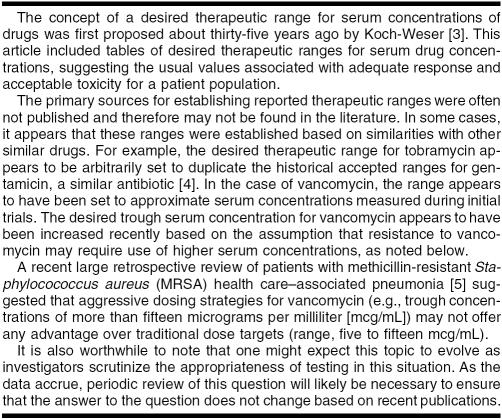
THE CASE
At morning rounds in your hospital's intensive care unit, a resident from the team presents a 55-year-old woman (weight 129 lbs) with a past medical history of multiple sclerosis, cerebellopontine angle meningioma, hypothyroidism, and a neurogenic bladder requiring a Foley catheter. This patient was transferred from her nursing home 3 days ago with a fever and altered mental status. Results from the nursing home bacterial culture of the patient's urine revealed Gram negative rods. Bacterial culture of blood drawn from her peripheral intravenous (IV) line at the nursing home indicated Gram positive cocci. Blood cultures redrawn upon hospital admission are still pending and require confirmation.
According to the patient's chart, she began empiric treatment at the nursing home with vancomycin (1,000 milligrams [mg] intravenously every 12 hours) and piperacillin-tazobactam (3.375 g IV every 6 hours) for urosepsis 4 days ago. The patient's current serum creatinine is 0.56 micrograms per deciliter (mg/dL) (normal range: 0.6–1.1 mg/dL) [1], and her estimated creatinine clearance is 104 milliliters per minute (mL/ min) (normal range: 88–128 mL/min) [2]. Her current body temperature is 97.2° Fahrenheit. Today is day 4 of this patient's vancomycin and piperacillin-tazobactam regimen and hospital day 3.
In reviewing the plan for the next twenty-four hours, the attending physician notes that the patient currently has a standing order for a laboratory test of the vancomycin trough level in her serum, with the blood sample to be taken just prior to the next dose of the drug. On day three of antibiotic therapy, the patient's serum vancomycin trough level was eleven mcg/mL, and, on day four, the trough was eighteen mcg/mL. The institution's target range for the serum trough level of vancomycin is five to twenty mcg/mL.
The attending physician initiates a discussion with the team—including a fellow, three residents, a pharmacist, a dietitian, the unit's nurses, and you, as the team's librarian—about monitoring of vancomycin. The clinician queries the team about the rationale for the standing order for vancomycin trough monitoring. The residents indicate that they often order this lab test when a patient is receiving vancomycin in an attempt to ensure therapeutic effectiveness and to prevent adverse effects of the drug but are not aware of any documentation behind the practice. The pharmacist comments that clinical practice can sometimes evolve before supporting evidence exists and that standards of practice at a hospital may not always be supported by evidence from the literature. In response to this discussion, the group asks you to identify any evidence supporting or disproving the practice of routine monitoring of trough levels in patients being treated with vancomycin in the adult critical care setting. Figures 1 and 2 provide elaboration from the team's attending physician and pharmacist on the significance of this question to clinical practice on the unit.
Figure 1.
Attending physician commentary
Figure 2.
Pharmacist comments
THE QUESTION
Is there evidence that monitoring serum vancomycin trough levels prevents drug toxicity and/or ensures therapeutic dosing of the drug?
