Abstract
• Background and Aims Most plant species are visited by a diversity of floral visitors. Pollen transfer of the four most common pollinating bee species and one nectar-robbing bee of the distylous plant Gelsemium sempervirens were compared.
• Methods Naturally occurring pollen loads carried by the common floral visitor species of G. sempervirens were compared. In addition, dyed pollen donor flowers and sequences of four emasculated recipient flowers in field cages were used to estimate pollen transfer, and the utility of fluorescent dye powder as an analogue for pollen transfer was determined.
• Key Results Xylocopa virginica, Osmia lignaria and Habropoda laboriosa carried the most G. sempervirens pollen on their bodies, followed by Bombus bimaculatus and Apis mellifera. However, B. bimaculatus, O. lignaria and H. laboriosa transferred significantly more pollen than A. mellifera. Nectar-robbing X. virginica transferred the least pollen, even when visiting legitimately. Dye particles were strongly correlated with pollen grains on a stigma, and therefore provide a good analogue for pollen in this system. The ratio of pollen : dye across stigmas was not affected by bee species or interactions between bee species and floral morphology. However, dye transfer was more sensitive than pollen transfer to differences in floral morphology.
• Conclusions The results from this study add to a growing body of literature highlighting that floral visitors vary in pollination effectiveness, and that visitors carrying the most pollen on their bodies may not always be the most efficient at depositing pollen on stigmas. Understanding the magnitude of variability in pollinator quality is one important factor for predicting how different pollinator taxa may influence the evolution of floral traits.
Keywords: Apis mellifera, Bombus bimaculatus, fluorescent dye, Gelsemium sempervirens, gene flow, Habropoda laboriosa, heterostyly, honey bee, nectar robber, Osmia lignaria, pollen transfer, Xylocopa virginica
INTRODUCTION
The majority of flowering plants require insects or other animals to mediate pollen transfer, and different floral visitors can vary widely in their ability to transfer pollen (Primack and Silander, 1975; Schemske and Horvitz, 1984; Herrera, 1987; Conner et al., 1995; Mayfield et al., 2001; Ivey et al., 2003). The flowers of most plant species are visited by a diverse array of floral visitors (Parker, 1982; Jordano, 1987; Memmott, 1999; Memmott and Waser, 2002; Olesen and Jordano, 2002; Vazquez and Simberloff, 2002; Jordano et al., 2003), and pollen transfer by these visitors varies along a continuum from highly efficient to larcenous (Waser et al., 1996). Some floral visitors that appear to be pollinators are more properly classified as ‘nectar thieves’ (Inouye, 1980), because their body shape prevents contact with floral reproductive structures. On the other hand, nectar robbers that pierce corollas to obtain nectar can still effect pollination in some plant species (for reviews, see Maloof and Inouye, 2000; Irwin et al., 2001), and thus act more as mutualists than antagonists. Despite the wealth of studies on plant–pollinator interactions, surprising little is known about the magnitude of variation in the efficiency of different pollinator species, or what determines such magnitude (Waser et al., 1996). For example, previous studies have generally found little support for the prediction that specialized bees will be more effective pollinators than generalists (e.g. Motten et al., 1981; Tepedino, 1981; Fishbein and Venable, 1996; Olsen, 1997; Gomez and Zamora, 1999; but see Parker, 1982). Understanding the magnitude and causes of variation in pollinator transfer efficiency is critical because the strength of plant–pollinator interactions is dependent, at least in part, on the ability of a pollinator species to remove pollen from anthers and deposit it on receptive stigmas (Schemske and Horvitz, 1984; Inouye et al., 1994). Furthermore, models predicting floral evolution in the context of pollination rely on quantifying the relationship between floral traits and pollen export or receipt for each pollinator species (e.g. Aigner, 2001).
Pollination efficiency is defined as the amount of conspecific pollen deposited on a stigma per visit, following Inouye et al. (1994). Floral visitors can vary in their pollination efficiency for a variety of reasons, none of which are mutually exclusive, including their ability to pick up and deposit pollen (Conner et al., 1995), the degree to which they groom pollen from their bodies (Rademaker et al., 1997), and their behaviour while visiting flowers (from legitimate to larcenous as described above; Inouye, 1980). Variation in pollen deposition per visit may also vary with floral morphology (Waser and Price, 1984; Campbell, 1989; Conner et al., 1995).
Heterostylous species provide an extreme form of floral variation, in which there are two or more consistent, genetically determined floral morphs that vary in the length of styles (female structure) and filaments (male structure). Distylous plants have two morphs, one with anthers protruding beyond the corolla tube and the stigma concealed within the tube (‘thrum’) and the other with the stigma protruding and anthers within the corolla tube (‘pin’). Due to stigma–anther placement, differential attraction of pollinators, and/or differences in pollen production, floral morphs in heterostylous plants may experience consistent differences in pollen donation and receipt (Ganders, 1979), and thus experience consistently greater reproduction through male or female function (e.g. Wolfe and Barrett, 1989; Ree, 1997; Barrett et al., 2000; Thompson, 2001; Pailler et al., 2002).
Understanding the importance of variation in pollinator taxa and floral morphology to pollen transfer dynamics requires understanding the movement of pollen. Unfortunately, assessing pollen movement is no easy task, as pollen grains are not easily distinguishable among plants without the use of genetic or morphological markers (Snow and Lewis, 1993). One way to indirectly measure pollen flow in natural populations is to mark pollen grains or use pollen surrogates (Kearns and Inouye, 1993). Powdered fluorescent dyes brushed onto dehiscing anthers can provide estimates of pollen flow. A careful comparison of dye and pollen movement has been made in only a few systems (Waser and Price, 1982; Thomson et al., 1986; Waser, 1988; Campbell et al., 1991; Rademaker et al., 1997). These studies have generally found that dye and pollen movement are correlated and that dye provides a good qualitative predictor of pollen movement. However, dye can have longer (Thomson et al., 1986), shorter (Waser and Price, 1982), or more variable (Campbell et al., 1991) movement patterns than pollen, and the ability of dye to predict pollen movement may vary with pollinator species (Waser and Price, 1982). Thus, it cannot be assumed a priori that dye will provide a good analogue for pollen movement. Nonetheless, fluorescent dye has been used to address a variety of ecological questions in the field, including the role of plant size and pollen presence on pollen transfer (Price and Waser, 1982; Rademaker and de Jong, 1998), the effect of floral variability or plant size on male function (Waser and Price, 1984; Campbell, 1989; Dudash, 1991), the effect of interspecific competition on pollination (Campbell, 1985), and the efficiency of diurnal compared with nocturnal pollinators (Young, 2002).
