Abstract
• Background and Aims Abiotic stresses stimulate formation of active oxygen species in plant tissues. Among antioxidant mechanisms, H2O2 detoxication by ascorbate peroxidases (APX) plays an important role. Several APX isoforms exist in plant cells, and they have rarely been studied separately. The aim of this work was to study changes in cytosolic, peroxisomal, stromatic and thylakoid APX gene expression in response to progressive drought, rapid desiccation and application of exogenous abscisic acid in the leaves of cowpea (Vigna unguiculata) plants.
• Methods Two cowpea (V. unguiculata) cultivars, ‘EPACE-1’ which is drought-tolerant and ‘1183’which is drought-sensitive, were submitted to drought stress by withholding irrigation. Detached leaves were air-dried or treated with exogenous abscisic acid. APX cDNAs were isolated by PCR and cloned in plasmid vectors. Changes in gene expression were studied using reverse-transcription PCR.
• Key Results Four new V. unguiculata cDNAs encoding putative cytosolic, peroxisomal and chloroplastic (stromatic and thylakoidal) APX were isolated and characterized. In response to the different treatments, higher increases in steady-state transcript levels of the cytoplasmic and peroxisomal APX genes were observed in ‘1183’ compared with ‘EPACE-1’. On the other hand, the expression of the chloroplastic APX genes was stimulated earlier in the tolerant cultivar when submitted to progressive drought.
• Conclusions Water deficit induced differences in transcript accumulation of APX genes between the two cultivars that were related to their respective tolerance to drought. Chloroplastic APX genes responded early to progressive water deficit in the tolerant plant, suggesting a capacity to efficiently detoxify active oxygen species at their production site. The more sensitive ‘1183’ was also able to respond to drought by activating its whole set of APX genes.
Keywords: Active oxygen species, drought tolerance, ascorbate peroxidase, abscissic acid, gene expression, Vigna unguiculata
INTRODUCTION
Water deficit (drought and desiccation) is known to generate active oxygen species (AOS). Among these, H2O2 is produced mainly in the chloroplasts and mitochondria of stressed cells and is the source of important cell damage (Foyer et al., 1994; Dat et al., 2000). Protection against AOS involves water-soluble and lipophilic antioxidants, as well as the ascorbate–glutathione cycle. In this cycle, ascorbate peroxidase (APX; EC 1.11.1.11) catalyses the first reaction between H2O2 and ascorbate, giving rise to monohydroascorbate and H2O. APX is responsible for H2O2 detoxication in green leaves (Foyer and Harbinson, 1994; Chaudière and Ferrari-Iliou, 1999) and is considered as a key antioxidant enzyme in plants (Orvar and Ellis, 1997). APX activities are located in chloroplasts, cytosol, mitochondria and peroxisomes, each cellular compartment possessing one or several APX isoforms. APX cDNAs have now been isolated and characterized from several plant species (for review see, Shigeoka et al., 2002). The isoforms are named cytosolic APX (cAPX), peroxisomal or microbody APX (pAPX), chloroplastic, i.e. thylakoidal and stromatic APX (tAPX and sAPX, respectively). In arabidopsis, the same protein is dually targeted to mitochondria and chloroplast stroma (Chew et al., 2003). Recent studies have focused on changes in activity and gene expression for APX isozymes in higher plants subjected to environmental stresses, such as ozone, high light, extreme temperatures and salt (Shigeoka et al., 2002). As far as is known, the only report concerned with the effect of water deficit on the expression of different APX isoform genes is in air-desiccated spinach leaves (Yoshimura et al., 2000) and there is not one dealing with plants submitted to progressive drought.
To understand better the role of the different forms of APX under water deficit, comparative studies were carried out using two Vigna unguiculata (Vu) cultivars: ‘EPACE-1’ which is drought-tolerant and ‘1183’ which is drought-sensitive. When submitted to drought, the two cultivars showed different physiological responses: EPACE-1 closed its stomata at lower leaf water potentials and therefore maintained photosynthetic activity longer than 1183 did (Cruz de Carvalho et al., 1998). At the cellular level, EPACE-1 could maintain cellular homeostasis at lower leaf water potentials compared with 1183 (Vasquez-Tello et al., 1990). This resulted from a lesser stimulation of lipolytic and proteolytic activities (Roy-Macauley et al., 1992; Sahsah et al., 1998) and from lower expression of the corresponding genes (El Maarouf et al., 1999; Cruz de Carvalho et al., 2001; Matos et al., 2001). Also, EPACE-1 was less prone to photooxidative damage (Ferrari-Iliou et al., 1994) and could accumulate more protective molecules (d'Arcy-Lameta et al., 1996).
In this study, first APX activities were measured in the leaves of cowpea plants submitted to drought, then four cowpea APX cDNAs corresponding to Vu1183 cytosolic (cAPX), peroxisomal (pAPX) and chloroplastic (thylakoid and stromatic, tAPX and sAPX, respectively) leaf compartments were isolated and characterized. The APX gene expression was studied in response to progressive drought and air desiccation. Exogenous abscisic acid (ABA) treatments were also carried out, as ABA is known to be involved in drought-induced signalling pathways leading to adaptive antioxidant processes (Zhu, 2002).