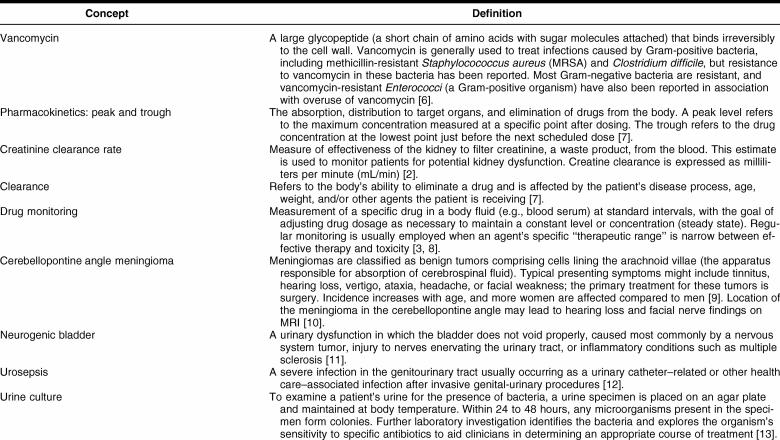
UNDERSTANDING THE CONCEPTS
Before you begin your literature search, you first consider the nuances of this patient's case, described further in Table 1. She is a nursing home resident and has significant comorbidities including a benign brain tumor and multiple sclerosis with unknown disabilities. The etiology of her currently altered mental status is unknown, but nursing home staff reports it to be a change from her baseline condition. The presence of a Foley catheter is a risk factor for a urinary tract infection; however, this patient's diagnosis of urosepsis, referring to a serious systemic infection caused by bacteria originating in the urinary tract, is much more severe and demands aggressive therapy [12]. The data presented on rounds also indicate that the patient is not obese, an important consideration for both drug dosing and prognosis. Also, her serum creatinine and estimated creatinine clearance are normal, suggesting that the patient does not have renal impairment.
Table 1 Key concepts for this case's medical concepts
In addition to developing familiarity with this patient's specific circumstances, understanding a few basic pharmacokinetic concepts and the therapeutic agent being used in this case will aid you in searching, reading, and interpreting the literature. Peak and trough values are important concepts in understanding drug disposition, the way an individual therapeutic agent or drug distributes in and is managed by the human body. The peak level refers to the predicted highest concentration between doses, and the trough refers to the lowest predicted concentration just before the next dose. Serum levels of the antibiotic must be high enough during the dosing interval (i.e., the period in between doses of the agent) to kill the infecting organism and prevent further growth. After IV dosing, a drug may be distributed throughout the body, may only target certain organs, or may not be absorbed at certain sites. Knowing the pattern and timing of drug distribution is vital to identifying correct dosing intervals. In critical care, in which an infection typically needs to be treated aggressively to avert significant patient morbidity and mortality, clinicians attempt to infuse the highest possible dose as rapidly as possible without causing side effects. Understanding the pharmacokinetics of an antibiotic helps to effectively treat the infection and prevent adverse effects.
Developing a better understanding of the history of this agent may also help guide your literature search and interpretation of the citations you find. Vancomycin was approved for use in the United States by the Food and Drug Administration (FDA) in 1958 for treating penicillin-resistant Staphylococcus aureus infection, was nicknamed “Mississippi Mud” due to its color from impurities, and was initially isolated from soil in Borneo and India [14]. Because vancomycin has been used since the 1950s, you find background information in a number of sources, with varying content and level of detail. For example, CRLOnline discusses the drug's mechanism of action and pharmacodynamics and provides a dosage recommendation [15]. This text also provides reference ranges for laboratory monitoring of patients being treated with vancomycin, recommending timing of blood draws to assess peak and trough levels; however, no reference to supporting evidence is provided.
The Micromedex DrugPoint summary for vancomycin [16] includes a “Monitoring” category, which cites target peak and trough levels along with a recommendation about measuring only troughs, but also lists exceptions:
peak and trough level monitoring is still warranted in certain high-risk patients such as those receiving concomitant nephrotoxic agents, sites of infection with difficult penetration (CNS, endocarditis), patients with rapidly changing renal function, or patients with a poor therapeutic response. [7]
The Drug Point summary does not provide references but does include a link to a full drug monograph in DrugDex [17]. The “Pharmacokinetics” section of the monograph discusses measuring serum peak and trough and mentions a guide for therapeutic monitoring, noting that the recommendations are derived from “observations” of recommended doses and citing a 1999 study [18].
An UpToDate search of vancomycin retrieves “Vancomycin dosing and serum concentration monitoring in adults,” which includes the subsections “Serum concentration monitoring” and “Utility of monitoring serum concentration” [19]. The UpToDate review discourages routine monitoring and trough monitoring, recommending such practices if the patient has end stage renal disease and is likely to receive more than one dose of vancomycin, has other apparent instability or changes in renal function, is receiving another nephrotoxic agent, or is morbidly obese. The section describing indications for monitoring cites no supporting references, however.
At this point, you realize that there is likely to be a large body of literature discussing vancomycin dosing (over 600 references in the Micromedex bibliography alone) and the literature is likely to be controversial, due to the acknowledged lack of evidence to support monitoring.
