Abstract
Information on the life span of organisms in the field is essential for elucidating the evolution of life span and aging. We present mark-recapture data (>30 000 marked individuals, >4000 recaptured at least once) on forty-seven species of fruit-feeding butterflies in a tropical forest in Uganda. The data reveal adult life spans in the field for several species that are significantly longer than previously recorded in Lepidoptera (butterflies and moths). Longevity records for species of which more than 100 individuals were recaptured ranged from 67 (Bicyclus auricruda) to 293 days (Euphaedra medon). In contrast to the majority of Lepidoptera which are short-lived, these all show exceptionally long life spans, and may thus help to better identify factors that affect aging, particularly when combined with information on temporal patterns in reproduction, strategies to avoid predation, and nutritional ecology. These key traits are readily measurable in butterflies and thus studies on fruit-feeding butterflies have much potential for gaining insight into the evolution of life span and aging, especially given the tradition of field-research on butterflies.
Keywords: adult phenology, baited traps, captive life span, dispersal, inter sexual differences, mark-recapture
INTRODUCTION
For elucidating the evolution of life span and aging, information on the natural life span of organisms in wild populations is essential. Field studies generate important new perspectives and ideas because in the field one can determine what sorts of conditions are actually experienced, and hence what sorts of selection pressures might be relevant. Moreover, novel mechanisms/strategies may be discovered that are not apparent in laboratory populations (Bronikowski and Promislow, 2005). In addition, the species diversity that field biologists deal with can be used for comparative studies that test the generality of results obtained using model organisms. Because the vast majority of aging studies are based exclusively on controlled experiments in the laboratory on a small number of model organisms, there is a need for studies of free-living animals to provide context, and assess the generality of these results (Austad, 1993, Tatar et al., 1997). Because it is life span that evolves and aging is one of the factors affecting mortality, measuring life span in natural populations may help determining the forces that shape life span and aging. Life span in the field will also affect fluctuations in population density and observed species diversity, and information on life span is, therefore, important for understanding population dynamics and population-genetical processes, as well as for the interpretation of monitoring data.
Aging has been expected to have negligible impact on organisms in their natural environment (Williams, 1957) and its natural history is poorly known. However, it has now been recognized as an intrinsic feature of wild populations (Kirkwood & Austad, 2000). African explorer Dr. David Livingstone (2001) noted in 1860 that “sometimes wild animals suffer a great deal from disease, and wearily drag on a miserable existence before relieved of it by a ravenous beast”, after shooting an old pallah (Antilope melampus) that “was stone blind, had several tumors and a broken leg, which showed no symptoms of ever having begun to heal”. Ricklefs (2000) argued that in birds, the component of mortality related to aging does not differ significantly between birds in the wild and in captivity. Mark-recaptures studies showed age-specific acceleration of mortality in male guppies (Bryant and Reznick, 2004) and ungulates (Gaillard et al., 2004, Loison et al., 1999). Senescence was also found in a demographic analyses of wild baboon populations (Bronikowski et al. 2002). Especially for invertebrates however, the prevalence of aging in the wild is not well known. It may well be an underestimated phenomenon, especially because the stage of frailty can be shortened by predation, but the ultimate cause of the mortality remains senescence.
Among adult insects, life spans differ by 5000 fold (one day for mayflies, twenty years for ant queens). Lepidoptera are among the short-lived orders and show life spans that can differ by 50 fold (Carey, 2001, Jervis et al., 2005). A host of data on butterflies has been generated by the work of aficionados and butterflies are an important insect field model in the study of insect life history evolution. Interspecies comparisons in butterflies offer opportunities to exploit the variety of strategies used to avoid predation, and variation in adult nutritional ecology, both likely important for life span evolution. Their often bright and intricate color patterns contributed much to butterfly popularity, and their function, often interpreted in the context of predator avoidance, has been the focus of much research (e.g. (Hill and Vaca, 2004, Kassarov, 2003, Pinheiro, 1996, Pinheiro, 2003, Windig et al., 1994). Nutritional ecology has also been a focus of butterfly research (e.g. (Boggs, 1987, Boggs and Freeman, 2005).
While most butterfly species are primarily nectar feeders, in tropical forests many species feed on fruit (DeVries, 1987, Larsen, 1991, Norris, 1936). These fruit-feeding butterflies range from small satyrines to large and powerful charaxines. They are suitable for studies of life span because they can be followed individually in the field through mark-recapture studies using fruit-baited traps as they generally do not recognize the traps as food sources after release, thus not biasing sampling (Hughes et al., 1998). In addition, most species can be kept in captivity allowing a combination of laboratory and field work.
Longevity is a complex trait. Even in the absence of predation, longevity is dependent on sex and environment (Carey and Liedo, 1995, Promislow, 2003) and there is extensive variation in life span among individuals even in genetically identical organisms reared in a constant environment (Kirkwood et al., 2005). Determining patterns of age specific mortality provides the most insight into aging and puts single measures of longevity into perspective (Horiuchi, 2002). Such studies, however, require large numbers of individuals of which both begin and end time of their life span is known or precise age measures are available (Promislow et al., 1999). More simple measures of longevity include mean life expectancy and longevity records. The latter do not represent so-called species specific maximal ages (Carey and Judge, 2000), but represent an estimate of the age that can be obtained in a benign environment where senescence can be expected to be the cause of death. However, as is also demonstrated and discussed in this paper, longevity records are sensitive to sample size.
