Abstract
Drosophila imaginal discs, the primordia of the adult fly appendages, are an excellent system for studying developmental plasticity. Cells in the imaginal discs are determined for their disc-specific fate (wingness, legness) during embryogenesis. Disc cells maintain their determination during larval development, a time of extensive growth and proliferation. Only when prompted to regenerate do disc cells exhibit lability in their determined identity. Regeneration in the disc is mediated by a localized region of cell division, known as the regeneration blastema. Most regenerating disc cells strictly adhere to their disc-specific identity; some cells however, switch fate in a phenomenon known as transdetermination. Similar regeneration and transdetermination events can be induced in situ by misexpression of the signaling molecule wingless. Recent studies indicate that the plasticity of disc cells during regeneration is associated with high morphogen activity and the reorganization of chromatin structure. Here we provide both a historical perspective of imaginal disc transdetermination, as well as discuss recent findings on how imaginal disc cells acquire developmental plasticity and multipotency. We also highlight how an understanding of imaginal disc transdetermination can enhance an understanding of developmental potency exhibited by stem cells.
Keywords: Drosophila, transdetermination, imaginal disc, stem cell, potency
INTRODUCTION
The most potent and versatile cell is the totipotent fertilized egg which generates all embryonic and extra-embryonic cell types. Developmental potency is similarly observed in mammalian embryonic stem (ES) cells---cells derived from the inner cell mass of the blastocyst. These cells are able to produce all three embryonic germ layers (ectoderm, endoderm and mesoderm) and thus differentiate numerous cell types. As development proceeds, the developmental potency of most cells in an organism declines. This occurs as cells become increasingly restricted in their potential and determined towards specific cell fates.
Cell determination is an operational term that reflects the ability of a group of cells to continue along a specific developmental path and then differentiate accordingly, irrespective of the surrounding environment or neighboring cells. Transplantation experiments have been utilized in many different systems to assess cell determination. In these experiments, cells with known fates are transplanted to an ectopic location within the developing animal. If the fate of the cells does not alter after being placed in the new site, then the cells are determined. Cell determination is typically established at the transcriptional level in response to transient developmental cues and is immediately followed by differentiation (specialized function and histotypic structure). This is not always the case as some cells are determined long before differentiation. For example, adult somatic stem cells (SSCs) are a small population of determined yet undifferentiated cells that reside in many differentiated tissues (skin, central nervous system, muscle, bone marrow, liver, gastrointestinal tract) (Alison & Sarraf, 1998; Gage, Ray, & Fisher, 1995; Schultz & McCormick, 1994; Watt, 1998; Weissman, 2000). These cells both self-renew as well as differentiate in order to replace cells lost to normal physiological turnover or injury (e.g. satellite cells in muscle) (Moss & Leblond, 1970, 1971; Wagers & Conboy, 2005). Therefore, it is important in normal development to stably maintain cell determination. This feat is made remarkable because during development cells divide, move, change gene expression and are exposed to a variety of extracellular signals. Nevertheless, once established, cells rarely change their established state of cell determination. Failure to do so can have severe consequences ranging from developmental defects to cancer (Slack, 1985).
Cell determination is not completely irreversible and under special circumstances, such as tissue and organ regeneration, cells can change their developmental program and generate structures that radically differ from their normal fate. In order to regenerate complex adult structures, different organisms utilize various modes of regeneration. For instance, limb regeneration in urodele amphibians involves de-differentiation of existing cells at the wound site, while planarians regenerate by the migration, multiplication and differentiation of adult stem cells (Sanchez Alvarado, 2006; Tanaka, 2003). In contrast, tail regeneration in Xenopus laevis involves the re-entry of differentiated cells into the cell-cycle (Gargioli & Slack, 2004). The fact that regeneration is a widespread phenomenon in metazoans indicates that, despite being determined or differentiated, cells remain remarkably plastic.
Drosophila imaginal discs: a model system to study cell plasticity
Imaginal discs, in Drosophila and other higher dipterans, are epithelial sacs that serve as the primordia for the adult appendages and other cuticular structures (Fig. 1a). The discs are formed and become determined (wingness, legness) during embryogenesis (Simcox & Sang, 1983). They maintain their determination throughout larval development. During this time, disc identity becomes more rigidly determined in order to generate specific cell fates, such as wing blade and hinge, or femur, tibia and tarsus as well as specific bristles present in segments (Schubiger, 1968). Some disc cells, such as sensory organ precursor cells, begin to differentiate by expressing tissue-specific genes (Nolo, Abbott, & Bellen, 2000; Skeath & Carroll, 1994). In response to hormonal cues at metamorphosis disc cells initiate terminal differentiation and form the adult fly exoskeleton (Fristrom & Fristrom, 1993).
Fig. 1. Method for in vivo culture of Drosophila imaginal discs.

(a) DIC images of third instar wing (W), haltere (H), first thoracic leg (L), eye-antennal (E/A), maxillary palp (MP) (primordia marked by arrowhead) and genital (G) imaginal discs. (b) Imaginal discs are dissected from a wandering third instar larva and fragmented into two pieces. One disc fragment (test fragment) is transplanted into the body cavity of a larval host which will metamorphose and differentiate adult structures (yellow cuticle). The other disc fragment (stem fragment) is transplanted into the abdominal cavity of a fertilized adult female host. There the disc cells will continue to proliferate in an undifferentiated state and regenerate. After several days of in vivo culture, the disc implant can be isolated, cut again and transplanted into another female host where the cells will continue to proliferate. Disc cells can be propagated indefinitely by repetitive fragmentation and in vivo culture. To examine the developmental potency of disc cells after long term culture, disc fragments are transplanted into the body cavities of larval hosts, where they will metamorphose and differentiate with the larval host. The figure is adapted and modified from (Hadorn, 1965).
Since imaginal disc determination and differentiation are temporally separated in development it was expected that disc cells would maintain their disc-specific fates prior to metamorphosis. Many researchers tested this idea, first by transplantation experiments (Gehring, 1978) and then using an in vivo culture system, in which disc fragments were transplanted and cultured in the abdominal cavity of an adult female host (Fig. 1b) (Gehring, 1978; Hadorn, 1963). Under such conditions, disc cells remain undifferentiated and continue to proliferate and regenerate lost structures. Disc cells will proliferate indefinitely if repeatedly cut and transplanted from one adult host to another. To determine whether disc cells maintained their disc-specific determination, they were transplanted into a larva where they differentiated along with the host during metamorphosis. Even after extensive periods in culture (up to 5 years), nearly all disc cells maintained their original disc-specific determination and differentiated appropriate adult structures. There were however, rare instances in which determined disc cells increased their developmental potential and switched cell fate (e.g. leg-to-wing), in a phenomenon known as transdetermination (TD).
TD events are infrequent, however when discs are fragmented at specific locations known as ‘weak points’, TD occurs with high frequency (Fig. 2) (Gehring, 1966; Gehring, 1978; Hadorn, 1963) (see below in The weak point). For example, leg disc cells fragmented at the dorsal-proximal region of the disc (weak point) readily TD to ventral wing. Fragmentation at weak points revealed that TD occurs in specific, reproducible directions and with particular frequencies (Fig. 2), indicating that disc cells prefer certain developmental switches over others. Overall, these switches suggest that disc determination is labile and that some cells are capable of following an alternative developmental path.
Fig. 2. Transdetermination events in Drosophila imaginal discs.

Schematic shows the transdetermination (TD) events (white ovals) that are induced by disc fragmentation and regeneration (shown in green arrows). Dashed arrows indicate infrequent events. Ubiquitous wingless signaling induces only a subset of the TD events that occur by disc fragmentation (shown in orange arrows). TD that arises from the gain (+) or loss (−) of selector gene activity are shown in red (Antp, Antennapedia; Ubx, Ultrabithorax; pb proboscipedia; Scr, Sex combs reduced; Dll, Distal-less; ey, eyeless; vg, vestigial). Black triangles represent weak points that are located at distinct sites within each disc that will TD frequently upon fragmentation. Note that maxillary palp, antenna and eye primordia are in one imaginal disc (see Fig. 1). (a-c) Examples of wg-induced TD events: (a) maxillary palp-to-antenna, (b) maxillary palp-to-proboscis and (c) leg-to-wing TD. Note that only first thoracic leg discs TD to wing after fragmentation (Steiner, 1981), while leg discs from all three thoracic segments transdetermine to wing and haltere upon ubiquitous wg signaling. The figure is modified from (Hadorn, 1978; Wei, Schubiger, Harder, & Muller, 2000).