Here, the importance of a diverse pollinator assemblage and floral morphology to variation in pollen and dye transfer of the perennial vine Gelsemium sempervirens (Carolina jessamine; Loganiaceae) was examined. Gelsemium sempervirens is distylous and self-incompatible, and its flowers have a diversity of visitors including several bees that visit legitimately (i.e. through the corolla opening) and the nectar-robbing carpenter bee Xylocopa virginica (Adler and Irwin, 2005). Both the amount of pollen carried and pollen transfer efficiency of each floral visitor species were quantified on a sequence of flowers. Also the relative importance of the floral morphology of donor and recipient flowers to pollen transfer was explored. Pollen transfer was estimated using pollen as well as fluorescent dye. Therefore, the question was asked whether fluorescent dye served as a reliable analogue for pollen movement in G. sempervirens. Specifically, the following questions were addressed. (1) Do bee species vary in the amount of naturally occurring loads of G. sempervirens and other pollen on their bodies? (2) To what degree do bee species vary in their ability to deposit pollen on a sequence of stigmas? (3) Does the morphology of pollen donor or recipient flowers affect pollen deposition by different floral visitors? (4) Does fluorescent dye serve as a reliable analogue for pollen deposition?
MATERIALS AND METHODS
Study system
Gelsemium sempervirens is native to the south-eastern United States and occurs along woodland margins, fences, roadsides and open pine forests (Ornduff, 1970; Phillips, 1985). The study site is located at the University of Georgia Botanical Gardens in Athens, Georgia, USA. In this area, G. sempervirens blooms from early March into late April, producing up to several hundred yellow, tubular flowers per plant. Individual flowers have a standing crop of 0–3 μL of nectar produced by nectaries on the base of the carpel, and flowers typically senesce after 4–5 d.
Gelsemium sempervirens is self-incompatible and distylous; each plant has either long styles and short filaments (‘pin’ plants) or short styles and long filaments (‘thrum’ plants). Thrum pollen can only fertilize pin ovules, and vice versa (Ornduff, 1970, 1980). Pollen grains from the two morphs overlap in size, so that grains cannot be identified to morph, as in some other distylous species (Ornduff, 1979). Thrum flowers of G. sempervirens tend to have longer corollas than pin flowers (Irwin and Adler, 2005). Variation in flower size between morphs may differentially alter the per-visit effectiveness of different floral visitors, especially if bees are more likely to contact floral organs in smaller flowers.
The most common legitimate floral visitors of G. sempervirens in Georgia include bumble bees (Bombus bimaculatus; Apidae), honey bees (Apis mellifera; Apidae), blue orchard bees (Osmia lignaria; Megachilidae) and blueberry bees (Habropoda laboriosa; Apidae). Gelsemium sempervirens is also visited by carpenter bees (Xylocopa virginica; Xylocopidae) that sometimes enter flowers legitimately through the corolla opening but also often make slits near the corolla base and insert their proboscis to steal nectar (Adler and Irwin, 2005). For simplicity, hereafter only the genus name will be used to refer exclusively to each species. In prior experiments, all the pollinating bees were observed foraging for both pollen and nectar, but only Xylocopa was seen to forage for nectar (Adler and Irwin, 2005); these behavioural differences could affect the amount of pollen transferred by different species. In addition, these visitors vary in body length and abdomen width (Mitchell, 1962; Michener et al., 1994), factors that may affect their ability to remove or deposit pollen. Ordered by size, the largest floral visitors are Xylocopa (abdomen length 19–23 mm, width 9·5–10·5 mm), followed by Bombus (17–22 mm, 8·5–10 mm), Apis (16–20 mm, 12 mm), Habropoda (15·5–16 mm, 6·5–7 mm) and Osmia (10–11 mm, width not given) (Michener et al., 1994). The largest of these visitors are as long and nearly as wide as the average G. sempervirens flower, while the smallest can crawl freely within the corolla tube. Variation in pollinator size, among other traits, could strongly impact pollen transfer and plant reproductive success, of which relatively little is known in this or other systems with diverse pollinator assemblages (but see Herrera, 1987; Conner et al., 1995; Mayfield et al., 2001; Ivey et al., 2003). It is hypothesized that the largest bees, when visiting legitimately, would carry the most pollen on their bodies and would be the most effective at transferring pollen, as they may be more likely to contact the sexual organs of flowers.
Field tests
Pollen loads carried by different floral visitors
Forty-seven bees were captured on seven days between 24 Mar. 2004 and 16 Apr. 2004 to compare naturally occurring pollen loads of G. sempervirens and other pollen. There was no control for the number or species of plants visited by the bees prior to collection; the present study was only interested in the naturally occurring pollen loads that may be available for transfer. Sample sizes were 13 Bombus, 11 Osmia, five Habropoda, four Apis and 14 Xylocopa. Bees were caught during late afternoon on sunny days within 500 m of G. sempervirens plants at the Botanical Gardens. Bees were placed in individual small plastic containers in coolers with ice for approx. 30 min. Cooled bees were wiped over their entire bodies with a small piece of fuchsin jelly (Kearns and Inouye, 1993). Care was taken to ensure that the same amount of time and effort were used for each bee and that similar amounts of jelly were used. Each piece of jelly was melted using a hot plate and mounted on a glass slide in the laboratory, and pollen grains were counted under a compound microscope (Nikon Eclipse E400, Melville, NY, USA). Pollen grains were identified as originating from G. sempervirens or other species using a reference collection of locally flowering plants.
Analysis using one-way ANOVAs was carried out to see if bee species varied in the number of G. sempervirens pollen grains and the proportion of G. sempervirens to total pollen. Individual bees were the unit of replication. Pollen counts were log(x + 1) transformed to improve normality; the proportion of G. sempervirens pollen was normal without transformation. The collection date did not explain significant variation in pollen counts (F4,38 < 0·8, P > 0·5) and was removed from analysis.
Pollen and dye deposition by different floral visitors
Pollen and dye deposition on stigmas by the five bee species visiting G. sempervirens was examined using trials with one pollen donor flower and up to four emasculated recipient flowers per insect. Pollen transfer was measured as deposition on receptive stigmas for a sequence of flowers (‘stigma pollen load per visit’; Inouye et al., 1994). Because bees were cleaned with ethanol prior to trials to remove any naturally occurring pollen loads on their bodies, these measures should represent a combination of ability to remove and deposit pollen grains. Trials were conducted in metal-framed flight cages (30 cm × 30 cm × 30 cm) draped with polyester netting (Bioquip Products, Gardena, CA, USA) with an opening that permitted flowers to be offered by hand. Both donor and previously emasculated recipient flowers were picked from horticultural vines at the Botanical Gardens directly before use. The anthers of donor flowers were dusted with green or orange fluorescent powder (Series JST-300, Radiant Color, Richmond, CA, USA) using a toothpick. Care was taken to apply similar amounts of dye to all anthers within and between flowers. Recipient flowers were emasculated with fine-point forceps while on the vine at least 1 d prior to opening, and then covered with mesh bridal veil bags to prevent insect visits.