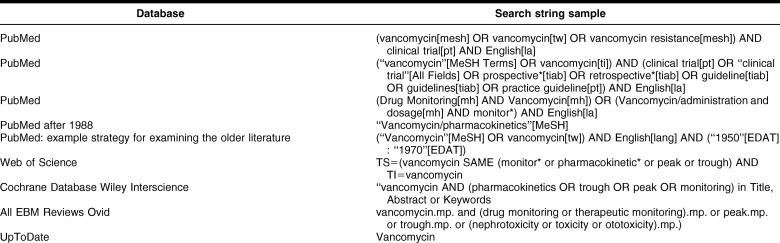
CONSTRUCTING A LITERATURE SEARCH
Table 2 includes example search strategies for a number of resources that may provide useful information describing vancomycin monitoring practices.
Table 2 Search strategy examples
Searching the earliest years after vancomycin approval is problematic due to the lack of abstracts (eliminating a key textword source) and subheadings. The majority of the 1950–1965 OLDMEDLINE citations [20] have been incorporated into PubMed, and a recent NLM Technical Bulletin news item notes that the Other Terms (subject terms from the original print indexes) have been mapped to Medical Subject Headings (MeSH) for approximately 75% of the 1.7 million OLDMEDLINE records [21], which will likely increase the chances that a MeSH-based search strategy will still find relevant citations from this portion of the PubMed dataset. However, given that the older citations were indexed with different practices due to the nature of the print indexes (e.g., sometimes including fewer subject terms and/or subheadings), you will likely find it useful to supplement your more focused search strategies with broader strategies. For example, a classic article by Anderson et al. is currently indexed in PubMed with only the major subject term and substance “Anti-Bacterial Agents” [22], necessitating your use of a much broader search than a simple vancomycin-based strategy. Due to the volume of the literature, you may want to consider these older citations separately, using the broader search only in OLDMEDLINE to obtain a more manageable retrieval set and using the narrower search in the more recent literature.
In addition to the work by Anderson et al. noted above, other older items will likely also provide essential background information for understanding how the biomedical community's knowledge of vancomycin pharmacokinetics has evolved since the drug's discovery in the 1950s [23–25]. Though these initial studies may not prove useful for your overall summary of the literature (discussed further below), they provide excellent insight into the origins of the vancomycin pharmacokinetic levels examined in the more recent literature.
The Fairbrother and Williams 1956 article, for example, reports testing results of vancomycin sensitivity for 1,350 organisms, including 540 strains of S. aureus, with all proving sensitive [23]. This paper notes a key limitation of in-vitro studies, however, in terms of the generalizability of the results to a clinical setting, emphasizing “in-vitro sensitivity tests do not provide an absolute criterion of clinical efficiency; they only indicate activity … under laboratory conditions which tend to be favorable to the antibiotic.” You also find that the limitations of generalizing the results of in-vitro studies echo through much of the literature debating the utility of therapeutic drug monitoring in patients receiving vancomycin [24–27].
Another example of an early in-vitro study of vancomycin's effects is provided by a 1956 paper by Geraci et al. examining Micrococcus pyogenes in several populations, both human and animal, detailing measurement of vancomycin concentrations in body fluids after both IV and oral dosing and examining the sensitivities of multiple pathogens [28]. These investigators established that a serum vancomycin concentration of 2.5 mcg/mL completely inhibited 110 of 112 strains of M. Pyogenes. This finding became an important standard established for vancomycin sensitivity for many organisms, despite the lack of validation in subsequent clinical trials, providing some insight into the weaknesses of the evidence base supporting vancomycin dosing and monitoring practices.
A 1970 study by Washington et al. is key due to the authors' perspective on vancomycin dosing and monitoring. These authors noted there was no experimental evidence or even “universal agreement” defining in-vitro susceptibility or resistance of a microorganism to an antibiotic [29]. Washington went on to say that as a general rule if the antibiotic blood levels exceeded the in-vitro MIC level by two to four times, then “the organism is usually considered to be sensitive.” As with the work by Geraci et al., this study highlights the way in which experimental in-vitro results became the foundation for clinical practice in vancomycin monitoring and dosing, without the strength of validation through clinical trials.