Here we focus on the developmental plasticity exhibited by Drosophila imaginal disc cells when prompted to regenerate. We discuss extracellular signals that induce regeneration and consider the possible molecular mechanisms that govern this plasticity. Comparisons will be made to the plasticity exhibited by vertebrate stem cells.
POLYCLONAL ORIGIN OF TD
Direct evidence that TD is a clear change from one determined state into another came from clonal analysis of regenerating antennal disc fragments (Gehring, 1967). In these experiments, genetically labeled cell clones were randomly induced in the antennal disc prior to fragmentation (Fig. 3). After a brief period of in vivo culture the discs underwent metamorphosis and the adult cuticular structures were analyzed. Using this method, Gehring (1967) identified labeled cell clones containing both TD wing tissue and non-TD antennal tissue (Fig. 3). Such clones indicated not only that determined antennal cells were capable of producing progeny with different states of determination, but that the switch occurred without the formation of intermediary cell types. The clonal analysis additionally showed that only 3-5 cells (founder cells) were responsible for generating the TD tissue (Hadorn, 1978).
Fig. 3. TD occurs in determined disc cells.

Antennal disc cells with black nuclei are of the genotype (+/y); cells with yellow nuclei are homozygous for the yellow gene (y/y). Antennal cell clones of the y/y genotype are randomly induced via somatic recombination prior to disc fragmentation and regeneration. To allow for regenerative proliferation, the antennal primordia is separated from the eye (dashed line) and cultured in an adult host. After metamorphosis, the differentiated adult tissue contains both non-TD antennal and TD wing tissue (indicated by solid line) without the formation of intermediary cell types. Both y/+ and y/y cells contribute to the TD wing tissue, providing evidence that TD is a polyclonal event. The figure is adapted and modified from (Gehring, 1967).
PROLIFERATION AND TD
After fragmentation, regeneration proceeds from a localized region of cell division, known as the regeneration blastema (Fig. 4B,C) (Gehring, 1978; Johnston & Schubiger, 1996; Sustar & Schubiger, 2005). Cells in the blastema restore the integrity of the disc by replacing all of the missing pattern elements (regeneration) or by reiterating (duplication) the existing disc pattern elements, both processes are considered regenerative growth (Bryant, 1970; Schubiger, 1971) (Fig. 5A,B). Strong evidence indicates that blastema formation and proliferation are required for TD. In normal Drosophila development, the proboscis forms from a pair of labial discs with each generating half of the proboscis. When a single labial disc is cultured in an adult host it regenerates by duplication and forms a symmetrical and complete proboscis (Wildermuth, 1968). If TD occurs in this experiment, it is confined to the newly regenerated labial disc. This experiment revealed that an increase in developmental potency occurs only after regenerative growth and proliferation (Fig. 4D,E). This hypothesis is strengthened by the findings that the frequency and area of TD increase when cell division is further stimulated by additional disc injury (Tobler, 1966). Conversely, TD is not observed when disc fragments are cultured under starvation conditions that inhibit cell division (Schubiger, 1973).
Fig. 4. The regeneration blastema and TD.

(A-C) First thoracic leg discs are labeled with a short pulse (20 minutes) of thymidine analog, 5-Bromo 2-deoxy-Uridine (BrdU), which labels cells in S-phase. (A) Cell division is asynchronous and random in discs harvested from third instar. (B) A regenerating leg disc fragment that shows localized cell division in the regeneration blastema (white arrow); cell division ceases in the remainder of the disc. (C) A pair of leg discs that have been subjected to ubiquitous wg expression show similar regeneration blastemas (white arrows) that localize to the proximal-most dorsal region (weak point) of the leg disc. Note that cells not contributing to the blastema are not labeled. (D,E) A subset of regenerating disc cells will TD. (D) A regenerating leg disc fragment that shows TD cells that express the wing selector gene vg (white asterisk). (E) A pair of leg discs that show ectopic expression of vg (white asterisks) following ubiquitous wg expression. The confocal images were taken at the same magnification.
Fig. 5. Regeneration of Drosophila imaginal disc fragments.

(A) The eye-antennal disc is cut into antenna (A) and eye (E) fragments. After in vivo culture, the antennal fragment will duplicate (dark gray), while the eye fragment will regenerate the missing antenna (dark gray). The figure is adapted from (Gehring & Schubiger, 1975). (B) The first thoracic leg disc is cut into three-quarter posterior (3/4P) and one-quarter anterior (1/4A) fragments. After in vivo culture, the 3/4P fragment will duplicate itself (dark gray), while the 1/4A fragment will regenerate the missing disc elements (dark gray). The figure is adapted from (Gibson & Schubiger, 1999).
The adult appendages in Drosophila are subdivided into anterior (A) and posterior (P) compartments (Garcia-Bellido, Ripoll, & Morata, 1973). Cells of the disc primordia form A/P compartments by the expression of the engrailed (en) and invected (inv) genes in all posterior founder cells. Heritable expression of en/inv during larval development prevents posterior cells from mixing with anterior cells and ensures that posterior cells maintain their fate and develop into posterior instead of anterior adult structures (Gustavson, Goldsborough, Ali, & Kornberg, 1996; Lawrence & Morata, 1976; Morata & Lawrence, 1975). Contrary to normal development, during disc regeneration anterior blastema cells can regenerate posterior cells (Abbott, Karpen, & Schubiger, 1981). Thus, compartment identity is not maintained and is plastic during regeneration. Additionally, if the newly formed blastema is isolated from the disc fragment and then cultured in vivo, it is programmed to form a complete adult leg (Karpen & Schubiger, 1981). Such a result indicates that blastema cells in the disc harbor tremendous developmental autonomy and plasticity, a property observed in vertebrate limb blastemas (Brockes & Kumar, 2005).
DO TD AND REGENERATION REQUIRE A DE-DETERMINATION?
In urodele limb regeneration, a regeneration blastema forms at the site of injury (Tanaka, 2003). To form the blastema, cells de-differentiate displaying some characteristics of embryonic cells such as migratory behavior, differential adhesion and an increase in cell division. Additionally, blastema cells re-express many developmentally regulated genes (Gardiner, Carlson, & Roy, 1999). It has been suggested that limb blastema cells de-differentiate to become more developmentally potent in order to replace lost or damaged structures (Tanaka, 2003). The possibility of ‘rejuvenation’ in disc regeneration was initially tested by examining TD tissue in fly mutants exhibiting a phenotypic variegation for the yellow (y) locus (Hadorn, Gsell, & Schultz, 1970). In these mutants, the decision of yellow activation (y+) or inactivation (y) occurs randomly during the first larval instar of development and is thereafter clonally propagated (Lindsley, 1968). Mosacism for the yellow gene (y+ or y) in TD tissue would indicate that disc cells had rejuvenated to the first larval instar as they acquired a new disc-specific identity. However, Gsell (1971) found that TD cells maintained their wild-type or y phenotype indicating that these cells do not revert to developmental decisions of the first instar.
The temperature sensitive allele of the homeotic gene aristapedia (ssa40a) proved to be ideal for examining the question of whether limited rejuvenation occurs during regeneration and TD. When ssa40a mutant animals are raised at the permissive temperature (28°C) during the mid-third larval instar they develop wild-type antenna. However, if the animals are raised at the restrictive temperature (17°C) during this time, they develop leg tissue in place of antennal tissue (Grigliatti & Suzuki, 1971). Thus, the critical temperature sensitive period (TSP) for ssa40a expression is mid-third larval instar (Grigliatti & Suzuki, 1971). Schubiger and Alpert (1975) raised ssa40a mutant larvae at either the permissive (wild-type, antenna) or restrictive (aristapedia, leg) temperatures. After the TSP for ssa40a expression was past they isolated eye-antennal discs from the larvae. The eye discs were then separated from the antennal discs and subsequently cultured in vivo at different temperatures to allow for antennal regeneration (Fig. 5A). For example, eye discs initially exposed to the permissive temperature during larval development were then shifted to the restrictive temperature during regeneration. Without exception Schubiger and Alpert (1975) found that the phenotype of the regenerating antenna solely depended upon the temperature during regeneration. For example, eye discs isolated from larvae raised at the permissive temperature (wild-type, antenna) displayed the aristapedia (leg) phenotype after they were shifted to the restrictive temperature during regeneration. These findings suggest that regenerating antennal disc cells undergo a second TSP for ssa40a expression and thus have reverted to the developmental stage of the mid-third larval instar, indicating limited rejuvenation during regeneration.