Trials were conducted on ten separate days between 20 Mar. 2002 and 19 Apr. 2002. Trials were conducted with 12 Bombus, 17 Osmia, six Habropoda, seven Apis and 12 Xylocopa. First bees of each species were caught with nets in the field, quickly transferred to small plastic containers, and then placed in coolers with ice. Cooling followed by gradual warming of the insects diminishes behavioural disturbances resulting from transport into flight cages (Kearns and Inouye, 1993). After 30 min, one cooled bee was placed in the flight cage, its body wiped gently but thoroughly with ethanol on a Kimwipe (Kimberly-Clark Corporation, Roswell, GA, USA) to remove pollen, and then allowed to dry, warm up and begin flying. Because ethanol evaporated before trials and all bees were treated in the same manner, the cleaning treatment was not expected to affect pollen adherence or to bias the results. The bee was allowed to forage on a donor flower with dehiscing pollen and dye and then on up to four emasculated recipient flowers with receptive stigmas. Hereafter, this is referred to this as a ‘sequence’. All flowers were offered sequentially by hand and immediately removed after one visit. The order of floral morphs in a sequence was haphazard, but pin flowers were rare at the field site. Thus there were unbalanced sample sizes for both donor morph (one donor flower per sequence; eight pin and 46 thrum) and recipient morph (29 pin and 157 thrum flowers). However, natural populations often have ratios of flowering pin and thrum plants as skewed as 2 : 1 in a given year (Irwin and Adler, 2005), indicating that such biases in availability of a floral morph are not uncommon.
The technique of offering flowers by hand to floral visitors in flight arenas has been used in previous studies for hummingbirds and bumble bees (Price and Waser, 1979; Waser and Price, 1982; Waser, 1988) and did not appear to disturb floral visitor behaviour. In the present study, once bees began foraging, they made the transition easily between flowers without grooming or clinging to the cage. Moreover, most interest was in the relative difference among bee species in pollen deposition, rather than absolute values. Because all flowers were hand-held, the same bias was introduced into all trials, and there is no a priori reason to suspect that different bees would behave differently to hand-held flowers. The exception is for Xylocopa, which typically performs a different behaviour (robbing) than the other species. It was found that in the flight cage, Xylocopa only visited flowers legitimately (entering via the corolla opening) and did not rob; this behaviour is also sometimes observed in the field. Thus, the pollen counts for Xylocopa represent the maximum amount they are likely to transfer.
Stigmas from each recipient flower were collected using forceps into individual 1·5-ml plastic microcentrifuge tubes immediately after visitation. Forceps were cleaned in ethanol after each stigma collection to avoid pollen transfer. Dye particles were counted the same day using a dissecting microscope (Nikon model SMZ800, Melville, NY, USA). Each stigma was then mounted in fuchsin stain (Kearns and Inouye, 1993) on a glass slide. Pollen grains were counted under a compound microscope (Nikon Eclipse E400). Pollen from species other than G. sempervirens was sometimes found on stigmas, indicating that either not all the pollen was successfully cleaned from the bees' bodies before trials or some pollen was deposited on the stigmas through the mesh bags from an unknown source or during emasculation. Both conspecific and heterospecific pollen receipt were counted. Because heterospecific pollen receipt was rare (only 4·9 % of all pollen grains), it was not included in statistical analyses.
Because unexpected heterospecific pollen on stigmas was found, two additional control experiments were conducted to try to determine the pollen source. First, to assess how well the ethanol wiping removed pollen from the bees' bodies, an additional 67 bees (17 Bombus, 14 Osmia, 16 Habropoda, four Apis, and 16 Xylocopa) were captured on seven days between 24 Mar. 2004 and 16 Apr. 2004. The bees were cooled for 30 min, their bodies wiped with ethanol, and then their bodies wiped with a small piece of fuchsin jelly (see the section ‘Pollen loads carried by different floral visitors’) to remove any residual pollen. The pieces of fuchsin jelly were individually mounted on slides and conspecific and heterospecific pollen grains were counted as described above. Naturally occurring pollen on bees' bodies (collected at the same time; from ‘Pollen loads carried by different floral visitors’) was compared with pollen loads after swabbing the bees with ethanol using ANOVA with cleaning treatment, bee species and their interactions as main effects, and gelsemium pollen grains and other pollen grains as response variables. It was found that swabbing removed 70 % of G. sempervirens pollen grains (F1,104 = 3·97, P = 0·049) and 89 % of other pollen grains (F1,104 = 53·20, P = 0·0001) from the bees' bodies. In addition, there was no interaction between bee species and the degree to which the ethanol wiping removed G. sempervirens pollen (F4,104 = 0·7, P > 0·5), indicating that cleaning was equally effective across all bee species. Thus, while cleaning did not remove all pollen grains, there was no bias in terms of how much G. sempervirens pollen was removed by the cleaning treatments on the different bee species.
As a second control, 18 control stigmas were collected on four dates in March and April 2002 to determine whether the stigmas of unvisited, bagged, emasculated flowers received unsuspected pollen. Despite best efforts being made to prevent pollen deposition on emasculated flowers, low but consistent numbers of pollen grains were found, but never dye particles, on control stigmas (mean between 7·5 and 30·3 grains day−1). Due to this unexpected pollen deposition on bagged control flowers, the data (see ‘Statistical analysis’ section) were analysed in two ways: (1) with correction by subtracting the number of pollen grains on control stigmas on each date from the experimental pollen counts for that date; and (2) without correction. Qualitatively similar results were obtained for both analyses, and all results are presented using uncorrected data to avoid issues of nonindependence for stigmas collected on the same date.
Statistical analysis
In previous studies by other researchers, pollen and dye deposition were standardized (by dividing by the mean or total pollen receipt per sequence) to control for variation in deposition between sequences (e.g. Waser and Price, 1982; Campbell, 1985). However, in this study the interest was in measuring differences in deposition between bee species, and standardizing counts between sequences would mask such variation. Three stigmas from the experimental data, each visited by a different Bombus at a different point in the sequence, were omitted from all analyses as outliers because they had extremely high pollen counts (each over 900 % higher than the mean pollen deposition). In addition, on two stigmas dye could be counted but not pollen because the slides were broken while mounting the stigmas in fuchsin stain. Pollen and dye counts were log(x + 1) transformed to improve normality in all ANCOVAs (described below). Untransformed data were used in the correlation analysis.