Here we present life span and longevity records derived from a mark-recapture data set from a moderately diverse community of fruit-feeding butterflies in Uganda (Molleman et al., 2006). Because of the exceptionally high individual abundances and the five year duration of the study, this probably represents one of the largest mark-recapture data sets of multiple species in a single habitat. From these data we extract general principles and compare with captive life spans of the same species when available.
MATERIAL AND METHODS
Field site
This study was conducted from April 2000 to July 2005 at Makerere University Biological Field Station in Kibale Forest National Park, Western Uganda (0° 35' N 30° 20' E). The field station borders selectively logged moist evergreen forest at an altitude of around 1500m and is therefore classified as a transition towards montane forest. The mean maximum temperature is 23.8 °C and the mean annual rainfall is 1749 mm and is bimodal in distribution but with only mild seasonality (Chapman et al., 2005).
Mark-recapture
Butterflies were marked on the ventral side of the forewing with an individual number of up to 4 digits, using a marking pen. We included only species that could be identified with certainty in the field (excluding for example the Bicyclus smithi, golo, istaris-species group) and excluded some further species that were hard to mark and closely related to other species included (e.g. Bicyclus buea) or were migratory (e.g. Sevenia species). For every capture, we noted; number, species, sex (when easy to determine in the field), a rough index of wing-wear, location, and date.
In the main marking effort, the majority of butterflies were marked between March 2000 and December 2003 when once every 4 weeks traps at 22 locations in an area of one kilometer square of forest were baited and butterflies were marked from these traps for four consecutive days. Additional butterflies were marked at other times from the same traps in 2000-2003. Moreover, between January 2004 and July 2005 a more limited subset of common representative species and all Charaxinae was marked once every 4 weeks from these 22 locations, with each an understory and a canopy trap, for four consecutive days, and these species were also marked in nearby (<2 km) areas.
Recaptures and resightings were noted whenever they occurred during various experiments and surveys in the area (Molleman et al., 2005a, Molleman et al., 2005b, Molleman et al., 2005d). However, the bulk of recaptures were recorded during the main marking effort and during the butterfly monitoring that followed a similar schedule, but started two weeks afterwards and had an understory and a canopy trap at each of the 22 locations (Molleman, et al., 2006). Thus, marked butterflies could be recaptured on the days within the same week, 2 weeks or 4 weeks et cetera later in 2000-2003, and on days within the same week or 4 weeks later in 2004-2005.
For all recapture events, the number of days between marking and recapturing was calculated. Subsequently, for each individual the longest time between marking and recapturing was taken as minimum life span and these times were categorized in 30-day periods as summarized in table 1. The longest minimum life span of an individual of a particular species was taken as the longevity record. When possible, the sexes were treated separately. We have put the maximum time between marking and recapturing into the perspective of the distribution of time-spans between marking and recapturing to identify outliers, and the data were corrected for likely erroneous observations.
Table 1.
Summary of mark recapture data on fruit-feeding butterflies in Kibale NP, Uganda. The sexes are represented separately only when established in the field and totals can exceed sum of males and females.
| Time between release and recapature (days) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| genus | species | sex | # marked | # recaptures | # recaptured indiviuals | percent indiv. recaptured | Maximum life span (days) | 1-30 | 31-60 | 61-90 | 91-120 | 121-150 | 151-180 | 181-210 | 211-240 | 241-270 |
| Sum/average | 29740 | 6961 | 4759 | 13.8 | 3565 | 551 | 198 | 54 | 19 | 16 | 10 | 3 | 5 | |||
| Charaxinae | Max/percent of indiv. | 3808 | 1036 | 624 | 33.3 | 293 | 75 | 12 | 4 | 1 | 0.40 | 0.34 | 0.21 | 0.06 | 0.11 | |
| Charaxes | brutus | both | 3 | 0 | 0 | 0.0 | ||||||||||
| candiope | both | 68 | 2 | 2 | 2.9 | 12 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| etheocles s.l. | both | 47 | 1 | 1 | 2.1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| etheocles s.l. | f | 5 | 0 | 0 | 0.0 | |||||||||||
| etheocles s.l. | m | 35 | 1 | 1 | 2.9 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| fulvescens | both | 2255 | 474 | 329 | 14.6 | 230 | 273 | 34 | 14 | 5 | 0 | 2 | 0 | 1 | 0 | |