More recently Sustar and Schubiger (2005) revisited the question of an increase in developmental potency during regeneration and TD, as they assessed the developmental age of cells in the regeneration blastema. They analyzed the cell-cycle profile, doubling time and size of blastema disc cells. Young disc cells (first and second instar) are large in size, have a distinct cell-cycle profile and divide rapidly compared to older disc cells. Sustar and Schubiger (2005) found that disc cells in the blastema acquired neither a faster cell doubling time nor a more ‘youthful’ cell-cycle profile. Instead blastema cells displayed a cell cycle that was consistent with the developmental age of a late-third instar larva. The most dramatic cell-cycle change was observed in the population of disc cells that did not participate in regeneration; these cells arrested in G1 or G2 (Fig. 4B,C). Such findings indicate that developmental plasticity during disc regeneration is not accomplished by blastema cells reverting to an earlier, more potent developmental stage. Blastema cells which TD however, behaved differently. These cells displayed a transient and novel cell-cycle profile with an extended S phase that is not observed during normal disc development. Additionally, TD cells were larger in size, a characteristic of young disc cells, indicating only one aspect of rejuvenation. Similar changes in morphology, including an increase in cell size is also observed in muscle cells during vertebrate limb regeneration (Hay, 1971). These studies indicate that disc regeneration does not recapitulate early developmental events.
GENES INVOLVED IN TD
(Kauffman, 1973) predicted that imaginal disc determination is established and maintained by a unique combination of developmental control genes, each of which regulates one of five bistable control circuits. Each of these circuits acts as a developmental switch with distinct on-off states. Different imaginal discs share the same circuits, however the activities of the circuits (on or off state) differs between the discs. Kauffman (1973) proposed that TD could arise when one of the circuits switches to its alternative state. Indeed, by switching the on-off states of individual circuits, Kauffman's model predicted the directionality of nearly all TD events. Selector genes are likely candidates for such switches. Selector genes are transcription factors whose activity initiates and maintains a developmental program that determines segmental and organ identity (Mann & Morata, 2000). These genes include eyeless, Distal-less, vestigial (vg) and Hox genes. The expression of selector genes where they are not normally active can cause transformations that phenotypically resemble TD (Fig. 2). For example, misexpression of the wing selector gene vestigial (vg), along with its DNA binding partner scalloped (sd), can induce outgrowths of wing tissue in parts of the eyes, legs, genitalia and antenna (Halder & Carroll, 2001). Such observations suggest that TD arises when there is inappropriate activation of selector gene expression. Indeed, this is observed in fragmented antennal discs where some regenerating cells will misexpress Antp and form leg structures (Gehring & Schubiger, 1975). Similarly, regenerating leg disc cells will ectopically activate the wing selector gene vg and switch to wing identity (Maves & Schubiger, 1995).
More recently it has been observed that secreted signaling molecules which spatially pattern the imaginal discs can induce regenerative growth and TD. Ubiquitous expression of wingless (wg) throughout the larva during the second or third instar induces a subset of TD events that are observed after disc fragmentation (Fig. 2) (Johnston & Schubiger, 1996; Maves & Schubiger, 1995). Misexpression of wg leads to TD by inducing ectopic expression of selector genes, such as vg expression in leg disc cells and proboscipedia (pb) expression in the maxillary palpus (Johnston & Schubiger, 1996; Maves & Schubiger, 1995). Wg is a secreted glycoprotein and a Wnt family member (Wnt1) that acts as a morphogen to direct many cell fate decisions in several diverse developmental processes (Nusse, 2005).
Wnt signaling has also been linked to cell fate changes and TD in vertebrates. Mouse lung progenitor cells, for example, TD to intestinal cell types by elevated and/or prolonged Wnt signaling (Okubo & Hogan, 2004). Lineage switches were also observed when Wnt signaling is altered in hair follicle and epidermal cells (Merrill, Gat, DasGupta, & Fuchs, 2001; Niemann, 2006) and in the mammary gland and prostate (Bierie et al., 2003; Miyoshi et al., 2002).
THE WEAK POINT
As mentioned earlier here, there are specific locations in the leg, labial, eye-antennal and genital discs that are particularly susceptible to TD. Disc cells stimulated to regenerate in these locations exhibit a high degree of plasticity. Such regions were aptly named ‘weak points’ (Fig. 2) (Gehring, 1966; Johnston & Schubiger, 1996; Maves & Schubiger, 1998; Schneuwly, Kuroiwa, & Gehring, 1987; Wildermuth, 1968). Weak points are located along the A/P compartment boundaries in different imaginal discs and are identified by their proliferative ability during regeneration (Johnston & Schubiger, 1996; Maves & Schubiger, 2003). For example, two days after leg disc fragmentation, cell division ceases throughout the entire disc except for cells at the weak point (Kiehle & Schubiger, 1985; Sustar & Schubiger, 2005) (Fig. 4B). Localized cell division is similarly observed at the weak point in leg discs exposed to ubiquitous wg signaling (Fig. 4C) (Johnston & Schubiger, 1996; Maves & Schubiger, 1995; Sustar & Schubiger, 2005). The weak point is thus a region of intense cell division which can lead to TD (Fig. 4D,E).
Recent work on leg-to-wing transdetermination has led to a molecular description of the weak point. Cells in the weak point TD when they inappropriately express high levels of both wg and decapentapelagic (dpp) (Johnston & Schubiger, 1996; Maves & Schubiger, 1995, 2003) (Fig. 6B). In leg discs from wandering third instar larvae, dpp is expressed in a stripe of cells that extends the length of the disc along the A/P compartment boundary, with the level of expression higher in the dorsal half of the disc where it specifies dorsal cell identity (Masucci, Miltenberger, & Hoffmann, 1990; Raftery, Sanicola, Blackman, & Gelbart, 1991) (Fig. 6B). In contrast, wg expression is confined to a ventral wedge within the A compartment where it functions to specify ventral cell fates (Fig. 6B) (Ng, Diaz-Benjumea, Vincent, Wu, & Cohen, 1996; Struhl & Basler, 1993). The ability of these two signals to induce vg expression and TD is complex. Antagonistic interactions between Wg and Dpp, in leg, genital and eye-antennal discs, normally prevent wg expression from overlapping with high levels of dpp expression (Johnston & Schubiger, 1996; Morimura, Maves, Chen, & Hoffmann, 1996). When wg is ubiquitously expressed in the leg disc, dpp expression is repressed throughout the entire disc, except for the proximal-most dorsal cells (cells of the weak point) (Johnston & Schubiger, 1996). It is only in leg cells which retain dpp expression that vg is activated (Fig. 6B). Ubiquitous expression of dpp however, is not sufficient on its own to induce ectopic vg expression and TD (Maves & Schubiger, 1995). Instead, Dpp represses Wg signaling and thus fails to induce TD. These findings indicate that a delicate balance between Wg and Dpp signaling is necessary to induce either leg or wing development (Fig. 7C).
Fig. 6. Expression of vestigial in normal wing discs and TD leg disc cells.
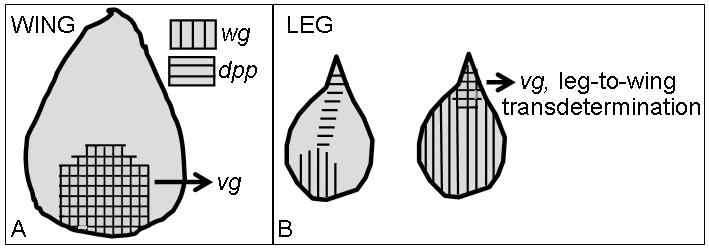
(A) The endogenous expression patterns of wg and dpp in the wing disc during the second and early third larval instars. vg (cross-hatched) is expressed where wg and dpp signals intersect and is required for proper wing and haltere development (Williams, Bell, & Carroll, 1991). (B) The left leg disc in the panel depicts the endogenous expression patterns of wg and dpp. Upon ubiquitous wg expression (right leg disc in panel), dpp expression is repressed throughout the entire leg disc, except for the proximal-most dorsal region (weak point) leading to ectopic activation of vg (cross-hatched) and TD to wing. The figure is adapted from (Wei et al., 2000).