Effect of visitor species and floral morph on pollen transfer
The effect of pollinator species, donor floral morph and recipient floral morph on mean pollen transfer and slope of transfer across a sequence of flowers was examined using ANCOVA. Bee species, donor morph and recipient morph were main effects and flower sequence number (1–4) was the covariate. Two-way interactions between bee species × flower sequence, bee species × donor morphology and bee species × recipient morphology were included. A main effect of bee species would indicate that different species transfer different numbers of pollen grains on average, while a bee species × flower sequence interaction would indicate that the slope of pollen transfer across flowers within a sequence varied between bee species. Additional interaction terms were not significant and their exclusion did not affect analyses. Duncan's multiple range tests (Littell et al., 2002) were used as a post hoc comparison of mean pollen transfer between bee species.
Comparing dye and pollen transfer
To determine whether dye transfer provides a reliable analogue for pollen transfer, three different approaches were used: (1) Pearson correlation coefficients were calculated for pollen and dye particles, using each recipient flower as an independent replicate; (2) presence/absence data of pollen and dye were compared to determine whether the presence of dye was a good indicator of the presence of pollen (Thomson et al., 1986; Waser, 1988) using a chi-squared test; (3) the ratio of (pollen) : (dye + 1) on each stigma was used as the dependent variable in an ANCOVA model (as described above) to understand whether flower sequence, bee species, or floral morphology had differential effects on pollen compared with dye. For example, a significant effect of flower sequence would indicate that the ratio of pollen to dye changes across a sequence. Log transformation was used to improve normality for the ANCOVA.
RESULTS
Pollen loads carried by different floral visitors
Naturally collected bee species varied significantly in the number (F4,42 = 5·13, P < 0·002) and proportion (F4,42 = 5·83, P < 0·001) of G. sempervirens pollen grains that they carried. Xylocopa, Osmia and Habropoda carried the most G. sempervirens pollen, followed by Bombus and Apis (Fig. 1A). All bee species carried approximately the same proportion of G. sempervirens pollen (57–69 %) except Apis (8 %; Fig. 1B).
Fig. 1.
Variation between naturally collected bee species in (A) the number and (B) the proportion of G. sempervirens grains carried by Bombus bimaculatus (bumble bees), Habropoda laboriosa (blueberry bees), Osmia lignaria (blue orchard bees), Apis mellifera (honey bees), and Xylocopa virginica (carpenter bees). Different letters represent significantly different means at α = 0·05. Means are plotted, and error bars indicate s.e.
Effect of visitor species and floral morph on pollen transfer
Visitor species varied significantly in their mean pollen transfer. Bombus, Osmia and Habropoda transferred significantly more pollen per flower than Apis (Table 1 and Fig. 2A). Xylocopa transferred significantly less pollen than all other bee species, even though they visited flowers through the corolla opening rather than robbing. Despite the difference in mean amount of pollen transferred, the slopes of pollen deposition across a sequence of flowers did not differ between bee species (bee species × flower sequence interaction; Table 1). Thus, the pattern of pollen deposition across a floral sequence did not differ between species.
Table 1.
Effect of bee species, donor and recipient floral morphology (pin versus thrum) and flower sequence on stigmatic pollen deposition, stigmatic dye deposition, and the ratio of pollen to dye per stigma
| Pollen |
Dye |
Pollen : dye |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | d.f. | SS | F | d.f. | SS | F | d.f. | SS | F |
| Bee species | 4 | 20·01 | 3·04* | 4 | 38·0 | 4·61** | 4 | 14·3 | 1·7 |
| Donor morph | 1 | 0·19 | 0·11 | 1 | 19·3 | 9·35** | 1 | 18·2 | 8·65** |
| Recipient morph | 1 | 2·28 | 1·39 | 1 | 16·3 | 7·88** | 1 | 7·1 | 3·38† |
| Flower number | 1 | 15·2 | 9·24** | 1 | 104·1 | 50·43**** | 1 | 40·3 | 19·2**** |
| Bee × flower | 4 | 2·68 | 0·41 | 4 | 13·73 | 1·66 | 4 | 12·6 | 1·5 |
| Bee × donor | 1 | 0·02 | 0·01 | 1 | 0·36 | 0·17 | 1 | 0·0 | 0·0 |
| Bee × recipient | 3 | 1·62 | 0·33 | 3 | 9·6 | 1·55 | 3 | 3·6 | 0·6 |
| Error | 168 | 276·1 | 170 | 350·93 | 164 | 454·3 | |||
P < 0·05;
P < 0·01;
P < 0·00·1;
P = 0·07.
Fig. 2.
Mean (A) pollen transfer and (B) dye transfer. Different letters represent significantly different means at α = 0·05. Numbers underneath labels refer to sample sizes per bee species. Means are plotted, and error bars indicate s.e.
Although not statistically significant, mean pollen transfer was higher for pin than thrum flowers across bee species (mean ± standard error, here and below: donor flowers, pin 169·4 ± 32·9 grains, thrum 104·3 ± 10·8 grains; recipient flowers, pin 174·1 ± 32·9 grains, thrum 106·5 ± 11·0 grains; Table 1). However, due to highly unbalanced sample sizes for pin and thrum morphs (see Materials and methods), power was low to detect such an effect. A retrospective power analysis was conducted, and then solved for the least significant number of samples or replicates that would be needed to find a significant effect of recipient and donor floral morph on pollen receipt. The analyses use and assume the same structure and estimates as in the dataset, taking into account the sample size, the specified alpha and the standard deviation of the error. It was found that this study had a power of 0·22 to detect a significant difference in pollen receipt as a function of recipient floral morphology at α = 0·05, and a power of 0·05 to detect a significant difference in pollen receipt as a function of donor morph. With the unbalanced design of this study, over 600 and 3000 flowers would have been needed to detect an effect of recipient and donor morph, respectively, at α = 0·05.
Neither donor nor recipient morph significantly affected the slope of pollen deposition across a sequence of floral morphs (Table 1). Finally, bee species did not differ in the amount of pollen transferred to and from flowers of each morph (donor morph × bee and recipient morph × bee interactions; Table 1). However, this study had relatively low power to detect effects of interactions with floral morph.
Comparing dye and pollen transfer
Pollen and dye deposition were strongly correlated when averaging across all pollinators and flowers (N = 186, r = 0·62, P < 0·0001). This correlation was slightly stronger when only the first flower in a sequence was analysed to ensure sample independence (N = 52, r = 0·68, P < 0·0001). Out of 183 stigmas, 180 received pollen. Of these, 69 % also had dye. Of the three stigmas without pollen, two also had no dye. Given that only two-thirds of stigmas consistently had or lacked both pollen and dye, the absence of dye was not a reliable indicator of the absence of pollen (N = 183, χ2 = 1·72, P = 0·19). However, the significant positive correlation between dye and pollen deposition indicates that when dye is present, the number of dye particles provides a reasonable indicator of the number of pollen grains deposited.