In addition to understanding the growth of the vancomycin-related literature by examining these older citations, perusing the bibliographies of the relevant articles you identify during your searching process is another way to find additional items not retrieved by your search strategy. Though it can be considerably time consuming, this hand-searching step is essential if you wish to conduct a comprehensive search and produce a high-quality and thorough end product.
As you scan through your search retrieval sets, you realize that a number of studies examine the use of vancomycin without quantitative sensitivity or pharmacokinetic data. Such studies may be useful to support selecting vancomycin as a therapeutic agent but omit data that would support informed clinical decision making about how or when such patients should be monitored during vancomycin therapy. In your results, you also note a lack of clinical trials examining correlation between vancomycin monitoring and clinical outcomes, finding that most primary data on the topic focus on either laboratory testing results or retrospective considerations of vancomycin pharmacokinetics in critically ill populations.
Of the items you peruse in your search results, you focus most closely on the studies that include either in-vitro sensitivity data or research examining associations between therapeutic drug monitoring and clinical outcomes (e.g., duration of therapy, cumulative dose, resolution of infection, mortality) in adult patients receiving vancomycin. You also consider key opinion pieces and guideline statements to reflect areas of consensus and debate related to laboratory monitoring during vancomycin therapy. To address the question, you select a final group of articles including a set of practice guidelines that contain recommendations for vancomycin monitoring [30], a review that provides an excellent summary of the debate surrounding this issue [31], and several studies from more recent years that yield in-vitro and observational clinical data illustrating potential utility and limitations of vancomycin monitoring [32–39].
SUMMARIZING THE LITERATURE
One of your key challenges in summarizing this literature lies in illustrating for the clinical team the areas of consensus and debate related to this question. Figure 3 provides a sample overall summary of the literature, beginning with a synthesis of the perspective you developed in your detailed examination of the older literature from the time period closer to the Food and Drug Administration's approval of vancomycin for use in the United States, followed by brief summarization of:
a set of 2005 practice guidelines from the American Thoracic Society and the Infectious Diseases Society of America, noting the rationale for the guidelines and the acknowledged paucity of evidence to inform decision making on the issue [30]
a thorough review from 1994 that emphasized the lack of data supporting vancomycin testing practices and the potential economic impact of over-testing [31]
individual studies highlighting various aspects of this debate [32–39]
an overall conclusion regarding your understanding of the literature on the topic
Figure 3.
Overall summary of the literature
CONCLUDING REMARKS
When you present the literature to your team, they are not surprised at your evaluation of the scant evidence to support connections between routine peak and trough monitoring and actual clinical outcomes or toxicity and realize that this is a huge and confusing literature. They are particularly interested in the specific articles used to support your synthesis of the literature and your skill in distinguishing real evidence from anecdotal or opinion-based recommendations and practices.
At a local charge of $250 for each peak and trough measurement for a patient receiving vancomycin, the charges for this testing combination approached $1.2 million at your institution in one recent year. Debating routine measurement of vancomycin levels and its associated costs without evidence of many benefits, as revealed by your examination of the literature in conjunction with the clinical team's interpretation and expertise, leads to changes in your institution's computerized provider order entry system (CPOE). To support informed clinician decision making on vancomycin monitoring, the CPOE system now advises against measuring peak values. If an order for a trough level is requested, a new popup screen called the “Vancomycin Trough Level Order Advisor” appears. The clinician must select an exception criterion (e.g., renal dysfunction, obesity, > 7 days duration of therapy, suspected treatment failure due to low serum levels) to order the drug monitoring and complete a consult form for pharmacist advice before the order is accepted. Such standardization of practices is one strategy for facilitating the systematic incorporation of evidence into clinical practices.
Your comprehensive review of the evidence in the literature—read and critiqued by physicians, pharmacists, or others—has become a catalyst for practice change in your institution. In this case, the attending physician and pharmacist concur about the existing lack of evidence and move to systematize changes in vancomycin test-ordering practices throughout the institution. Using your skills as part of the clinical team can assist the library in proving that your services have a direct return on investment and make you a highly valued team member.