Fig. 7. A model for the ectopic activation of vg expression in leg-to-wing TD.

(A) The PcG and trxG genes normally maintain the on/off transcriptional states of many developmentally regulated genes. In leg disc cells, the PcG proteins have been proposed to maintain the transcriptional off state of wing selector gene vg by binding to the vg-PRE (PcG Response Element) (Lee et al., 2005). (B) Following disc fragmentation and regeneration, leg-to-wing TD may be caused by the reduction of PcG silencing in a JNK-dependent manner, specifically at the vg-PRE (Lee et al., 2005). (C) Exposure to high levels of both Wg and Dpp causes leg disc cells of the weak point to activate vg expression and TD to wing (Johnston & Schubiger, 1996; Maves & Schubiger, 1998). These signals activate ectopic vg expression in the leg disc through the vgBE (vg- Boundary Enhancer) (Maves & Schubiger, 1998). Ectopic wg signaling also alters the expression of several PcG and trxG genes in leg disc cells that have TD to wing (Klebes et al., 2005). Changing the composition of these proteins within leg disc cells may result in whole-scale transcriptional changes, including the transcriptional activation of vg and wg, leading to leg-to-wing TD (Lee et al., 2005).
If Wg and Dpp synergistically cooperate to induce leg-to-wing TD, then misexpression of both signals should cause all leg disc cells to switch to wing identity. However, ectopic expression of dpp and wg only leads to leg-to-wing TD within a second ‘ventral’ weak point (Maves & Schubiger, 1998). It is possible that additional inputs from other sources of patterning information are required for all leg disc cells to TD to wing. Notch signaling (together with Wg and Dpp) could be one such factor, since it has been previously shown to alter selector gene expression, specifically vg expression, in the eye-antennal disc (Kurata, Go, Artavanis-Tsakonas, & Gehring, 2000). Alternatively, only a small cluster of cells in the disc may remain plastic and under certain conditions are capable of TD, while the vast majority of cells rigidly adhere to their disc-specific state of determination.
VG ACTIVATION IN NORMAL WING DISC DEVELOPMENT AND TD
Vg expression is first activated in the embryo and continues until the third larval instar. The enhancer elements that regulate vg expression in the embryo are unknown. In the wing disc, however, vg expression requires two separate transcriptional enhancers: the boundary enhancer (vgBE) and the quadrant enhancer (vgQE) (Kim et al., 1996; Williams, Paddock, Vorwerk, & Carroll, 1994). During the mid-second instar, the vgBE activates vg expression at the dorsoventral (DV) boundary of the wing disc, while the vgQE acts during the third instar and is responsible for vg expression in the developing wing blade primordium (Kim et al., 1996; Williams et al., 1994). Control of vg expression by the vgBE is in response to various signaling pathways, such as Notch, Wg and Vg itself (Kim et al., 1996; Williams et al., 1994). In contrast, vg expression by the vgQE is cooperatively stimulated by Dpp and Wg signals (Klein & Arias, 1999; Neumann & Cohen, 1997; Zecca, Basler, & Struhl, 1996). The ectopic expression of vg in wg-induced leg-to-wing TD requires function of the vgBE (Fig. 7C). Ubiquitous expression of wg during the third larval instar causes a high frequency of leg-to-wing TD (>50% of leg discs) (Maves & Schubiger, 1998). In contrast, wg-induced leg-to-wing TD is rare (<2% of leg discs) in animals homozygous for a deletion of the vgBE (Kim et al., 1996; Maves & Schubiger, 1998). This result is surprising because it has never been shown that Dpp signaling activates vg expression through the vgBE during normal wing disc development. However, it is possible that Dpp (together with Wg) stimulates the vgBE, since the initial activation of the vgBE coincides with overlapping expression of wg and dpp in the wing disc (Burke & Basler, 1996; Kim et al., 1996) (Fig. 6A).
DECIPHERING THE GENES INVOLVED IN TRANSDETERMINATION
New insight into putative downstream genes that initiate and elaborate TD comes from analyzing the transcriptional profile of leg disc cells that TD to wing following ectopic wg expression (Klebes et al., 2005). Using microarray, approximately 140 ‘TD’ genes were identified whose expression levels were enriched in vg-expressing leg cells (Klebes et al., 2005). Interestingly, many candidate ‘TD’ genes are known to suppress differentiation. For example, unpaired (upd) was significantly up-regulated in TD leg disc cells. Upd encodes a ligand for the JAK/STAT (janus kinase/signal transducer and activator of transcription) signaling cascade that suppresses differentiation and promotes self-renewal of mammalian embryonic stem cells and in Drosophila stem cells of the testis (Forrai et al., 2006; Harrison, McCoon, Binari, Gilman, & Perrimon, 1998). lamina ancestor (lama) transcripts were also enriched in TD leg disc cells. lama encodes a novel protein that is expressed in glial and neuronal precursor cells in the Drosophila lamina prior to differentiation (Perez & Steller, 1996). A similar pattern of lama expression is observed in developing leg discs, with expression observed in early but not late third instar leg discs (Klebes et al., 2005). The reactivation of lama expression in TD leg cells suggests that lama might prevent differentiation, a potential step required for acquiring disc cell multipotency.
The microarray analysis also identified several genes that may be involved in pluripotency. Sox15, for example, was identified as a putative TD gene. There are 26 distinct Sox proteins found in vertebrates (Bowles, Schepers, & Koopman, 2000; Pevny & Lovell-Badge, 1997; Wegner, 1999), these proteins are transcription factors that play essential roles in cell differentiation, development and organogenesis (Pevny & Lovell-Badge, 1997). While only Sox2 has been shown essential for the establishment and maintenance of pluripotency in ES cells (Avilion et al., 2003) evidence suggests that Sox15 may also maintain ES cell pluripotency (Maruyama, Ichisaka, Nakagawa, & Yamanaka, 2005). While little known about Sox15 function during normal Drosophila development, these data intriguingly suggest that it may play an important role in developmental potency.
DEVELOPMENTAL POTENCY AND CHROMATIN REORGANIZATION
Recent studies have drawn attention to the idea that ES cells restrict their developmental potency during cell determination and cell differentiation by implementing tissue-specific gene expression programs. The pluripotency of ES cells is associated with an unfettered and plastic genome, accessible to transcriptional regulation and thus poised to differentiate into any cell type (Meshorer & Misteli, 2006). As pluirpotent cells are specified into tissue lineages they selectively activate specific subsets of genes (Eiges & Benvenisty, 2002; Loebel, Watson, De Young, & Tam, 2003; Weiss & Orkin, 1996). Regions of the genome that are not involved in the emerging cell lineage become silenced and thereafter remain inaccessible to transcriptional regulation (Eckfeldt, Mendenhall, & Verfaillie, 2005). Such fate-specific transcriptional programs are maintained, during development and the lifespan of the organism, through chromatin remodeling proteins such as the Polycomb Group (PcG) and trithorax Group (trxG) proteins (Ringrose & Paro, 2004). These proteins sustain lineage-specific gene expression patterns over multiple cell divisions through epigenetic modification of chromatin structure, notably through covalent histone modifications (Francis & Kingston, 2001). It is commonly thought that PcG proteins act as repressors of gene activity, while trxG proteins promote gene activity.