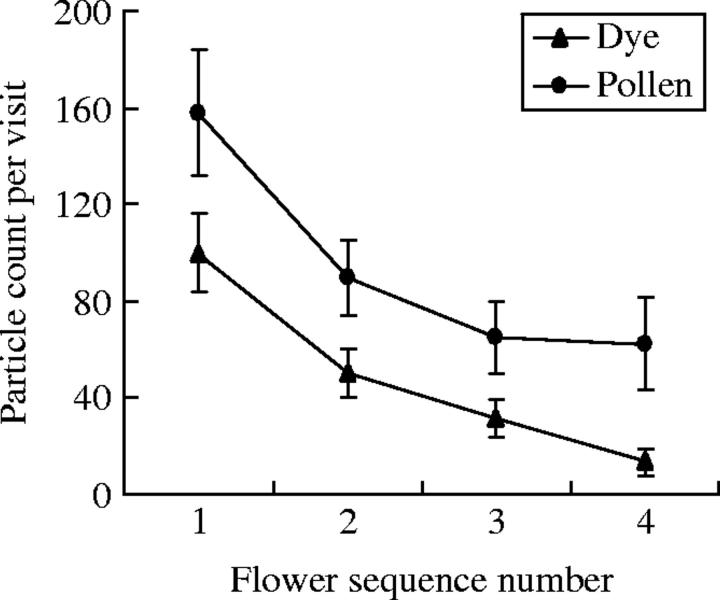
The ratio of pollen to dye particles was significantly affected by flower sequence and by donor morphology (Table 1). An examination of pollen compared with dye deposition shows that the rate of pollen decay is shallower than the rate of dye decay (Fig. 3). Additionally, dye deposition appears to be more sensitive to floral morph than pollen deposition (Table 1; dye deposition: pin 135·4 ± 27·3 dye particles, thrum 37·9 ± 4·6 dye particles; pollen deposition: pin 169·4 ± 32·9 pollen grains, thrum 104·3 ± 10·8 pollen grains), and there was a marginally significant effect of recipient morph on the ratio of pollen to dye received, despite the low power to detect morphology effects (Table 1; uncorrected means: pin, 6·25 ± 2·7; thrum, 17·54 ± 4·1). There were no other significant effects on the pollen : dye ratio, indicating that bee species do not transfer pollen at rates different from dye particles (Table 1).
Fig. 3.
Rate of decay across flower sequence for pollen and dye particles, averaged across all bee species. Sample sizes for each flower sequence number are 53, 52, 47 and 37, respectively. Means are plotted, and error bars indicate s.e.
DISCUSSION
Comparison of bee species as effective pollinators
It was found that Bombus, Osmia and Habropoda transferred equivalent amounts of pollen, and significantly more than Apis or Xylocopa. This work joins an increasing number of studies demonstrating that floral visitor species vary in their ability to transfer pollen between plants. For example, bees were more effective pollinators of the butterfly-weed, Asclepias tuberosa, than butterflies (Fishbein and Venable, 1996) and bumble bees were more effective pollinators of Ipomopsis aggregata than hummingbirds (Waser, 1988; Mayfield et al., 2001). Introduced honey bees transfer less pollen per flower compared with native pollinators on almond, cranberry, onions and the tropical dry forest annual Kallstroemia grandiflora (Bosch and Blas, 1994; Osorio-Beristain et al., 1997; Cane and Schiffhauer, 2003), a result consistent with the present study. However, honey bees are not always inefficient at transferring pollen. For example, honey bees have comparable efficiency with other bees when visiting the milkweeds Asclepias tuberosa and A. incarnata (Fishbein and Venable, 1996; Ivey et al., 2003) and oilseed rape, Brassica napus (Cresswell et al., 1995). In an extensive field survey of 29 insect species from three orders on Lavandula latifolia, Herrera (1987) found relatively high pollinator efficiency by Hymenoptera, including honey bees and a Xylocopa carpenter bee. One cautionary note about the present results is that some visitors are effective at removing but not at depositing pollen or vice versa (Wilson and Thomson, 1991; Conner et al., 1995). Thus, studies that tease apart the removal versus deposition components of pollinator efficiency will provide additional ecological insight.
For G. sempervirens, Xylocopa were the least effective at transferring pollen (even when visiting legitimately as in this study; Fig. 2). However, field-caught Xylocopa near the study site carried the highest amounts of G. sempervirens pollen (Fig, 1A), and a high proportion of their total pollen came from G. sempervirens (Fig. 1B). Furthermore, Xylocopa were one of the most frequent floral visitors in a related study (Adler and Irwin, 2005). The net effect of carpenter bees on plant reproduction will depend on whether the low transfer efficiency is counterbalanced by the high amount of G. sempervirens pollen carried by Xylocopa, whether small amounts of pollen can still effect fruit set, and the abundances of more efficient pollinators in the community (Lau and Galloway, 2004). In many cases, low pollen deposition and/or tissue damage due to nectar robbing can result in no fruit set and thus a negative impact on plant reproduction (Maloof and Inouye, 2000; Irwin et al., 2001). Determining whether the outcome of Xylocopa floral visitation is positive or negative for G. sempervirens would require following flowers visited by Xylocopa (robbing and legitimate visits) over the season to determine fruit set efficiency relative to visits by pollinating bees.
One surprising result was that bee size did not predict bees' ability to transfer pollen. The original hypothesis was that larger bees (i.e. Xylocopa and Bombus) when visiting legitimately would be better at transferring pollen due to a greater likelihood of contacting plant sexual organs. Why Apis and Xylocopa (a smaller and larger species, respectively) were the least efficient visitors is not clear, especially given that bees did not have the opportunity to groom between floral visits. Apis may be ineffective pollinators due to their highly generalist foraging patterns, indicated by the low proportion of Gelsemium pollen carried on their bodies (Fig. 1B). Xylocopa, on the other hand, rarely visits flowers legitimately in nature (L. S. Adler and R. E. Irwin, personal observation) and so may be less effective at foraging through the corolla opening. Additionally, the amount or proportion of Gelsemium pollen carried on bees' bodies did not predict pollen transfer efficiency (compare Figs 1 and 2A); Xylocopa carried large amounts and a high proportion of Gelsemium pollen but was very poor at transferring pollen to stigmas. Thus, pollen carried on bees' bodies is not a reliable proxy for pollinator efficiency. Other species-level factors, such as hairiness or morphological fit, may play a larger role in pollinator efficiency than has been previously recognized. Such a hypothesis warrants further attention, but could be more easily addressed using a plant species with a large pollinator assemblage (e.g. Herrera, 1987; Fishbein and Venable, 1996; Gomez and Zamora, 1999) where the relationship between morphology, size, hairiness and pollinator species efficiency could be compared within a phylogenetic context.