REFERENCES
- American Society for Clinical Chemistry. Creatinine. [Web document]. The Society, 2005. [rev. 22 Jun 2005; cited 5 Jun 2007]. <http://labtestsonline.org/understanding/analytes/creatinine/test.html>. [Google Scholar]
- Creatinine clearance. In: MedlinePlus medical encyclopedia. [Web document]. Atlanta, GA: A.D.A.M., 2006. [rev. 3 Feb 2006; cited 5 Jun 2007]. <http://www.nlm.nih.gov/medlineplus/ency/article/003611.htm#Normal%20Values>. [Google Scholar]
- Koch-Weser J. Drug therapy. serum drug concentrations as therapeutic guides. N Engl J Med. 1972 Aug 3; 287(5):227–31. [DOI] [PubMed] [Google Scholar]
- Begg EJ, Barclay ML, and Kirkpatrick CJ. The therapeutic monitoring of antimicrobial agents. Br J Clin Pharmacol. 1999 Jan; 47(1):23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffres MN, Isakow W, Doherty JA, McKinnon PS, Ritchie DJ, Micek ST, and Kollef MH. Predictors of mortality for methicillin-resistant Staphylococcus aureus health-care-associated pneumonia: specific evaluation of vancomycin pharmacokinetic indices. Chest. 2006 Oct; 130(4):947–55. [DOI] [PubMed] [Google Scholar]
- Anderson PO, Knoben JE, and Troutman WG. eds. Handbook of clinical drug data. 10th ed. Stamford, CT: Appleton & Lange, 2002. [Google Scholar]
- Brunton L. ed. Goodman & Gilman's the pharmacological basis of therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006. [Google Scholar]
- Lab tests online. Therapeutic drug monitoring. [Web document]. American Association for Clinical Chemistry, 2006. [rev. 19 Apr 2006; cited 5 Jun 2007]. <http://www.labtestsonline.org/understanding/analytes/thdm/glance.html>. [Google Scholar]
- Haddad G, Chamoun RB. Meningioma. [Web document]. Omaha, NE: eMedicine, 2006. [rev. 14 Nov 2006, cited 5 Jun 2007]. <http://www.emedicine.com/neuro/topic209.htm>. [Google Scholar]
- Conrad CB. Cerebellopontine angle meningioma. [Web document]. ImagesMD, 2002. [cited 5 Jun 2007]. <http://www.images.md>. [Google Scholar]
- Neurogenic bladder. In: MedlinePlus medical encyclopedia. [Web document]. Atlanta, GA: A.D.A.M., 2006. [rev. 16 May 2006; cited 5 Jun 2007]. <http://www.nlm.nih.gov/medlineplus/ency/article/000754.htm>. [Google Scholar]
- Balk RA, Ely EW, and Goyette RE. Sepsis handbook. Knoxville, TN: National Initiative in Sepsis Education, 2001. [Google Scholar]
- Lab tests online. Urine culture. [Web document]. American Association for Clinical Chemistry, 2006. [rev. 11 May 2005; cited 1 Jun 2007]. <http://www.labtestsonline.org/understanding/analytes/urine_culture/test.html>. [Google Scholar]
- Vancomycin. Lancet. 1957 Jan 12; 272(6959):85–6. [PubMed] [Google Scholar]
- Vancomycin. In: Clinical reference library online (CRLonline). [Web document]. Hudson, OH: Lexi-Comp, 2001. [rev. 23 May 2007; cited 5 Jun 2007]. <http://www.crlonline.com/crlonline>. [Google Scholar]
- Vancomycin. [Web document]. In: DrugPoint. [cited 5 Jun 2007]. Greenwood Village, CO: Thomson Micromedex, 2006. <http://www.thomsonhc.com>. [Google Scholar]
- Vancomycin. [Web document]. In: DRUGDEX. [cited 5 Jun 2007]. Greenwood Village, CO: Thomson Micromedex, 2006. <http://www.thomsonhc.com>. [Google Scholar]
- Wilhelm MP, Estes L. Symposium on antimicrobial agents—part XII. vancomycin. Mayo Clin Proc. 1999 Sep; 74(9):928–35. [DOI] [PubMed] [Google Scholar]
- UpToDate. Vancomycin dosing and serum concentration monitoring in adults. Waltham, MA: UpToDate. [Google Scholar]
- National Library of Medicine. OLDMEDLINE data. [Web document]. Bethesda, MD: National Library of Medicine, 2004. [rev. 16 Mar 2007; cited 5 Jun 2007]. <http://www.nlm.nih.gov/databases/databases_oldmedline.html>. [Google Scholar]