MATERIALS AND METHODS
Plant material and stress treatments
Two Vigna unguiculata (L.) Walp. (Vu) cultivars were used: ‘EPACE-1’ which is drought-tolerant originated from semi-arid north-eastern Brazil and ‘1183’ which is drought-sensitive originated from humid areas of China. Plants were grown in pots (9 cm diameter), in a mixture of peat and vermiculite (50 : 50, v/v), in a greenhouse under conditions described previously (El Maarouf et al., 1999). They were watered daily with tap water and twice a week with a modified Hewitt's nutrient solution. At 5 weeks, second and third expanded leaves were harvested at 10 a.m. after 4 h of illumination, frozen in liquid nitrogen and stored at −80 °C until needed.
Different harmful conditions known to cause oxidative stress were applied to cowpea EPACE-1 and 1183 plants to characterize expression of the VuAPX cDNA isolated here: air desiccation, exogenous ABA treatment and progressive drought. Desiccated leaves were air-dried at 24 °C under dim light for 30 min, 2 h and 5 h. Exogenous ABA was applied by immersing the petioles of excised leaves in a 0.1 mm aqueous solution of mixed ABA isomers (Sigma, St Quentin Fallavier, France) for 30 min, 2 h and 24 h under ambient conditions (24 °C and 250 µmol s−1 m−2 light intensity). Progressive drought was applied by withholding irrigation from treated plants for 2–15 d. Leaf water potentials (ψw) were measured using a pressure bomb (PMS instrument, Corvallis, USA) (Scholander et al., 1964). In control leaves, water potentials were −0·3 to −0·5 MPa. Treated leaves were harvested at ψw = −1·0 ± 0·1 MPa (S1), ψw = −1·5 ± 0·2 MPa (S2) and ψw = −2·0 ± 0·2 MPa (S3).
Ascorbate peroxidase activity
To prepare APX extracts, 1 g of fresh leaf tissue was homogenized in 10 ml of 50 mm phosphate buffer pH 7·8, containing 2 mm EDTA, 0·5 mm phenylmethylsulfonyl fluoride and 2 mm ascorbate, in the presence of 1·2 % insoluble PVP and 0·1 % Triton X-100. Leaf homogenates were centrifuged at 30 000 g for 20 min at 4 °C, APX activity was measured in the supernatant as the decrease in A290 per mg of protein and per minute (Nakano and Asada, 1981). Protein contents were determined according to Bradford (1976) with the Bio-Rad protein assay reagent, using bovine serum albumin as a standard.
RNA extraction and cDNA identification
Total RNA was isolated from leaf samples using RNeasy plant midi or maxi Kit (Qiagen, Courtaboeuf, France). Poly (A)+ RNA was isolated from total RNA using Oligotex columns (Qiagen, France), following manufacturer's instructions. Total and mRNA were quantified using a Nanodrop ND-1000 spectrophotometer (Starlab, Paris, France) at 260 nm.
Previously isolated APX sequences were aligned using the CLUSTALW program (Thompson et al., 1994). Degenerated oligonucleotides were designed from conserved regions and used as primers. Reverse-transcription PCR (RT–PCR) was performed from 100-ng 1183 mRNA template, using One Step RT–PCR kit (Qiagen, France). cDNA fragments were PCR amplified in 35 cycle reactions at 50 °C annealing temperature for cAPX and 60 °C for pAPX, tAPX and sAPX. Amplified APX cDNA fragments were purified by Wizard PCR Prep (Promega, Charbonnières, France) after electrophoretic separation on 1·2 % (w/v) agarose gels and cloned in pGEM-T Easy plasmid, according to the manufacturer's instructions (Promega, France). DNA sequencing was performed on both strands (ESGS, Lille, France).
The four putatively full-length APX cDNA (cAPX, pAPX and chloroplastic t and sAPX) were obtained, using 3′- and 5′-RACE method (5′/3′ RACE kit, Roche Diagnostics, Meylan, France), following the manufacturer's protocols. 1183 purified poly (A)+ mRNA was used as a template.
RT–PCR and gene expression
APX gene expression analysis was carried out by RT–PCR reactions, using One-Step RT–PCR Kit (Qiagen, France). Fifty or 100 ng of total leaf RNA were used as templates. Vu APX specific primers were used to amplify cDNA fragments of 790 bp (cAPX), 387 bp (pAPX), 396 bp (tAPX) and 551 bp (sAPX). To evaluate the amount of template RNA in each RT–PCR reaction, a region of Vu 18S gene encoding a ribosomal RNA with near constitutive expression under water deficit conditions was amplified. All PCR reactions included an initial denaturation step at 94 °C, followed by 30–35 cycles depending on the template with a denaturation step (40 s at 94 °C), an annealing step (40 s at 60 °C) and an extension step (40 s at 72 °C). A final extension was carried out for 7 min at 72 °C. Amplification products were visualized on 1·2 % (w/v) agarose gels, using a UV light transilluminator (Snapshot, Syngene, Ozyme, Saint Quentin en Yvelines, France). Densitometric evaluation of DNA bands were performed with the Imager 1D/2D software (Appligene, Strasbourg, France). Preliminary experiments (not shown) with various PCR cycle numbers indicated that in the RT–PCR conditions in this study (50 ng RNA, 30 cycles for cAPX and pAPX, 250 ng RNA and 35 cycles for chloroplastic APX), amplifications were not in the plateau phase, and therefore allowed for semi-quantitative estimations of transcript levels.