Strong evidence indicates that the PcG/trxG proteins are involved in the control of disc cell multipotency. Recent studies have shown that PcG and trxG proteins bind to and potentially regulate the expression of many developmentally regulated genes (167 genes), including transcription factors and key signal transduction pathway molecules (Ringrose, Rehmsmeier, Dura, & Paro, 2003; Tolhuis et al., 2006). Therefore changing the activity or composition of these proteins, either during or preceding TD, may have profound effects on selector gene expression, and thus the developmental potential of a cell. In support of this idea, Lee et al. (2005) found that following disc fragmentation, blastema cells activate the JNK signaling pathway which down-regulates some PcG proteins. The frequency of TD is lowered in a JNK heterozygote mutant background, indicating that down-regulation of PcG repression is necessary for the cell fate switches. Similarly, Klebes et al. (2005) found that upon ubiquitous wg signaling the expression levels of 15 of the 32 PcG and trxG genes were specifically altered in vg-expressing leg disc cells. Heterozygous mutations in several of these genes (Polycomb, Enhancer of zeste, Enhancer of polycomb, Sex comb on midleg, Su(z)2, brahma and osa) dominantly modified the frequency and area of wg-induced leg-to-wing TD (Klebes et al., 2005; Lee, Maurange, Ringrose, & Paro, 2005). These observations indicate that modulation of PcC/trxG proteins, specifically in regenerating disc cells, may facilitate a switch in fate, possibly by changing the transcriptional accessibility of chromatin to a more reprogrammable state.
The PcG proteins were observed to bind specific cis-regulatory DNA sequences termed PcG Response Elements (PREs) found adjacent to both the wg and vg genes (Lee et al., 2005). PREs often reside within promoters of PcG targets (Ringrose & Paro, 2004). They enable PcG proteins to maintain transcriptional silencing over numerous cell divisions, as seen when the wg-PRE and vg-PRE were able to cause in vivo silencing of a white eye color reporter gene (Lee et al., 2005). Since wg and vg are continuously expressed in many cells and shut off (or not activated or both) in other cells, the wg-PRE and vg-PRE enhancers are likely to be active only in repressed tissues. We hypothesize that PcG complexes maintain vg repression at the vg-PRE in leg disc cells (Fig. 7A). Downregulation of PcG proteins by JNK signaling in some leg cells may reactivate vg and/or wg expression leading to TD (Fig. 7B) (Lee et al., 2005). Alternatively, ubiquitous wg signaling has been shown to regulate the expression of several chromatin remodeling genes (Klebes et al., 2005). Changing the composition of these proteins may render cells of the weak point more susceptible to TD by de-repressing several PRE-controlled genes, including vg and wg (Fig 7C). It is likely that TD occurs in disc cells with both high morphogen activity and a more accessible chromatin configuration (Fig. 7C). We propose this as a general model, indeed the switch from leg to wing requires many genes (not just vg and wg) to be switched on and off (Klebes et al., 2005).
Further evidence that demonstrates the relationship between disc cell plasticity and chromatin remodeling comes from the identification of winged eye (wge) (Katsuyama et al., 2005). Misexpression of wge induces eye-to-wing TD by altering both wg and vg expression. wge displays trxG-like characteristics and encodes a chromatin-associated protein that includes a bromo-adjacent homology (BAH) domain implicated in epigenetic regulation of gene expression (Callebaut, Courvalin, & Mornon, 1999). Taken together, these studies indicate that developmental potency and the reprogramming of cellular fate are closely allied with the maintenance of both chromatin organization and fate-specific transcriptional programs.
Another mechanism by which PcG proteins could affect developmental plasticity is through cell-cycle regulation. Many PcG targets are genes involved in cell-cycle regulation (Brumby et al., 2002; Trimarchi, Fairchild, Wen, & Lees, 2001). As mentioned previously, TD cells in the blastema display a transient and novel cell-cycle profile that is not observed during normal development. Down-regulation of PcG activity following disc regeneration may initiate these cell-cycle changes by directly modifying the transcription of cell-cycle genes (Lee et al., 2005; Sustar & Schubiger, 2005). Such cell-cycle changes may allow for transcriptional reprogramming and TD. This idea is based on the fact that as stem cells progress through the cell cycle their decision to become a stem cell or commit to a particular lineage is most vulnerable during S phase, when there are dramatic structural changes made to the chromatin, resulting in numerous transcriptional changes (Holtzer et al., 1975; Quesenberry, Colvin, & Lambert, 2002).
TD AND TRANSDIFFERENTIATION
The phenomenon of TD in Drosophila shares many interesting parallels to transdifferentiation in vertebrates. Transdifferentiation is a form of metaplasia or the conversion of one already differentiated cell type to another. Both TD and transdifferentiation involve changes in cell fate, and such changes are attributed to altered gene expression. Once cells of a tissue convert to another fate they will exhibit their newly specialized function and normal histology. Transdifferentiation, like TD, usually arises during regenerative growth after tissue injury.
To illustrate the similarities between TD and transdifferentiation, comparisons can be made between leg-to-wing TD in Drosophila and liver-to-pancreas transdifferentiation in vertebrates. First, both cell fate changes are believed to occur because the two organs share a close developmental origin. The pancreas and liver both arise from the embryonic foregut endoderm (Jung, Zheng, Goldfarb, & Zaret, 1999; Rossi, Dunn, Hogan, & Zaret, 2001). Lineage separation of the liver emerges when some endodermal cells receive an FGF-like signal from the cardiac mesoderm (Deutsch, Jung, Zheng, Lora, & Zaret, 2001). Without this signal, the ventral foregut endoderm becomes pancreas. In Drosophila, wing and leg appendages arise from a common primordium in the embryo (Steiner, 1978; Wieschaus & Gehring, 1976). The formation of distinct wing and leg disc primordia occurs when a group of distal-less expressing cells within the common leg-wing primordium migrate dorsally and begin to express vg (Cohen, 1993). Interestingly, like leg-to-wing TD, the liver-to-pancreas transdifferentiation can be induced by ectopic expression of a single selector gene. Targeted expression of an activated form of Pancreatic-duodenal homeobox-1 (Pdx-1) in liver cells can induce the formation of pancreas (Li, Horb, Tosh, & Slack, 2005). Therefore, like TD, the identification of molecules which cause transdifferentiation will provide information on the molecular basis of how cellular identity is established as well as enhance our understanding of how cells can be coaxed into adopting alternative fates and become more stem-cell like.
CONCLUSION: TRANSDETERMINATION--A STEM CELL CONNECTION
Cells in the weak point exhibit many stem cell-like characteristics. Embryonic and adult stem cells exhibit unlimited proliferative capacity--the ability to divide and create additional stem cells (Schofield, 1978). Similar endless proliferation is observed in some imaginal disc cells; these cells can be propagated indefinitely in culture while maintaining their diploidy and ability to differentiate (Hadorn, 1978). Only a few cells (founder cells) in the imaginal discs are capable of TD (multipotency) (Gehring, 1978). Similarly, in many tissues and organs only a small sub-population of cells, the adult stem cells, have the ability to regenerate and give rise to several specialized cell types (multipotency). Adult stem cells are commonly embedded in a special microenvironment known as the niche (Fuchs, Tumbar, & Guasch, 2004). Signals within the niche instruct stem cell fate, either to self-renew or commit to transiently proliferate and differentiate. Signaling pathways common to the niche include BMPs/TGFβ, Jak/Stat, Notch and Wnt signals (Alonso & Fuchs, 2003; Calvi et al., 2003; Kiger, Jones, Schulz, Rogers, & Fuller, 2001; Sancho, Batlle, & Clevers, 2003; Xie & Spradling, 1998). Intriguingly, the same signal transduction pathways have been identified to cause cell fate changes in the weak point of imaginal discs (Johnston & Schubiger, 1996; Klebes et al., 2005; Maves & Schubiger, 1998). Thus, weak points within imaginal discs may represent a niche-like environment, providing signals that either maintain disc-specific determination or under certain conditions trigger disc cell multipotency. In conclusion, TD in Drosophila imaginal discs provides an excellent model system for investigating how cells acquire multipotency as well as decide between different fates during development.
ACKNOWLEDGEMENTS
We are grateful to members of the Schubiger lab, Margrit Schubiger and Laura Johnston for their thoughtful comments and suggestions on the manuscript. This work was supported by grant RO1 GM058282 from the National Institutes of Health to G.S.
Abbreviations
- ES cells
embryonic stem cells
- SSC
somatic stem cells
- PRE
PcG Response Element
- vg
vestigial
- wg
wingless
- dpp
decapentapelagic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kimberly D McClure, University of Washington, Department of Biology 24 Kincaid Hall, Box 351800 Seattle, WA 98195 (206)-543-8159 Email: kmcclure@u.washington.edu.
