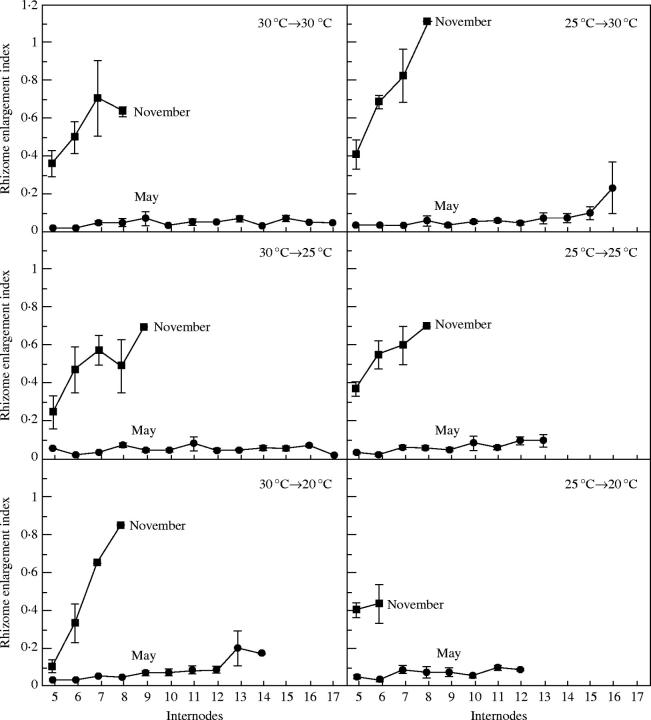
Figure 8 shows the distal rhizome internode in plants sown in May and November and grown at 25 °C for 1 month after 30 °C for 1 month. Regardless of cultivation temperature, rhizome enlargement was seen in the plants sown in November but not in those sown in May. Both temperature (P < 0·001) and photoperiod (P < 0·001) were found to affect the number of rhizome nodes (Table 1). The number of rhizome nodes ranged from 12 to 17 in the plants sown in May, but from six to nine in those sown in November. In May-sown plants, greater numbers of nodes were produced with higher cultivation temperatures. Rhizome enlargement indices from the fifth internodes were lower than 0·2 in the plants sown in May (Fig. 9). When seeds were sown in November, enlargement indices increased from the fifth internodes and the maximum index for each treatment was greater than 0·3. The highest index value (1·11) was for plants grown at 30 °C for 1 month after 25 °C for 1 month.
Fig. 8.
Distal parts of rhizomes of plants grown at 25 °C for 1 month after 30 °C for 1 month under natural daylength in a phytotron room. Seeds were sown in May (A) and November (B). Scale bars = 1 cm.
Fig. 9.
Changes in enlargement index of main rhizomes of plants grown under controlled temperature with natural daylength in a phytotron room. Seeds were sown in May and November. Vertical bars represent ±s.e.
DISCUSSION
For seeds sown in August or October, short daylength and/or a decline in temperature appeared to promote rhizome enlargement. As the November- and May-sowings, irrespective of temperature treatments, promoted rhizome enlargement and elongation, respectively, in the second experiment, it is assumed that short daylength rather than temperature is the main environmental factor leading to rhizome enlargement in lotus. The critical photoperiod for rhizome enlargement seemed to be approximately from 11 h 40 min to 13 h 50 min for August-sown plants in the first experiment.
It is clear that production of floating leaves was affected by daylength. In the second experiment, plants without rhizome enlargement produced upright leaves, whereas those with rhizome enlargement produced no upright leaves. By contrast, in the first experiment, plants sown in August produced upright leaves although their distal internodes enlarged after lower internode elongation. From these results, it may be concluded that long daylength accelerates rhizome elongation and upright leaf production, and short daylength promotes rhizome enlargement and inhibits upright leaf production. In the plants sown in August in the first experiment, rhizomes elongated and upright leaves were produced under the long daylength in the early stage of plant growth, and the distal internodes enlarged as the daylength became shorter. Leaf proliferation ceased in plants whose rhizomes began to enlarge.
There was a significant difference in the number of rhizome branches between May- and November-sown plants in the second experiment. When cultivation temperature was high, more rhizome branches were produced in May. It can be assumed that long daylength and higher temperature promote rhizome branching.
In the second experiment, plants cultivated under long daylength had more nodes on the main stem than those cultivated under short daylength. Although the effect of temperature on the number of nodes was not clear under short daylength, numbers increased as the temperature became higher under long daylength.