RESULTS AND DISCUSSION
Effect of water withholding on APX activity
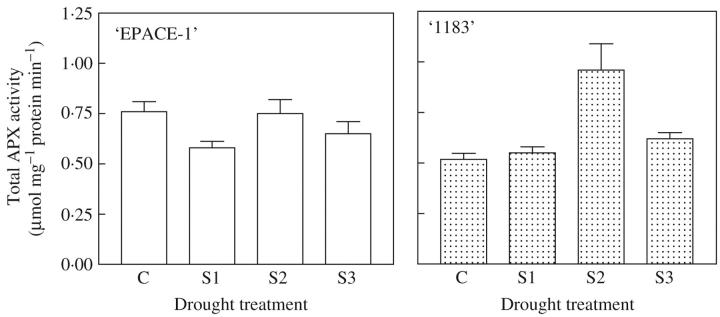
APX activity was 60 % higher in control EPACE-1 plants than in control 1183 plants (Fig. 1). In response to drought stress (S1, S2 and S3 plants), variations in APX activity were not significant in EPACE-1. Conversely, drought conditions induced significant changes in the levels of APX activity in 1183 since S2 plants showed a 78 % increase compared with control plants. In S3 plants, however, APX activity decreased back to control level (Fig. 1). Changes in APX activities in higher plants subjected to environmental stresses have been extensively studied (for a review see, Shigeoka et al., 2002). Results reported in the literature are contradictory and it is of no doubt that the plant response largely depends on its tolerance or susceptibility to stress and on the way the stress is applied to plants. The present results on total APX activities showed different effects of water stress on the two cowpea cultivars. In response to mild water stress, APX activity was stimulated in the drought-susceptible plant and remained stable in the tolerant one. Several APX isoforms exist in plant cells (review by Shigeoka et al., 2002). Since drought induces damage to membranes leading to loss of cell compartmentation (Pham Thi and Vieira de Silva, 1975; Ferrari-Iliou et al., 1984), it was not possible to measure accurately APX activities in different cell compartments in plants submitted to water stress. Therefore, to understand better the respective role of each APX isoform, the corresponding genes were cloned and the effect of water deficit on their expression levels was studied.
Fig. 1.
Effect of drought treatment on total APX activity of extracts from cowpea (Vigna unguiculata) ‘EPACE-1’ and ‘1183’ leaves. Control plants C, ψw = –0.5 ± 0.1 Mpa; or droughted plants: S1, ψw = –1.0 ± 0.1 MPa; S2, ψw = –1.5 ± 0.2 MPa; S3, ψw = –2.0 ± 0.2 MPa. APX activity was assayed by following oxidation of ascorbate to dehydroascorbate (decrease in A290) and expressed in μmol mg−1 protein min−1. ANOVA analysis was carried out on APX activity corresponding to five to nine independent experiments.
Isolation and characterization of four APX cDNAs
Regarding cytosolic APX, a 1117-bp-long cDNA denoted VucAPX (Genbank, accession number U61379) was obtained. The open reading frame (ORF) started with an ATG codon at position 89 and was interrupted by a stop codon (TAA) at position 839. It was flanked by an 89-bp 5′ untranslated region (UTR) and a 278-bp 3′ UTR. A putative polyadenylation signal ‘AAATAA’ was found at position 984. The deduced protein had 250 amino acid residues and a calculated mol. wt of 27 kDa. At the amino acid level, it shared 92 % and 80·8 % identity with pea (X62077; Mittler and Zilinskas, 1991) and radish (X78452; Lopez et al., 1994) cAPX, respectively.
Regarding peroxisomal APX, a full-length cDNA of 1169 bp was obtained and denoted VupAPX (AY466858). Its ORF started with an ATG codon at position 130 and terminated with a stop codon (TAA) at position 1094. It was flanked by 172 additional nucleotides on the 3′ end. A putative polyadenylation signal ‘AAATAA’ was found at position 1109. The ORF encoded a deduced polypeptide of 320 amino acid residues (calculated mol. wt 31·7 kDa) that shared 84·7 % and 75·7 % sequence identity with Cucurbita (AB070626) and Hordeum (AB063117) pAPX, respectively.