- National Library of Medicine. OLDMEDLINE MeSH® mapping project—update May 15, 2007. [Web document]. NLM Tech Bull 2007 May–Jun;356. [cited 5 Jun 2007]. <http://www.nlm.nih.gov/pubs/techbull/mj07/mj07_technote.html#0>. [Google Scholar]
- Anderson RC, Worth HM, Harris PN, and Chen KK. Vancomycin, a new antibiotic. IV. pharmacologic and toxicologic studies. Antibiot Annu 1956–1957:75–81. [PubMed] [Google Scholar]
- Fairbrother RW, Williams BL. Two new antibiotics; antibacterial activity of novobiocin and vancomycin. Lancet. 1956 Dec 8; 271(6954):1177–8. [DOI] [PubMed] [Google Scholar]
- Ackerman BH. Clinical value of monitoring serum vancomycin concentrations. Clin Infect Dis. 1994 Dec; 19(6):1180–2. [DOI] [PubMed] [Google Scholar]
- Catchpole C, Hastings JG. Measuring pre- and post-dose vancomycin levels—time for a change? J Med Microbiol. 1995 May; 42(5):309–11. [DOI] [PubMed] [Google Scholar]
- Darko W, Medicis JJ, Smith A, Guharoy R, and Lehmann DE. Mississippi mud no more: cost-effectiveness of pharmacokinetic dosage adjustment of vancomycin to prevent nephrotoxicity. Pharmacotherapy. 2003 May; 23(5):643–50. [DOI] [PubMed] [Google Scholar]
- Moellering RC. Monitoring serum vancomycin levels: climbing the mountain because it is there? Clin Infect Dis. 1994 Apr; 18(4):544–6.Review. [DOI] [PubMed] [Google Scholar]
- Geraci JE, Heilman FR, Nichols DR, Ross GT, and Wellman WE. Some laboratory and clinical experiences with a new antibiotic, vancomycin. Mayo Clin Proc. 1956 Oct 17; 31(21):564–82. [PubMed] [Google Scholar]
- Washington JA, Hermans PE, and Martin WJ. In vitro susceptibility of Staphylococci and Streptococci and influence of agarmedia on minimum inhibitory concentration. Mayo Clin Proc. 1970 Jul; 45(7):527–35. [PubMed] [Google Scholar]
- Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005 Feb 15; 171(4):388–416. [DOI] [PubMed] [Google Scholar]
- Cantu TG, Yamanaka-Yuen NA, and Lietman PS. Serum vancomycin concentrations: reappraisal of their clinical value. Clin Infect Dis. 1994 Apr; 18(4):533–43. [PubMed] [Google Scholar]
- Iwamoto T, Kagawa Y, and Kojima M. Clinical efficacy of therapeutic drug monitoring in patients receiving vancomycin. Biol Pharm Bull. 2003 Jun; 26(6):876–9. [DOI] [PubMed] [Google Scholar]
- Karam CM, McKinnon PS, Neuhauser MM, and Rybak MJ. Outcome assessment of minimizing vancomycin monitoring and dosing adjustments. Pharmacotherapy. 1999 Mar; 19(3):257–66. [DOI] [PubMed] [Google Scholar]
- Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, and Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004 Jun; 42(6):2398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faculty of 1000 Medicine. [Web document]. London, UK: Medicine Reports, 2007. [cited 5 Jun 2007]. <http://www.f1000medicine.com>. [Google Scholar]
- Pea F, Bertolissi M, Di Silvestre A, Poz D, Giordano F, and Furlanut M. TDM coupled with Bayesian forecasting should be considered an invaluable tool for optimizing vancomycin daily exposure in unstable critically ill patients. Int J Antimicrob Agents. 2002 Nov; 20(5):326–32. [DOI] [PubMed] [Google Scholar]
- Murphy JE, Gillespie DE, and Bateman CV. Predictability of vancomycin trough concentrations using seven approaches for estimating pharmacokinetic parameters. Am J Health Syst Pharm. 2006 Dec 1; 63(23):2365–70. [DOI] [PubMed] [Google Scholar]
- Kitzis MD, Goldstein FW. Monitoring of vancomycin serum levels for the treatment of Staphylococcal infections. Clin Microbiol Infect. 2006 Jan; 12(1):92–5. [DOI] [PubMed] [Google Scholar]
- Hidayat LK, Hsu DI, Quist R, Shriner KA, and Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006 Oct 23; 166(19):2138–44. [DOI] [PubMed] [Google Scholar]