One caveat of the present study is that pollinator efficiency was considered solely in the context of pollen transfer rather than seed set, which is the ultimate measure of pollination effectiveness from the female plant perspective. Pollinators can vary in their per-visit effectiveness for seed set (e.g. Parker, 1982; Olsen, 1997, and references therein). However, pollen deposition may not translate directly into seed set, due to variation in pollen quality (Waser and Price, 1991), clogging from heterospecific pollen (Waser, 1978), and saturation of dose–response relationships (Waser and Price, 1991; Bosch and Waser, 1999; Cane and Schiffhauer, 2003). Thus, the conclusions regarding variation in effectiveness of pollinator species might be modified if consequences for seed set were considered as an additional measure.
Pollen transfer in heterostylous species
One of the challenges for self-incompatible flowering plants is to minimize the amount of pollen transferred within a plant (selfing or geitonogamy) and maximize the amount of outcross pollen (de Jong et al., 1993). Heterostyly, a floral polymorphism that is characterized by the reciprocal positioning of stigmas and anthers between morphs, occurs in approx. 28 angiosperm families (Barrett et al., 2000). Heterostyly may have evolved as a mechanism to promote disassortative pollen transfer between anthers and stigmas of floral morphs (Ganders, 1979), because pollen from long-filament flowers would most likely be transferred to long-styled flowers due to pollen placement on pollinators' bodies (Darwin, 1877). There has been some evidence for such disassortative mating in several distylous species (Wolfe and Barrett, 1989; Ree, 1997; Barrett et al., 2000). In the present study, however, no evidence was found that floral morph of donor or recipient flowers affected the amount of pollen transferred, although there was low power to detect such an effect. However, per-visit pollen transfer is just one mechanism that can promote disassortative pollen movement between floral morphs. The present experimental design excluded the possibility of disassortative movement among floral morphs due to pollinator foraging behaviour and discrimination within and among morphs. This could play a significant role in determining the amount of pollen transfer between morphs in natural populations.
Fluorescent dye as an analogue for pollen transfer
The utility of fluorescent dye as a pollen analogue has been studied in several other systems using hummingbirds and bumble bees as vectors (Waser and Price, 1982; Thomson et al., 1986; Waser, 1988; Fenster et al., 1996; Rademaker et al., 1997). The present results are within the range of those found in other studies. A correlation of 0·62 was found between the total amount of dye and pollen found on individual stigmas; most other studies have reported r-values between 0·4 and 0·8. Given that pollen or dye particles were not standardized between sequences, and that transfer was compared between several pollinators, the assertion is justified that fluorescent dye provides a reliable analogue for pollen movement in the present system. However, a difference in the ratio of pollen to dye across even a short flower sequence was found (Fig. 3), indicating that dye would underestimate pollen movement, especially across a longer sequence of flower visits. Thus, dye movement is likely to provide a conservative estimate of pollen movement in the present system, as has been found for hummingbirds visiting Delphinium nelsonii (Waser, 1988). However, dye and pollen movement were equivalent for bumble bees visiting D. nelsonii and hummingbirds visiting Ipomopsis aggregata (Waser and Price, 1982; Waser, 1988), and in Erythronium grandiflorum dye moves farther than pollen across a flower sequence (Thomson et al., 1986). Thus, the pattern of dye and pollen movement may vary between systems.
Pollinator species could vary in their ability to transfer dye compared with pollen due to morphological or behavioural differences. If this were the case, dye might be a better analogue for pollen movement in some pollinators compared with others. There appears to be only one other study that has expressly considered differences between pollinator species when determining the utility of dye as a pollen analogue. Waser (1988) compared dye and pollen movement by hummingbirds and bumble-bees, two very different pollinators of Delphinium nelsonii. He demonstrated that while dye movement closely approximated pollen movement by bumble-bees, hummingbird visitation caused more variable deposition of dye and pollen and longer pollen carryover on average than dye. By contrast, in the present study it was found that bee species did not differ in the ratio of pollen to dye transferred or in the rate of decay of pollen versus dye across a sequence of flowers (bee × flower interaction; Table 1). This suggests that dye is equally effective as a pollen analogue across this assemblage of pollinating bees.
Most other studies that have examined pollen and dye transfer in controlled experiments used longer flower sequences per visitor than the present study, which used sequences of four flowers per individual bee. For example, Waser (1988) used between ten and 36 flowers per sequence for individual bumble bees and hummingbirds, Rademaker et al. (1997) offered sequences of 32 emasculated flowers to bumble bees, and Fenster et al. (1996) used between five and 12 flowers per sequence of a single hummingbird. Shorter sequences were used because the goal of the present study was to compare pollen and dye transfer by different bee species, which requires greater numbers of replicate individual bees. With limited numbers of flowers, it was decided to increase the sample size (number of individual bees) rather than the sequence length. Whereas most studies use 10–20 individual pollinators to measure pollen and dye transfer of one or two pollinator species (Waser, 1988; Fenster et al., 1996; Rademaker et al., 1997), a total of 54 bees from five different pollinator species was used in the present study. Thus, the study was designed to emphasize comparing bee species in pollen transfer efficiency, rather than characterizing pollen and dye movement patterns over long flower sequences.
In conclusion, it was demonstrated that floral visitors can vary widely in the amount of pollen they carry on their bodies and in their ability to transfer pollen across a sequence of flowers. Moreover, it was found that fluorescent dye particles can provide an analogue of pollen transfer in this system. The present results add to a growing number of studies highlighting that floral visitors vary in pollination effectiveness (Waser et al., 1996), and that pollinators carrying the most pollen on their bodies may not be the most efficient at depositing pollen on stigmas. However, the present results are contrary to studies on many heterostylous species showing that anther and stigma position affect pollen removal and deposition (e.g. Wolfe and Barrett, 1989; Harder and Barrett, 1993). Taken together, these results suggest that more studies are needed to assess how variation in both visitor species and in floral morphology can alter pollen transfer in natural plant populations. Understanding the magnitude of variability in pollinator quality is one of several important factors for predicting how different pollinator taxa may influence the evolution of floral traits.