Regarding chloroplastic APX, two cDNA were obtained. The longest thylakoidal cDNA sequence, designated VutAPX (AY484492), was 1354 bp long. The ORF started with an ATG codon at position 61 and terminated with a TAG stop codon at position 1299. A putative polyadenylation signal ‘ATAAT’ was found at position 1329. The ORF encoded a deduced protein of 413 amino acid residues (calculated mol. wt 45·2 kDa) that shared 77·5 % and 79·3 % sequence identity with tAPX from spinach (D77997) and pumpkin (D83656), respectively. The longest stromatic cDNA sequence, denoted VusAPX (AY484493), was 1505 bp long. Its ORF started with an ATG codon at position 61 and terminated with a TAA stop codon at position 1153, the ORF encoded a deduced protein of 364 amino acid residues identical to that of VutAPX (calculated mol. wt 39·8 kDa). It was followed by 350 additional nucleotides. Among these, a 151-nucleotide sequence corresponded to an intron (Ishikawa et al., 1996; Mano et al., 1997) and a 195-bp span was identical to the end of the 3′-end region of the VutAPX sequence (Fig. 2).
Fig. 2.
Nucleotide sequence alignment of the 3′ ends of Vigna unguiculata ‘1183’ sAPX and tAPX cDNA (nucleotides 1081–1505). sAPX has an additional 151-bp nucleotide sequence with a stop codon TAA (⇓) at position 1153 bp. In tAPX, a stop codon TAG (⇓) is found at position 1297 bp. The asterisk indicates the putative polyadenylated signal.
The four cowpea APX deduced proteins have been aligned and compared with a pea cytosolic APX (Mittler and Zilinskas, 1991) (Fig. 3). Amino acid residues essential for enzymatic activity (Jespersen et al., 1997; Shigeoka et al., 2002) were conserved in the cowpea sequences and were referenced according to the pea cAPX (Y62077).
Fig. 3.

Comparison of Vu APX-deduced amino acid sequences with Pisum sativum (Ps) cAPX (Mittler and Zilinskas, 1991). VucAPX, cytosolic APX; VupAPX, peroxisomal APX; VutAPX, thylakoidal APX; VusAPX, stromatic APX. The asterisks at the bottom indicate conserved amino acids; dashes indicate gaps introduced to maximize alignment. The arrow at the top indicates the putative cleavage sites of transit peptides of the chloroplast isoforms. The arrows (⇓) at the top indicate essential amino acid residues: Arg38, Trp41, His42, Glu65, Asn71, Glu112 (cAPX) or Lys112 (chloroAPX), His143, His163, Arg172, Trp179, Asp208 (Shigeoka et al., 2002).
The thylakoidal and stromal APX sequences described herein were identical, except for the presence of a 49 amino acid region at the C-terminal end of the thylakoidal sequence with a very hydrophobic profile which could correspond to a membrane-spanning region (Shigeoka et al., 2002). As is the case in spinach (Ishikawa et al., 1996), pumpkin (Mano et al., 1997), Mesembryanthemum crystallinum and tobacco (Shigeoka et al., 2002), cowpea tAPX and sAPX could be coded by a single gene alternatively spliced. In Vu chloroplastic APX, Phe 175 (as referred to pea cAPX) was changed in Trp, as is the case in spinach (Shigeoka et al., 2002). Sequence alignment revealed the presence of 61 additional amino acid residues at the N-terminal end of the two chloroplastic APX compared with the other Vu APX. These regions were identified as chloroplastic targeting sequences (ChloroP program, //psort.nibb.ac.jp) with a putative cleavage site at Lys69 (Shigeoka et al., 2002) leading to putative mature polypeptides of 38·2 kDa and 32·8 kDa, for tAPX and sAPX, respectively.
Compared with VucAPX, VupAPX presented an additional C-terminal region of 39 amino acid residues that shared high sequence similarity with Cucurbita and arabidopsis pAPX C-terminal regions. In these putative membrane-spanning regions, the YEVKRXK-COOH motif seemed characteristic of peroxisomal (microbody) proteins (Mullen and Trelease, 2000). According to Bunkelman and Trelease (1996) the pAPX is bound to the external side of the membrane of peroxisomes.