It is also known that tuberization is promoted by short daylength and low temperature in potato, but is inhibited by long daylength and high temperature (Menzel, 1983, 1985; Reynolds and Ewing, 1989; Snyder and Ewing, 1989; Ewing and Struik, 1992; Jackson, 1999). Photoperiod responses have also been reported in other tuber and corm plants, e.g. Begonia evansiana, Helianthus tuberosus, Gladiolus and Colocasia antiquorum. In Begonia evansiana and Helianthus tuberosus, underground or aerial tubers formed under short but not long daylength (Hamner and Long, 1939; Esashi and Nagao, 1958). Corm formation was not affected by photoperiod, but the number of cormels was increased by short days (8–10 h) in Gladiolus (Asahira et al., 1968; Imanishi et al., 1970). Cormel formation in Gladiolus, however, progresses to some extent under long daylength. Tsukamoto and Inaba (1961) obtained similar results in taro (Colocasia antiquorum), namely that short daylength did not result in corm formation, but did result in cormel formation. Thus, such tuber plants are completely dependent on photoperiod for tuber induction, but corm plants such as Gladiolus and Colocasia antiquorum are not dependent on photoperiod for cormel formation. Therefore, it has been reported that photoperiod strongly influences enlargement only in tuber plants among stem-enlarging geophytes. The photoperiod responses in flowering of short-daylength plants have been widely investigated, whereas the responses in formation of dormancy organs have not been advanced except for a few plants such as S. tuberosum and B. evansiana. There are no previous reports suggesting that rhizome enlargement is dependent on daylength. This is the first report detailing that rhizome enlargement and elongation were induced by short and long daylength, respectively, in lotus.
High temperature and long daylength accelerate vegetative growth, such as leaf production, rhizome branches and rhizome nodes. It is clear that short daylength rather than temperature is the main environmental factor leading to induction of dormancy in lotus plants.
Acknowledgments
Financial support was provided by the Matsushima Horticultural Development Foundation, Japan, and a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan.
LITERATURE CITED
- Asahira T, Imanishi H, Tsukamoto Y. 1968. Studies on the cormel formation in gladiolus. Memoirs of the College of Agriculture, Kyoto University 93: 21–34.
- Esashi Y, Nagao M. 1958. Studies on the formation and sprouting of aerial tubers in Begonia evansiana Andr. I. Photoperiodic conditions for tuberization. The Science Reports of the Tohoku University Fourth Series (Biology) 24: 81–88.
- Esashi Y, Ogata K, Nagao M. 1964. Studies on the formation and sprouting of aerial tubers in Begonia evansiana Andr. IX. Effects of temperature on tuber initiation. Plant and Cell Physiology 5: 1–10. [Google Scholar]
- Ewing EE, Struik PC. 1992. Tuber formation in potato: induction, initiation and growth. Horticultural Reviews 14: 89–198. [Google Scholar]
- Hamner KC, Long EM. 1939. Localization of photoperiodic perception in Helianthus tuberosus. Botanical Gazette 101: 81–90. [Google Scholar]
- Imanishi H, Sasaki K, Oe M. 1970. Further studies on the cormel formation in gladiolus. Bulletin of the University of Osaka Prefecture, Series B Agriculture and Biology 22: 7–17.
- Jackson SD. 1999. Multiple signaling pathways control tuber induction in potato. Plant Physiology 119: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu E, Tsukahara A, Amagaya H, Okazawa N, Noguchi T, Okuyama T, et al. 1975. Lotus. In: Izaki M, ed. The cultivation and management in aquatic vegetables. Tokyo: Ie-No-Hikari Kyokai Press, 9–94 [in Japanese].
- Menzel CM. 1983. Tuberization in potato at high temperatures: interaction between shoot and root temperatures. Annals of Botany 52: 65–69. [Google Scholar]
- Menzel CM. 1985. Tuberization in potato at high temperatures: interaction between temperature and irradiance. Annals of Botany 55: 35–39. [Google Scholar]
- Reynolds MP, Ewing EE. 1989. Effects of high air and soil temperature stress on growth and tuberization in Solanum tuberosum. Annals of Botany 64: 241–247. [Google Scholar]
- Sakamoto Y. 1977. Lotus. Tokyo: Hosei University Press [in Japanese].
- Snyder RG, Ewing EE. 1989. Interactive effect of temperature, photoperiod, and cultivar on tuberization of potato cuttings. HortScience 24: 336–338. [Google Scholar]
- Toyoda K. 1981. Study on lotus. Tokyo: Ariakeshobo Press [in Japanese].
- Tsukamoto Y, Inaba K. 1961. The effect of daylength upon the cormel formation in taro (Colocasia antiquorum). Memoirs of the Research Institute for Food Science, Kyoto University 23: 15–21.