Acknowledgments
We thank J. Affolter and the Botanical Gardens of the University of Georgia for their generous accommodation during this research; J. Neff for bee identification; M. Price for advice with statistical analyses; J. Barron, L. Burkle, G. Crutsinger, B. DeGasperis, G. Gisler, A. Lentz, H. Norden, J. Tian and R. Wallace for their hard work in the field; J. Vineis, J. Barron and M. Johnson for counting pollen grains on stigmas; and S. Richardson, J. Rudgers, N. Waser and one anonymous reviewer for helpful comments on the manuscript. This research was funded by the Virginia Tech Deparatment of Biology (L.S.A.), NSF DEB-0089643 (R.E.I.), and NSF DEB-0211480 (L.S.A. and R.E.I.).
LITERATURE CITED
- Adler LS, Irwin RE. 2005. Ecological costs and benefits of defenses in nectar. Ecology 86: 2968–2978. [Google Scholar]
- Aigner PA. 2001. Optimality modeling and fitness trade-offs: when should plants become pollinator specialists? Oikos 95: 177–184. [Google Scholar]
- Barrett SCH, Jesson LK, Baker AM. 2000. The evolution and function of stylar polymorphisms in flowering plants. Annals of Botany 85:253–265. [Google Scholar]
- Bosch J, Blas M. 1994. Foraging behavior and pollinating efficiency of Osmia cornuta and Apis mellifera on almond (Hymenoptera, Megachilidae and Apidae). Applied Entomology and Zoology 29: 1–9. [Google Scholar]
- Bosch M, Waser NM. 1999. Effects of local density on pollination and reproduction in Delphinium nuttallianum and Aconitum columbianum (Ranunculaceae). American Journal of Botany 86: 871–879. [PubMed] [Google Scholar]
- Campbell DR. 1985. Pollen and gene dispersal: the influences of competition for pollination. Evolution 39: 418–431. [DOI] [PubMed] [Google Scholar]
- Campbell DR. 1989. Inflorescence size: test of the male function hypothesis. American Journal of Botany 76: 730–738. [Google Scholar]
- Campbell DR, Waser NM, Price MV, Lynch EA, Mitchell RJ. 1991. Components of phenotypic selection: pollen export and lower corolla width in Ipomopsis aggregata. Evolution 45: 1458–1467. [DOI] [PubMed] [Google Scholar]
- Cane JH, Schiffhauer D. 2003. Dose-response relationships between pollination and fruiting refine pollinator comparisons for cranberry (Vaccinium macrocarpon Ericaceae). American Journal of Botany 90: 1425–1432. [DOI] [PubMed] [Google Scholar]
- Conner JK, Davis R, Rush S. 1995. The effect of wild radish floral morphology on pollination efficiency by four taxa of pollinators. Oecologia 104: 234–245. [DOI] [PubMed] [Google Scholar]
- Cresswell JE, Bassom AP, Bell SA, Collins SJ, Kelly TB. 1995. Predicted pollen dispersal by honey-bees and three species of bumble-bees foraging on oil-seed rape: a comparison of three models. Functional Ecology 9: 829–841. [Google Scholar]
- Darwin C. 1877. The different forms of flowers on plants of the same species. London: John Murray.
- Dudash MR. 1991. Plant size effects on female and male function in hermaphroditic Sabatia angularis (Gentianaceae). Ecology 72: 1004–1012. [Google Scholar]
- Fenster CB, Hassler CL, Dudash MR. 1996. Fluorescent dye particles are good pollen analogs for hummingbird-pollinated Silene virginica (Caryophyllaceae). Canadian Journal of Botany 74: 189–193. [Google Scholar]
- Fishbein M, Venable DL. 1996. Diversity and temporal change in the effective pollinators of Asclepias tuberosa. Ecology 77: 1061–1073. [Google Scholar]
- Ganders FR. 1979. The biology of heterostyly. New Zealand Journal of Botany 17: 607–635. [Google Scholar]
- Gomez JM, Zamora R. 1999. Generalization vs. specialization in the pollination system of Hormathophylla spinosa (Cruciferae). Ecology 80: 796–805. [Google Scholar]
- Harder LD, Barrett SCH. 1993. Pollen removal from tristylous Pontederia cordata: effects of anther position and pollinator specialization. Ecology 74: 1059–1072. [Google Scholar]
- Herrera CM. 1987. Components of pollinator ‘quality’: comparative analysis of a diverse insect assemblage. Oikos 50: 79–90. [Google Scholar]
- Inouye DW. 1980. The terminology of floral larceny. Ecology 61:1251–1253. [Google Scholar]
- Inouye DW, Gill DE, Dudash MR, Fenster CB. 1994. A model and lexicon for pollen fate. American Journal of Botany 81: 1517–1530. [Google Scholar]
- Irwin RE, Adler LS. 2006. Correlations among traits associated with herbivore resistance and pollination: implications for pollination and nectar robbing in a distylous plant. American Journal of Botany 93 (in press).
- Irwin RE, Brody AK, Waser NM. 2001. The impact of floral larceny on individuals, populations, and communities. Oecologia 129: 161–168. [DOI] [PubMed] [Google Scholar]
- Ivey CT, Martinez P, Wyatt R. 2003. Variation in pollinator effectiveness in swamp milkweed, Asclepias incarnata (Apocynaceae). American Journal of Botany 90: 214–225. [DOI] [PubMed] [Google Scholar]
- de Jong TJ, Waser NM, Klinkhamer PGL. 1993. Geitonogamy: the neglected side of selfing. Trends in Ecology and Evolution 8: 321–325. [DOI] [PubMed] [Google Scholar]
- Jordano P. 1987. Patterns of mutualistic interactions in pollination and seed dispersal: connectance, dependence asymmetries, and coevolution. American Naturalist 129: 657–677. [Google Scholar]
- Jordano P, Bascompte J, Olesen JM. 2003. Invariant properties in coevolutionary networks of plant–animal interactions. Ecology Letters 6: 69–81. [Google Scholar]
- Kearns CA, Inouye DW. 1993. Techniques for pollination biologists. Niwot, CO: University Press of Colorado.
- Lau JA, Galloway LF. 2004. Effects of low-efficiency pollinators on plant fitness and floral trait evolution in Campanula americana (Campanulaceae). Oecologia 141: 577–583. [DOI] [PubMed] [Google Scholar]
- Littell RC, Stroup WW, Freund RJ. 2002. SAS for linear models, 4th edn. Cary, NC: SAS Institute.