Effect of stress conditions on APX gene expression
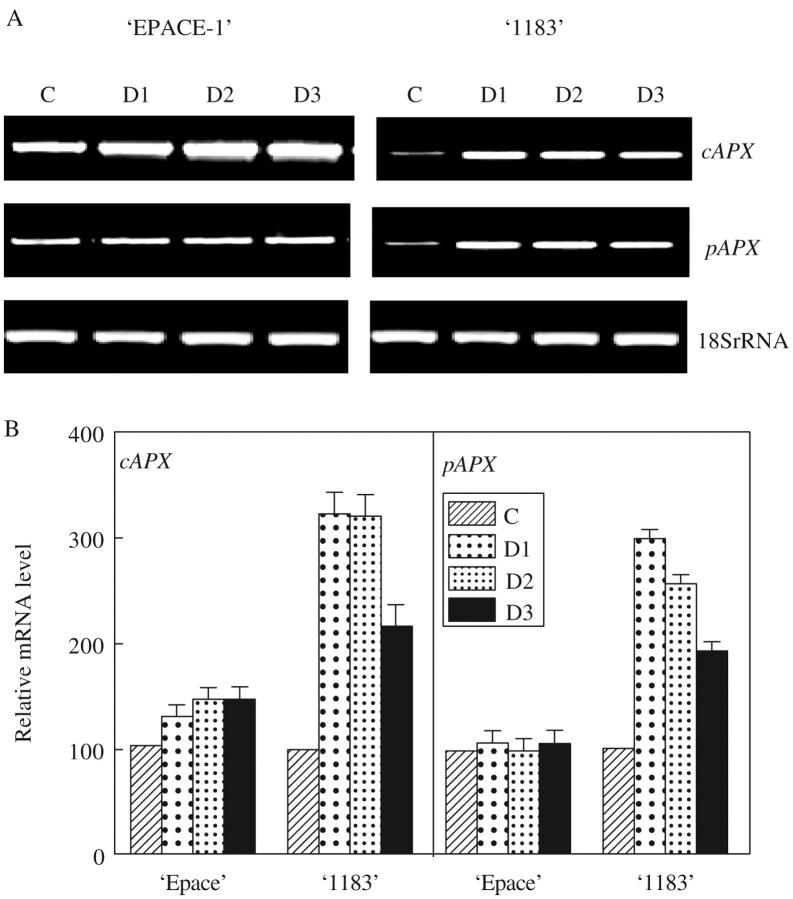
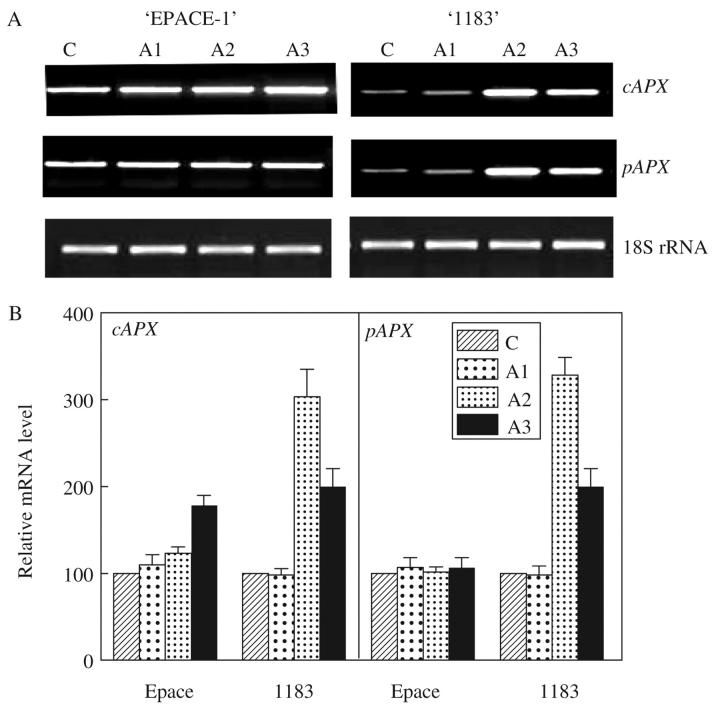
In EPACE-1 air-desiccated leaves, no significant changes were observed in steady-state levels of VucAPX and VupAPX transcripts in response to rapid water loss (Fig. 4) and exogenous ABA treatment (Fig. 5). In the case of 1183, important increases in steady-state transcript levels of cAPX and pAPX were observed after 2 h of ABA treatment and after 30 min of desiccation. It is interesting to note that VupAPX, which codes for a protein facing the cytosolic side of peroxisomes, showed an expression profile similar to that of VucAPX. The present results concerning EPACE-1 are equivalent to those obtained by Yoshimura et al. (2000) on spinach. However, results on the sensitive cowpea cultivar are different and indicate an up-regulation of APX gene expression by rapid water loss and ABA. ABA is implicated in signalling pathways induced by water stress (Shinozaki and Yamaguchi-Shinozaki, 1997; Chinnusamy et al., 2004) and APX gene expression was stimulated by ABA (Jiang and Zhang, 2002). However, since APX gene expression induced by ABA occurred later than drought-induced transcript accumulation a role for ABA in drought-induced APX transcript accumulation is not supported. The role of other mechanisms, such as changes to photosynthesis, have to be considered (Chang et al., 2004).
Fig. 4.
Effect of desiccation (D) treatment on mRNA abundance of cAPX and pAPX and 18S rRNA in Vigna unguiculata ‘EPACE-1’ and ‘1183’ leaves. Desiccated leaves were air-dried at 24 °C for 30 min (D1), 2 h (D2) and 5 h (D3). Control leaf samples (C) were taken prior to treatments. (A) Gel analysis of APX isoenzyme transcripts. After RT–PCR carried out on 50 ng of total RNA with 30 cycles, samples were visualized using UV light transilluminator. (B) Relative mRNA levels. The mRNA level of each sample was quantified with the Imager 1D/2D software and normalized to the respective 18S ribosomal RNA. The values represent the mean ± s.d. of three experiments.
Fig. 5.