Gerold Schubiger, University of Washington, Department of Biology 24 Kincaid Hall, Box 351800 Seattle, WA 98195 (206)-543-8159 Email: gerold@u.washington.edu.
REFERENCES
- Abbott LC, Karpen GH, Schubiger G. Compartmental restrictions and blastema formation during pattern regulation in Drosophila imaginal leg discs. Dev Biol. 1981;87(1):64–75. doi: 10.1016/0012-1606(81)90061-0. [DOI] [PubMed] [Google Scholar]
- Alison M, Sarraf C. Hepatic stem cells. J Hepatol. 1998;29(4):676–682. doi: 10.1016/s0168-8278(98)80165-7. [DOI] [PubMed] [Google Scholar]
- Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev. 2003;17(10):1189–1200. doi: 10.1101/gad.1086903. [DOI] [PubMed] [Google Scholar]
- Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17(1):126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierie B, Nozawa M, Renou JP, Shillingford JM, Morgan F, Oka T, Taketo MM, Cardiff RD, Miyoshi K, Wagner KU, Robinson GW, Hennighausen L. Activation of beta-catenin in prostate epithelium induces hyperplasias and squamous transdifferentiation. Oncogene. 2003;22(25):3875–3887. doi: 10.1038/sj.onc.1206426. [DOI] [PubMed] [Google Scholar]
- Bowles J, Schepers G, Koopman P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev Biol. 2000;227(2):239–255. doi: 10.1006/dbio.2000.9883. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Kumar A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310(5756):1919–1923. doi: 10.1126/science.1115200. [DOI] [PubMed] [Google Scholar]
- Brumby AM, Zraly CB, Horsfield JA, Secombe J, Saint R, Dingwall AK, Richardson H. Drosophila cyclin E interacts with components of the Brahma complex. Embo J. 2002;21(13):3377–3389. doi: 10.1093/emboj/cdf334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant PJ. Cell lineage relationships in the imaginal wing disc of Drosophila melanogaster. Dev Biol. 1970;22(3):389–411. doi: 10.1016/0012-1606(70)90160-0. [DOI] [PubMed] [Google Scholar]
- Burke R, Basler K. Dpp receptors are autonomously required for cell proliferation in the entire developing Drosophila wing. Development. 1996;122(7):2261–2269. doi: 10.1242/dev.122.7.2261. [DOI] [PubMed] [Google Scholar]
- Callebaut I, Courvalin JC, Mornon JP. The BAH (bromo-adjacent homology) domain: a link between DNA methylation, replication and transcriptional regulation. FEBS Lett. 1999;446(1):189–193. doi: 10.1016/s0014-5793(99)00132-5. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Cohen SM. Development of Drosphila. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 1993. [Google Scholar]
- Deutsch G, Jung J, Zheng M, Lora J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128(6):871–881. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- Eckfeldt CE, Mendenhall EM, Verfaillie CM. The molecular repertoire of the ’almighty’ stem cell. Nat Rev Mol Cell Biol. 2005;6(9):726–737. doi: 10.1038/nrm1713. [DOI] [PubMed] [Google Scholar]
- Eiges R, Benvenisty N. A molecular view on pluripotent stem cells. FEBS Lett. 2002;529(1):135–141. doi: 10.1016/s0014-5793(02)03191-5. [DOI] [PubMed] [Google Scholar]
- Forrai A, Boyle K, Hart AH, Hartley L, Rakar S, Willson TA, Simpson KM, Roberts AW, Alexander WS, Voss AK, Robb L. Absence of suppressor of cytokine signalling 3 reduces self-renewal and promotes differentiation in murine embryonic stem cells. Stem Cells. 2006;24(3):604–614. doi: 10.1634/stemcells.2005-0323. [DOI] [PubMed] [Google Scholar]
- Francis NJ, Kingston RE. Mechanisms of transcriptional memory. Nat Rev Mol Cell Biol. 2001;2(6):409–421. doi: 10.1038/35073039. [DOI] [PubMed] [Google Scholar]
- Fristrom D, Fristrom J. The Development of Drosophila melanogaster. Vol. 2. Cold Spring Harbor Press; Cold Spring Harbor: 1993. The Metamorphic Development of the Adult Epidermis. [Google Scholar]
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116(6):769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- Gage FH, Ray J, Fisher LJ. Isolation, characterization, and use of stem cells from the CNS. Annu Rev Neurosci. 1995;18:159–192. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A, Ripoll P, Morata G. Developmental compartmentalisation of the wing disk of Drosophila. Nat New Biol. 1973;245(147):251–253. doi: 10.1038/newbio245251a0. [DOI] [PubMed] [Google Scholar]
- Gardiner DM, Carlson MR, Roy S. Towards a functional analysis of limb regeneration. Semin Cell Dev Biol. 1999;10(4):385–393. doi: 10.1006/scdb.1999.0325. [DOI] [PubMed] [Google Scholar]
- Gargioli C, Slack JM. Cell lineage tracing during Xenopus tail regeneration. Development. 2004;131(11):2669–2679. doi: 10.1242/dev.01155. [DOI] [PubMed] [Google Scholar]
- Gehring W. Cell heredity and changes of determination in cultures of imaginal discs in Drosophila melanogaster. J Embryol Exp Morphol. 1966;15(1):77–111. [PubMed] [Google Scholar]
- Gehring W. Clonal analysis of determination dynamics in cultures of imaginal disks in Drosophila melanogaster. Dev Biol. 1967;16(5):438–456. doi: 10.1016/0012-1606(67)90058-9. [DOI] [PubMed] [Google Scholar]
- Gehring WJ. The Genetics and Biology of Drosophila. 2c. Academic Press; New York: 1978. [Google Scholar]
- Gehring WJ, Schubiger G. Expression of homeotic mutations in duplicated and regenerated antennae of Drosophila melanogaster. J Embryol Exp Morphol. 1975;33(2):459–469. [PubMed] [Google Scholar]
- Gibson MC, Schubiger G. Hedgehog is required for activation of engrailed during regeneration of fragmented Drosophila imaginal discs. Development. 1999;126(8):1591–1599. doi: 10.1242/dev.126.8.1591. [DOI] [PubMed] [Google Scholar]
- Grigliatti T, Suzuki DT. Temperature-sensitive mutations in Drosophila melanogaster. 8. The homeotic mutant, ss a40a. Proc Natl Acad Sci U S A. 1971;68(6):1307–1311. doi: 10.1073/pnas.68.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson E, Goldsborough AS, Ali Z, Kornberg TB. The Drosophila engrailed and invected genes: partners in regulation, expression and function. Genetics. 1996;142(3):893–906. doi: 10.1093/genetics/142.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadorn E. Differenzierungsleistungen wiederholt fragmentierter Teilstücke männlicher Genitalscheiben von Drosophila melanogaster nach Kultur in vivo. Dev Biol. 1963;6:617–629. [Google Scholar]
- Hadorn E. Genetic Control of Differentiation. Vol. 18. Brookhaven Symposia in Biology; Upton, New York: 1965. Problems of Determination and Transdetermination; pp. 148–161. [Google Scholar]
- Hadorn E. The Genetics and Biology of Drosophila. 2c. Academic Press; New York: 1978. [Google Scholar]
- Hadorn E, Gsell R, Schultz J. Stability of a position-effect variegation in normal and transdetermined larval blastemas from Drosophila melanogaster. Proc Natl Acad Sci U S A. 1970;65(3):633–637. doi: 10.1073/pnas.65.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder G, Carroll SB. Binding of the Vestigial co-factor switches the DNA-target selectivity of the Scalloped selector protein. Development. 2001;128(17):3295–3305. doi: 10.1242/dev.128.17.3295. [DOI] [PubMed] [Google Scholar]
- Harrison DA, McCoon PE, Binari R, Gilman M, Perrimon N. Drosophila unpaired encodes a secreted protein that activates the JAK signaling pathway. Genes Dev. 1998;12(20):3252–3263. doi: 10.1101/gad.12.20.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay ED. Skeletal-muscle regeneration. N Engl J Med. 1971;284(18):1033–1034. doi: 10.1056/NEJM197105062841812. [DOI] [PubMed] [Google Scholar]