- Maloof JE, Inouye DW. 2000. Are nectar robbers cheaters or mutualists? Ecology 81: 2651–2661. [Google Scholar]
- Mayfield MM, Waser NM, Price MV. 2001. Exploring the ‘most effective pollinator principle’ with complex flowers: bumblebees and Ipomopsis aggregata. Annals of Botany 88: 591–596. [Google Scholar]
- Memmott J. 1999. The structure of a plant-pollinator food web. Ecology Letters 2: 276–280. [DOI] [PubMed] [Google Scholar]
- Memmott J, Waser NM. 2002. Integration of alien plants into a native flower-pollinator visitation web. Proceedings of the Royal Society of London Series B 269: 2395–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michener CD, McGinley RJ, Danforth BN. 1994. The bee genera of North and Central America (Hymenoptera: Apoidea). Washington, DC: Smithsonian Institution Press.
- Mitchell TB. 1962. Bees of the Eastern United States. Raleigh, NC: North Carolina Agricultural Experiment Station.
- Motten AF, Campbell DR, Alexander DE, Miller HL. 1981. Pollination effectiveness of specialist and generalist visitors to a North Carolina population of Claytonia virginica. Ecology 62: 1278–1287. [Google Scholar]
- Olesen JM, Jordano P. 2002. Geographic patterns in plant–pollinator mutualistic networks. Ecology 83: 2416–2424. [Google Scholar]
- Olsen KM. 1997. Pollination effectiveness and pollinator importance in a population of Heterotheca subaxillaris (Asteraceae). Oecologia 109: 114–121. [DOI] [PubMed] [Google Scholar]
- Ornduff R. 1970. Systematics and breeding system of Gelsemium (Loganiaceae). Journal of the Arnold Arboretum 51: 1–17. [Google Scholar]
- Ornduff R. 1979. Features of pollen flow in Gelsemium sempervirens (Loganiaceae). Journal of the Arnold Arboretum 60: 377–381. [Google Scholar]
- Ornduff R. 1980. The probable genetics of distyly in Gelsemium sempervirens (Loganiaceae). Canadian Journal of Genetics and Cytology 22: 303–304. [Google Scholar]
- Osorio-Beristain M, Dominguez CA, Eguiarte LE, Benrey B. 1997. Pollination efficiency of native and invading Africanized bees in the tropical dry forest annual plant, Kallstroemia grandiflora Torr ex Gray. Apidologie 28: 11–16. [Google Scholar]
- Pailler T, Maurice S, Thompson JD. 2002. Pollen transfer patterns in a distylous plant with overlapping pollen-size distributions. Oikos 99: 308–316. [Google Scholar]
- Parker FD. 1982. Efficiency of bees in pollinating onion flowers. Journal of the Kansas Entomological Society 55: 171–176. [Google Scholar]
- Phillips HR. 1985. Growing and propagating wild flowers. Chapel Hill, NC: The University of North Carolina Press.
- Price MV, Waser NM. 1979. Pollen dispersal and optimal outcrossing in Delphinium nelsonii. Nature 277: 294–296. [Google Scholar]
- Price MV, Waser NM. 1982. Experimental studies of pollen carryover: hummingbirds and Ipomopsis aggregata. Oecologia 54: 353–358. [DOI] [PubMed] [Google Scholar]
- Primack RB, Silander JA. 1975. Measuring relative importance of different pollinators to plants. Nature 255: 143–144. [Google Scholar]
- Rademaker MCJ, de Jong TJ. 1998. Effects of flower number on estimated pollen transfer in natural populations of three hermaphroditic species: an experiment with fluorescent dye. Journal of Evolutionary Biology 11: 623–641. [Google Scholar]
- Rademaker MCJ, deJong TJ, Klinkhamer PGL. 1997. Pollen dynamics of bumble-bee visitation on Echium vulgare. Functional Ecology 11: 554–563. [Google Scholar]
- Ree RH. 1997. Pollen flow, fecundity, and the adaptive significance of heterostyly in Palicourea padifolia (Rubiaceae). Biotropica 29: 298–308. [Google Scholar]
- Schemske DW, Horvitz CC. 1984. Variation among floral visitors in pollination ability: a precondition for mutualism specialization. Science 225: 519–521. [DOI] [PubMed] [Google Scholar]
- Snow AA, Lewis PO. 1993. Reproductive traits and male fertility in plants: empirical approaches. Annual Review of Ecology and Systematics 24: 331–351. [Google Scholar]
- Tepedino VJ. 1981. The pollination efficiency of the squash bee (Peponapis pruinosa) and the honey bee (Apis mellifera) on summer squash (Cucurbita pepo). Journal of the Kansas Entomological Society 54: 359–377. [Google Scholar]
- Thompson JD. 2001. How do visitation patterns vary among pollinators in relation to floral display and floral design in a generalist pollination system? Oecologia 126: 386–394. [DOI] [PubMed] [Google Scholar]
- Thomson JD, Price MV, Waser NM, Stratton DA. 1986. Comparative studies of pollen and fluorescent dye transport by bumble bees visiting Erythronium grandiflorum. Oecologia 69: 561–566. [DOI] [PubMed] [Google Scholar]
- Vazquez DP, Simberloff D. 2002. Ecological specialization and susceptibility to disturbance: conjectures and refutations. American Naturalist 159: 606–623. [DOI] [PubMed] [Google Scholar]
- Waser NM. 1978. Interspecific pollen transfer and competition between co-occurring plant species. Oecologia 36: 223–236. [DOI] [PubMed] [Google Scholar]
- Waser NM. 1988. Comparative pollen and dye transfer by pollinators of Delphinium nelsonii. Functional Ecology 2: 41–48. [Google Scholar]
- Waser NM, Price MV. 1982. A comparison of pollen and fluorescent dye carry-over by natural pollinators of Ipomopsis aggregata (Polemoniaceae). Ecology 63: 1168–1172. [Google Scholar]
- Waser NM, Price MV. 1984. Experimental studies of pollen carryover: effects of floral variability in Ipomopsis aggregata. Oecologia 62:262–268. [DOI] [PubMed] [Google Scholar]
- Waser NM, Price MV. 1991. Outcrossing distance effects in Delphinium nelsonii: pollen loads, pollen tubes, and seed set. Ecology 72: 171–179. [Google Scholar]
- Waser NM, Chittka L, Price MV, Williams NM, Ollerton J. 1996. Generalization in pollination systems, and why it matters. Ecology 77: 1043–1060. [Google Scholar]
- Wilson P, Thomson JD. 1991. Heterogeneity among floral visitors leads to discordance between removal and deposition of pollen. Ecology 72: 1503–1507. [Google Scholar]
- Wolfe LM, Barrett SCH. 1989. Patterns of pollen removal and deposition in tristylous Pontederia cordata L. (Pontederiaceae). Biological Journal of the Linnean Society 36: 317–329. [Google Scholar]
- Young HJ. 2002. Diurnal and nocturnal pollination of Silene alba (Caryophyllaceae). American Journal of Botany 89:433–440. [DOI] [PubMed] [Google Scholar]