Effect of exogenous abscisic acid (ABA) treatment on mRNA abundance of cAPX and pAPX and 18S rRNA in Vigna unguiculata ‘EPACE-1’ and ‘1183’ leaves. Exogenous ABA (0.1 mm) was applied by immersing petioles of excised leaves for 30 min (A1), 2 h (A2) and 24 h (A3) at 24 °C and 250 μmol s−1 m−2 light intensity. (A) Gel analysis of APX isoenzyme transcripts. After RT–PCR carried out on 50 ng of total RNA with 30 cycles, samples were visualized using a UV light transilluminator. (B) Relative mRNA levels. The mRNA level of each sample was quantified with the Imager 1D/2D software and normalized to the respective 18S ribosomal RNA. The values represent the mean ± s.d. of three experiments.
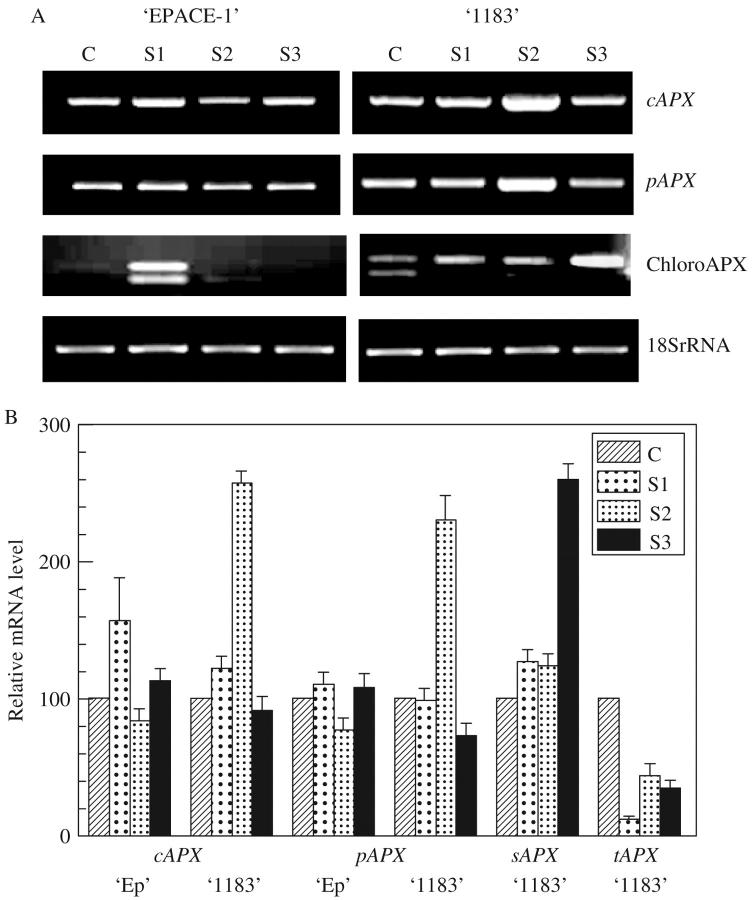
In response to progressive drought (Fig. 6), as in the case of rapid water loss, variations in VucAPX and VupAPX transcript levels were more important in the drought-sensitive cultivar than in the drought-tolerant one. Concerning chloroplastic APX, two signals were visualized on the agarose gel, since Vu chloroplastic APX primers were chosen so as to include the 151-bp insertion found at the 3′ end of the stromatic isoform (Fig. 2). In EPACE-1, slight water stress strongly stimulated both chloroplastic APX gene expression (Fig. 6), in 1183, stimulation of the stromal isoform occurred much later, at severe water deficits. Although chloroAPX gene expression was weak compared with those of VucAPX and VupAPX, their role may be important since they detoxify AOS at their production site. In transgenic tobacco plants, Yabuta et al. (2002) have shown that chloroAPX played an important role as the primary target of the AOS scavenging system and enabled leaf tissues to maintain the capacity of the water–water cycle and photosynthetic activity. However, cytosolic antioxidant proteins are also extremely important (Foyer and Noctor, 2003) as central components of AOS-scavenging gene network (Davletova et al., 2005). In the less-tolerant cowpea cultivar 1183, the stimulation of chloroplastic APX genes occurred later than for EPACE-1, but the plant was able to activate early the expression of genes coding for cytosolic isoforms. In fact, cowpea is a drought-tolerant species, compared with other cutivated plants, and even most sensitive cultivars are able to resist water deficits relatively well.
Fig. 6.
Effect of progressive drought on mRNA abundance of cAPX, pAPX, chloroplastic APX (upper signals sAPX, lower signals tAPX), 18S rRNA in Vigna unguiculata ‘EPACE-1’ and ‘1183’ leaves. Control plants C, ψw = –0.5 ± 0.1 MPa; or droughted plants S1: ψw = –1.0 ± 0.1 MPa; S2, ψw = –1.5 ± 0.2MPa; S3, ψw = –2.0 ± 0.2 MPa. (A) Gel analysis of APX isoenzyme transcripts. After RT–PCR carried out on 50 ng of total RNA with 30 cycles, in the case of cAPX and pAPX and 150 ng of tRNA and 35 cycles in the case of tAPX and sAPX, results were visualized using a UV light transilluminator. (B) Relative mRNA levels. The mRNA level of each sample was quantified with the Imager 1D/2D software and normalized to the respective 18S ribosomal RNA. The values represent the mean ± s.d. of three experiments.