- Holtzer H, Rubinstein N, Fellini S, Yeoh G, Chi J, Birnbaum J, Okayama M. Lineages, quantal cell cycles, and the generation of cell diversity. Q Rev Biophys. 1975;8(4):523–557. doi: 10.1017/s0033583500001980. [DOI] [PubMed] [Google Scholar]
- Johnston LA, Schubiger G. Ectopic expression of wingless in imaginal discs interferes with decapentaplegic expression and alters cell determination. Development. 1996;122(11):3519–3529. doi: 10.1242/dev.122.11.3519. [DOI] [PubMed] [Google Scholar]
- Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284(5422):1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- Karpen GH, Schubiger G. Extensive regulatory capabilities of a Drosophila imaginal disk blastema. Nature. 1981;294(5843):744–747. doi: 10.1038/294744a0. [DOI] [PubMed] [Google Scholar]
- Katsuyama T, Sugawara T, Tatsumi M, Oshima Y, Gehring WJ, Aigaki T, Kurata S. Involvement of winged eye encoding a chromatin-associated bromo-adjacent homology domain protein in disc specification. Proc Natl Acad Sci U S A. 2005;102(44):15918–15923. doi: 10.1073/pnas.0507945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman SA. Control circuits for determination and transdetermination. Science. 1973;181(97):310–318. doi: 10.1126/science.181.4097.310. [DOI] [PubMed] [Google Scholar]
- Kiehle CP, Schubiger G. Cell proliferation changes during pattern regulation in imaginal leg discs of Drosophila melanogaster. Dev Biol. 1985;109(2):336–346. doi: 10.1016/0012-1606(85)90460-9. [DOI] [PubMed] [Google Scholar]
- Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294(5551):2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- Kim J, Sebring A, Esch JJ, Kraus ME, Vorwerk K, Magee J, Carroll SB. Integration of positional signals and regulation of wing formation and identity by Drosophila vestigial gene. Nature. 1996;382(6587):133–138. doi: 10.1038/382133a0. [DOI] [PubMed] [Google Scholar]
- Klebes A, Sustar A, Kechris K, Li H, Schubiger G, Kornberg TB. Regulation of cellular plasticity in Drosophila imaginal disc cells by the Polycomb group, trithorax group and lama genes. Development. 2005;132(16):3753–3765. doi: 10.1242/dev.01927. [DOI] [PubMed] [Google Scholar]
- Klein T, Arias AM. The vestigial gene product provides a molecular context for the interpretation of signals during the development of the wing in Drosophila. Development. 1999;126(5):913–925. doi: 10.1242/dev.126.5.913. [DOI] [PubMed] [Google Scholar]
- Kurata S, Go MJ, Artavanis-Tsakonas S, Gehring WJ. Notch signaling and the determination of appendage identity. Proc Natl Acad Sci U S A. 2000;97(5):2117–2122. doi: 10.1073/pnas.040556497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence PA, Morata G. Compartments in the wing of Drosophila: a study of the engrailed gene. Dev Biol. 1976;50(2):321–337. doi: 10.1016/0012-1606(76)90155-x. [DOI] [PubMed] [Google Scholar]
- Lee N, Maurange C, Ringrose L, Paro R. Suppression of Polycomb group proteins by JNK signalling induces transdetermination in Drosophila imaginal discs. Nature. 2005;438(7065):234–237. doi: 10.1038/nature04120. [DOI] [PubMed] [Google Scholar]
- Li WC, Horb ME, Tosh D, Slack JM. In vitro transdifferentiation of hepatoma cells into functional pancreatic cells. Mech Dev. 2005;122(6):835–847. doi: 10.1016/j.mod.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Lindsley D. L. a. G. E. H. Genetic Variations of Drosophila melanogaster. Carnegie Instituation of Washington Publication; 1968. [Google Scholar]
- Loebel DA, Watson CM, De Young RA, Tam PP. Lineage choice and differentiation in mouse embryos and embryonic stem cells. Dev Biol. 2003;264(1):1–14. doi: 10.1016/s0012-1606(03)00390-7. [DOI] [PubMed] [Google Scholar]
- Mann RS, Morata G. The developmental and molecular biology of genes that subdivide the body of Drosophila. Annu Rev Cell Dev Biol. 2000;16:243–271. doi: 10.1146/annurev.cellbio.16.1.243. [DOI] [PubMed] [Google Scholar]
- Maruyama M, Ichisaka T, Nakagawa M, Yamanaka S. Differential roles for Sox15 and Sox2 in transcriptional control in mouse embryonic stem cells. J Biol Chem. 2005;280(26):24371–24379. doi: 10.1074/jbc.M501423200. [DOI] [PubMed] [Google Scholar]
- Masucci JD, Miltenberger RJ, Hoffmann FM. Pattern-specific expression of the Drosophila decapentaplegic gene in imaginal disks is regulated by 3′ cis-regulatory elements. Genes Dev. 1990;4(11):2011–2023. doi: 10.1101/gad.4.11.2011. [DOI] [PubMed] [Google Scholar]
- Maves L, Schubiger G. Wingless induces transdetermination in developing Drosophila imaginal discs. Development. 1995;121(5):1263–1272. doi: 10.1242/dev.121.5.1263. [DOI] [PubMed] [Google Scholar]
- Maves L, Schubiger G. A molecular basis for transdetermination in Drosophila imaginal discs: interactions between wingless and decapentaplegic signaling. Development. 1998;125(1):115–124. doi: 10.1242/dev.125.1.115. [DOI] [PubMed] [Google Scholar]
- Maves L, Schubiger G. Transdetermination in Drosophila imaginal discs: a model for understanding pluripotency and selector gene maintenance. Curr Opin Genet Dev. 2003;13(5):472–479. doi: 10.1016/j.gde.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Merrill BJ, Gat U, DasGupta R, Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15(13):1688–1705. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshorer E, Misteli T. Chromatin in pluripotent embryonic stem cells and differentiation. Nat Rev Mol Cell Biol. 2006;7(7):540–546. doi: 10.1038/nrm1938. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Shillingford JM, Le Provost F, Gounari F, Bronson R, von Boehmer H, Taketo MM, Cardiff RD, Hennighausen L, Khazaie K. Activation of beta -catenin signaling in differentiated mammary secretory cells induces transdifferentiation into epidermis and squamous metaplasias. Proc Natl Acad Sci U S A. 2002;99(1):219–224. doi: 10.1073/pnas.012414099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morata G, Lawrence PA. Control of compartment development by the engrailed gene in Drosophila. Nature. 1975;255(5510):614–617. doi: 10.1038/255614a0. [DOI] [PubMed] [Google Scholar]
- Morimura S, Maves L, Chen Y, Hoffmann FM. decapentaplegic overexpression affects Drosophila wing and leg imaginal disc development and wingless expression. Dev Biol. 1996;177(1):136–151. doi: 10.1006/dbio.1996.0151. [DOI] [PubMed] [Google Scholar]
- Moss FP, Leblond CP. Nature of dividing nuclei in skeletal muscle of growing rats. J Cell Biol. 1970;44(2):459–462. doi: 10.1083/jcb.44.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec. 1971;170(4):421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- Neumann C, Cohen S. Morphogens and pattern formation. Bioessays. 1997;19(8):721–729. doi: 10.1002/bies.950190813. [DOI] [PubMed] [Google Scholar]
- Ng M, Diaz-Benjumea FJ, Vincent JP, Wu J, Cohen SM. Specification of the wing by localized expression of wingless protein. Nature. 1996;381(6580):316–318. doi: 10.1038/381316a0. [DOI] [PubMed] [Google Scholar]
- Niemann C. Controlling the stem cell niche: right time, right place, right strength. Bioessays. 2006;28(1):1–5. doi: 10.1002/bies.20352. [DOI] [PubMed] [Google Scholar]
- Nolo R, Abbott LA, Bellen HJ. Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell. 2000;102(3):349–362. doi: 10.1016/s0092-8674(00)00040-4. [DOI] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15(1):28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- Okubo T, Hogan BL. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J Biol. 2004;3(3):11. doi: 10.1186/jbiol3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez SE, Steller H. Molecular and genetic analyses of lama, an evolutionarily conserved gene expressed in the precursors of the Drosophila first optic ganglion. Mech Dev. 1996;59(1):11–27. doi: 10.1016/0925-4773(96)00556-4. [DOI] [PubMed] [Google Scholar]