Regarding protective molecules against AOS, lipophilic extracts from EPACE-1 resisted photoperoxidation better than those from 1183 (Ferrari-Iliou et al., 1994). This phenomenon could be due partly to higher intrinsic levels of protective processes and/or to a better ability to accumulate protective molecules (d'Arcy-Lameta et al., 1996; Chaudière and Ferrari-Iliou, 1999). This metabolic stability is important for plant survival.
CONCLUSIONS
When comparing gene expression patterns (Fig. 6) and enzymatic activity (Fig. 1), the present results suggest that subtle changes in the intracellular distribution of protective enzymes and different sensitivities to AOS exhibited by APX isozyme forms might be more important for protection than an overall increase in total enzyme activity. Adjustments of isozyme form affinities for their substrates may be the main strategy to increase the efficiency and the fine-tuning of the antioxidant system (Foyer et al., 1994).
Acknowledgments
The authors thank Dr Anne Repellin for language correction and constructive comments on the manuscript.
The nucleotide sequence data are registered in Genbank under the accession numbers: cAPX, U61379; tAPX, AY484492; sAPX, AY484493; pAPX, AY466858.
LITERATURE CITED
- d'Arcy-Lameta A, Ferrari-Iliou R, Pham Thi AT, Lemoine Y, Zuily-Fodil Y. 1996. Involvement of photosynthetic pigments in total bean leaf lipid extract sensitivity to photoperoxidation. Plant Physiology and Biochemistry 34: 818–825. [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Bunkelmann JR, Trelease RN. 1996. Ascorbate peroxidase: a prominent membrane protein in oilseed glyoxysome. Plant Physiology 110: 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Ball L, Fryer MJ, Baker NR, Karpinski S, Mullineaux PM. 2004. Induction of ascorbate peroxidase 2 expression in wounded Arabidopsis leaves does not involve known wound-signalling pathways but is associated with changes in photosynthesis. The Plant Journal 38: 499–511. [DOI] [PubMed] [Google Scholar]
- Chaudière J, Ferrari-Iliou R. 1999. Intracellular antioxidants: from chemical to biochemical mechanisms. Food Chemical Toxicology 37: 949–962. [DOI] [PubMed] [Google Scholar]
- Chew O, Whelan J, Millar AH. 2003. Molecular definition of the ascorbate-glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants. Journal of Biological Chemistry 278: 46869–46877. [DOI] [PubMed] [Google Scholar]
- Chinnusamy V, Schumaker K, Zhu JK. 2004. Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. Journal of Experimental Botany 55: 225–236. [DOI] [PubMed] [Google Scholar]
- Cruz de Carvalho MH, Laffray D, Louguet P. 1998. Comparison of the physiological responses of Phaseolus vulgaris and Vigna unguiculata cultivars when submitted to drought conditions. Environmental and Experimental Botany 40: 197–207. [Google Scholar]
- Cruz de Carvalho MH, d'Arcy-Lameta A, Roy-Macauley H, Gareil M, El Maarouf H, Pham Thi AT, et al. 2001. Aspartic proteinase in leaves of common bean (Phaseolus vulgaris L.) and cowpea (Vigna unguiculata L. Walp): enzymatic activity, gene expression and relation to drought susceptibility. FEBS Letters 492: 242–246. [DOI] [PubMed] [Google Scholar]
- Dat J, Vandenabeele S, Vranova E, Van Montagu M, Inzé D, Van Breusegem F. 2000. Dual action of the active oxygen species during plant stress response. Cellular and Molecular Life Science 57: 779–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, et al. 2005. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen network of Arabidopsis. The Plant Cell 17: 268–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Maarouf H, Zuily-Fodil Y, Gareil M, d'Arcy-Lameta A, Pham Thi AT. 1999. Enzymatic activity and gene expression under water stress of phospholipase D in two cultivars of Vigna unguiculata L. Walp. differing in drought tolerance. Plant Molecular Biology 39: 1257–1265. [DOI] [PubMed] [Google Scholar]
- Ferrari-Iliou R, PhamThi AT, Vieira da Silva J. 1984. Effect of water deficit on the lipid and fatty acid composition of cotton (Gossypium hirsutum) chloroplasts. Physiologia Plantarum 62: 219–224. [Google Scholar]
- Ferrari-Iliou R, d'Arcy-Lameta A, Pham Thi AT, Zuily-Fodil Y, Mazliak P. 1994. Effect of drought on photodynamic peroxidation of leaf total lipophilic extracts. Phytochemistry 37: 1237–1243. [Google Scholar]
- Foyer CH, Harbinson J. 1994. Oxygen metabolism and the regulation of photosynthetic electron transport. In: Foyer CH, Mullineaux PM, eds. Causes of photo-oxidative stress and amelioration of defense systems in plants. Boca Raton, FL: CRC Press, 1–42.
- Foyer CH, Noctor G. 2003. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiologia Plantarum 119: 355–364. [Google Scholar]
- Foyer CH, Lelandais M, Kunert KJ. 1994. Photooxidative stress in plants. Physiologia Plantarum 92: 696–717. [Google Scholar]
- Ishikawa TK, Sakai K, Yoshimura T, Takeda T, Shigeoka S. 1996. cDNAs encoding spinach stromal and thylakoid-bound ascorbate peroxidase, differing in the presence or absence of their 3′-coding regions. FEBS Letters 384: 289–293. [DOI] [PubMed] [Google Scholar]
- Jespersen HM, Kjaersgard IVH, Ostergaard L, Welinder KG. 1997. From sequence analysis of three novel ascorbate peroxidases from Arabidopsis thaliana to structure, function and evolution of seven types of ascorbate peroxidase. Biochemical Journal 326: 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Zhang J. 2002. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. Journal of Experimental Botany 53: 2401–2410. [DOI] [PubMed] [Google Scholar]
- Lopez F, Vansuyt G, Fourcroy P, Casse-Delbart F. 1994. Accumulation of a 22-kDa protein and its mRNA in the leaves of Raphanus sativus in response to salt stress or water deficit. Physiologia Plantarum 91: 601–608. [Google Scholar]
- Mano S, Yamaguchi K, Hayashi M, Nishimura M. 1997. Stromal and thylakoid-bound ascorbate peroxidases are produced by alternative splicing in pumpkin. FEBS Letters 413: 21–26. [DOI] [PubMed] [Google Scholar]
- Matos AR, d'Arcy-Lameta A, França MG, Pêtres S, Edelman L, Kader JC, et al. 2001. A novel patatin-like gene stimulated by drought stress encodes a galactolipid acyl hydrolase. FEBS Letters 491: 188–192. [DOI] [PubMed] [Google Scholar]
- Mittler R, Zilinskas BA. 1991. Molecular cloning and nucleotide sequence analysis of a cDNA encoding pea cytosolic ascorbate peroxidase. FEBS Letters 289: 257–259. [DOI] [PubMed] [Google Scholar]
- Mullen RT, Trelease RN. 2000. The sorting signals for peroxisomal membrane-bound ascorbate peroxidase are within its C-terminal tail. Journal of Biological Chemistry 275: 16337–16344. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. 1981. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology 22, 867–880. [Google Scholar]
- Orvar BL, Ellis BE. 1997. Transgenic tobacco plants expressing antisense RNA for cytosolic ascorbate peroxidase show increased susceptibility to ozone injury. The Plant Journal 11: 1297–1305. [Google Scholar]
- Pham Thi AT, Vieira da Silva J. 1975. Action d'un traitement osmotique sur l'ultrastructure de feuilles de cotonniers (Gossypium hirsutum L. et G. anomalum Waw et Peyr). Comptes Rendus de l'Académie des Sciences, Paris 280: 2857–2860. [Google Scholar]
- Roy-Macauley H, Zuily-Fodil Y, Kidric M, Pham Thi AT, Vieira da Silva J. 1992. Effect of drought stress on proteolytic activities in Phaseolus and Vigna leaves from sensitive and resistant plants. Physiologia Plantarum 85: 90–96. [Google Scholar]
- Sahsah Y, Campos P, Gareil M, Zuily-Fodil Y, Pham Thi AT. 1998. Enzymatic degradation of polar lipids in Vigna unguiculata leaves and influence of drought stress. Physiologia Plantarum 104: 577–586. [Google Scholar]
- Scholander F, Hammel H, Hemmingsten E, Bradstreet E. 1964. Hydrostatic pressure and osmotic potential in leaves of mangrove and some other plants. Proceedings of the National Academy of Sciences of the USA 52: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, et al. 2002. Regulation and function of ascorbate peroxidase isoenzymes. Journal of Experimental Botany 53: 1305–1319. [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. 1997. Gene expression and signal transduction in water-stress response. Plant Physiology 115: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Research 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Tello A, Zuily-Fodil Y, Pham-Thi AT, Vieira da Silva J. 1990. Electrolyte, Pi leakages and soluble sugar content as physiological tests for screening resistance to water stress in Phaseolus and Vigna species. Journal of Experimental Botany 228: 827–832. [Google Scholar]
- Yabuta Y, Motoki T, Yoshimura K, Takeda T, Ishikawa T, Shigeoka S. 2002. Thylakoid membrane-bound ascorbate peroxidase is a limiting factor of antioxidative systems under photo-oxidative stress. The Plant Journal 32: 915–925. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Yabuta Y, Ishikawa T, Shigeoka S. 2000. Expression of ascorbate peroxidase isoenzymes in response to oxidative stresses. Plant Physiolology 123: 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK. 2002. Salt and drought stress signal transduction in plants. Annual Review of Plant Biology 53: 247–273. [DOI] [PMC free article] [PubMed] [Google Scholar]