- Pevny LH, Lovell-Badge R. Sox genes find their feet. Curr Opin Genet Dev. 1997;7(3):338–344. doi: 10.1016/s0959-437x(97)80147-5. [DOI] [PubMed] [Google Scholar]
- Quesenberry PJ, Colvin GA, Lambert JF. The chiaroscuro stem cell: a unified stem cell theory. Blood. 2002;100(13):4266–4271. doi: 10.1182/blood-2002-04-1246. [DOI] [PubMed] [Google Scholar]
- Raftery LA, Sanicola M, Blackman RK, Gelbart WM. The relationship of decapentaplegic and engrailed expression in Drosophila imaginal disks: do these genes mark the anterior-posterior compartment boundary? Development. 1991;113(1):27–33. doi: 10.1242/dev.113.1.27. [DOI] [PubMed] [Google Scholar]
- Ringrose L, Paro R. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet. 2004;38:413–443. doi: 10.1146/annurev.genet.38.072902.091907. [DOI] [PubMed] [Google Scholar]
- Ringrose L, Rehmsmeier M, Dura JM, Paro R. Genome-wide prediction of Polycomb/Trithorax response elements in Drosophila melanogaster. Dev Cell. 2003;5(5):759–771. doi: 10.1016/s1534-5807(03)00337-x. [DOI] [PubMed] [Google Scholar]
- Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15(15):1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Alvarado A. Planarian regeneration: its end is its beginning. Cell. 2006;124(2):241–245. doi: 10.1016/j.cell.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Sancho E, Batlle E, Clevers H. Live and let die in the intestinal epithelium. Curr Opin Cell Biol. 2003;15(6):763–770. doi: 10.1016/j.ceb.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Schneuwly S, Kuroiwa A, Gehring WJ. Molecular analysis of the dominant homeotic Antennapedia phenotype. Embo J. 1987;6(1):201–206. doi: 10.1002/j.1460-2075.1987.tb04739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(12):7–25. [PubMed] [Google Scholar]
- Schubiger G. Anlageplan, Determinationszustand und Transdeterminationsleistungen der männlichen Vorderbeinscheibe von Drosophila melanogaster. Wilhelm Roux's Arch. EntwMech. Org. 1968;160:9–40. doi: 10.1007/BF00573645. [DOI] [PubMed] [Google Scholar]
- Schubiger G. Regeneration, duplication and transdetermination in fragments of the leg disc of Drosophila melanogaster. Dev Biol. 1971;26(2):277–295. doi: 10.1016/0012-1606(71)90127-8. [DOI] [PubMed] [Google Scholar]
- Schubiger G. Regeneration of Drosophila melanogaster male leg disc fragments in sugar fed female hosts. Experientia. 1973;29(5):631–632. doi: 10.1007/BF01926712. [DOI] [PubMed] [Google Scholar]
- Schultz E, McCormick KM. Skeletal muscle satellite cells. Rev Physiol Biochem Pharmacol. 1994;123:213–257. doi: 10.1007/BFb0030904. [DOI] [PubMed] [Google Scholar]
- Simcox AA, Sang JH. When does determination occur in Drosophila embryos? Dev Biol. 1983;97(1):212–221. doi: 10.1016/0012-1606(83)90078-7. [DOI] [PubMed] [Google Scholar]
- Skeath JB, Carroll SB. The achaete-scute complex: generation of cellular pattern and fate within the Drosophila nervous system. Faseb J. 1994;8(10):714–721. doi: 10.1096/fasebj.8.10.8050670. [DOI] [PubMed] [Google Scholar]
- Slack JM. Homoeotic transformations in man: implications for the mechanism of embryonic development and for the organization of epithelia. J Theor Biol. 1985;114(3):463–490. doi: 10.1016/s0022-5193(85)80179-x. [DOI] [PubMed] [Google Scholar]
- Steiner E. Establishment of compartments in the developing leg imaginal discs of Drosophila melanogaster. Wilhelm Roux's Archives. 1978;180:9–30. doi: 10.1007/BF00848882. [DOI] [PubMed] [Google Scholar]
- Steiner EMK-W, Rolf Nothiger. Transdetermination in Leg Imaginal Discs of Drosophila melanogaster und Drosophila nigromelanica. Wilhelm Roux's Arch. 1981;190:156–160. doi: 10.1007/BF00867802. [DOI] [PubMed] [Google Scholar]
- Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72(4):527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Sustar A, Schubiger G. A transient cell cycle shift in Drosophila imaginal disc cells precedes multipotency. Cell. 2005;120(3):383–393. doi: 10.1016/j.cell.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Tanaka EM. Cell differentiation and cell fate during urodele tail and limb regeneration. Curr Opin Genet Dev. 2003;13(5):497–501. doi: 10.1016/j.gde.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Tobler H. Cell specific determination and the relationship between proliferation and transdetermination in leg and wing primordia in Drosophila melanogaster. J Embryol Exp Morphol. 1966;16(3):609–633. [PubMed] [Google Scholar]
- Tolhuis B, de Wit E, Muijrers I, Teunissen H, Talhout W, van Steensel B, van Lohuizen M. Genome-wide profiling of PRC1 and PRC2 Polycomb chromatin binding in Drosophila melanogaster. Nat Genet. 2006;38(6):694–699. doi: 10.1038/ng1792. [DOI] [PubMed] [Google Scholar]
- Trimarchi JM, Fairchild B, Wen J, Lees JA. The E2F6 transcription factor is a component of the mammalian Bmi1-containing polycomb complex. Proc Natl Acad Sci U S A. 2001;98(4):1519–1524. doi: 10.1073/pnas.041597698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers AJ, Conboy IM. Cellular and molecular signatures of muscle regeneration: current concepts and controversies in adult myogenesis. Cell. 2005;122(5):659–667. doi: 10.1016/j.cell.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Watt FM. Epidermal stem cells: markers, patterning and the control of stem cell fate. Philos Trans R Soc Lond B Biol Sci. 1998;353(1370):831–837. doi: 10.1098/rstb.1998.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res. 1999;27(6):1409–1420. doi: 10.1093/nar/27.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G, Schubiger G, Harder F, Muller AM. Stem cell plasticity in mammals and transdetermination in Drosophila: common themes? Stem Cells. 2000;18(6):409–414. doi: 10.1634/stemcells.18-6-409. [DOI] [PubMed] [Google Scholar]
- Weiss MJ, Orkin SH. In vitro differentiation of murine embryonic stem cells. New approaches to old problems. J Clin Invest. 1996;97(3):591–595. doi: 10.1172/JCI118454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100(1):157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- Wieschaus E, Gehring W. Clonal analysis of primordial disc cells in the early embryo of Drosophila melanogaster. Dev Biol. 1976;50(2):249–263. doi: 10.1016/0012-1606(76)90150-0. [DOI] [PubMed] [Google Scholar]
- Wildermuth H. Differenzierungsleistungen, Mustergliederung und Transdeterminationsmechanismen in hetero- und homoplastischen Transplantaten der Rüsselprimordien von Drosophila. Wilhelm Roux's Arch. EntwMech. Org. 1968;160:41–75. doi: 10.1007/BF00573646. [DOI] [PubMed] [Google Scholar]
- Williams JA, Bell JB, Carroll SB. Control of Drosophila wing and haltere development by the nuclear vestigial gene product. Genes Dev. 1991;5(12B):2481–2495. doi: 10.1101/gad.5.12b.2481. [DOI] [PubMed] [Google Scholar]
- Williams JA, Paddock SW, Vorwerk K, Carroll SB. Organization of wing formation and induction of a wing-patterning gene at the dorsal/ventral compartment boundary. Nature. 1994;368(6469):299–305. doi: 10.1038/368299a0. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94(2):251–260. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G. Direct and long-range action of a wingless morphogen gradient. Cell. 1996;87(5):833–844. doi: 10.1016/s0092-8674(00)81991-1. [DOI] [PubMed] [Google Scholar]