| fulvescens | f | 284 | 19 | 18 | 6.3 | 39 | 17 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| fulvescens | m | 786 | 121 | 80 | 10.2 | 230 | 58 | 14 | 5 | 0 | 0 | 2 | 0 | 1 | 0 | |
| paphianus | both | 5 | 0 | 0 | 0.0 | |||||||||||
| pleione | m | 136 | 7 | 6 | 4.4 | 14 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| pollux | both | 129 | 4 | 4 | 3.1 | 2 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| bipunctatus | both | 217 | 5 | 5 | 2.3 | 75 | 4 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| bipunctatus | f | 94 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| bipunctatus | m | 116 | 5 | 5 | 4.3 | 75 | 4 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| numenes | both | 93 | 6 | 4 | 4.3 | 69 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| numenes | f | 27 | 0 | 0 | 0.0 | |||||||||||
| numenes | m | 62 | 6 | 4 | 6.5 | 69 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| tiridates | both | 71 | 3 | 3 | 4.2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| tiridates | f | 34 | 1 | 1 | 2.9 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| tiridates | m | 33 | 2 | 2 | 6.1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| cynthia | both | 164 | 13 | 12 | 7.3 | 63 | 10 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| cynthia | f | 49 | 2 | 2 | 4.1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| cynthia | m | 110 | 11 | 10 | 9.1 | 63 | 8 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| protoclea | both | 45 | 3 | 3 | 6.7 | 8 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| protoclea | f | 5 | 0 | 0 | 0.0 | |||||||||||
| protoclea | m | 40 | 3 | 3 | 7.5 | 8 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Euxanthe | crossleyi | both | 9 | 1 | 1 | 11.1 | 42 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nymphalinae | ||||||||||||||||
| Aterica | galene | both | 639 | 204 | 128 | 20.0 | 159 | 108 | 13 | 5 | 1 | 0 | 1 | 0 | 0 | 0 |
| galene | f | 197 | 45 | 31 | 15.7 | 41 | 30 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| galene | m | 438 | 159 | 97 | 22.1 | 159 | 78 | 12 | 5 | 1 | 0 | 1 | 0 | 0 | 0 | |
| Bebearia | absolon | both | 108 | 15 | 14 | 13.0 | 36 | 13 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| sophus | both | 450 | 135 | 93 | 20.7 | 63 | 82 | 9 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | |
| sophus | f | 270 | 106 | 71 | 26.3 | 30 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| sophus | m | 167 | 29 | 22 | 13.2 | 63 | 60 | 9 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Cymothoe | caenis | both | 68 | 0 | 0 | 0.0 | ||||||||||
| herminia | both | 1226 | 70 | 65 | 5.3 | 46 | 64 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| herminia | f | 426 | 25 | 22 | 5.2 | 14 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| herminia | m | 795 | 45 | 43 | 5.4 | 32 | 42 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| hobarti | both | 109 | 6 | 5 | 4.6 | 2 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| hobarti | f | 28 | 1 | 1 | 3.6 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| hobarti | m | 81 | 5 | 4 | 4.9 | 2 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| lurida | both | 1186 | 180 | 149 | 12.6 | 67 | 118 | 9 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | |
| lurida | f | 396 | 49 | 42 | 10.6 | 67 | 23 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| lurida | m | 785 | 131 | 107 | 13.6 | 64 | 95 | 5 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Euphaedra | alacris | both | 2031 | 681 | 427 | 21.0 | 253 | 322 | 62 | 25 | 8 | 3 | 2 | 3 | 1 | 1 |
| alacris | f | 878 | 263 | 167 | 19.0 | 253 | 126 | 23 | 12 | 4 | 0 | 1 | 0 | 0 | 1 | |
| alacris | m | 1137 | 417 | 259 | 22.8 | 217 | 195 | 39 | 13 | 4 | 3 | 1 | 3 | 1 | 0 | |
| christyi | both | 654 | 302 | 192 | 29.4 | 85 | 167 | 21 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| christyi | f | 250 | 92 | 63 | 25.2 | 49 | 60 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| christyi | m | 404 | 210 | 129 | 31.9 | 85 | 107 | 18 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| edwardsi | both | 347 | 135 | 81 | 23.3 | 114 | 58 | 13 | 6 | 4 | 0 | 0 | 0 | 0 | 0 | |
| edwardsi | f | 101 | 27 | 19 | 18.8 | 58 | 17 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| edwardsi | m | 246 | 108 | 62 | 25.2 | 114 | 41 | 11 | 6 | 4 | 0 | 0 | 0 | 0 | 0 | |
| eusemoides | both | 319 | 96 | 69 | 21.6 | 64 | 122 | 0 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | |
| eusemoides | f | 110 | 47 | 31 | 28.2 | 57 | 57 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | |
| eusemoides | m | 198 | 49 | 38 | 19.2 | 64 | 64 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | |
| harpalyce | both | 1155 | 445 | 277 | 24.0 | 218 | 92 | 10 | 9 | 3 | 2 | 0 | 1 | 0 | 0 | |
| harpalyce | f | 360 | 103 | 75 | 20.8 | 188 | 61 | 6 | 5 | 2 | 0 | 0 | 1 | 0 | 0 | |
| harpalyce | m | 791 | 342 | 202 | 25.5 | 218 | 31 | 4 | 4 | 1 | 2 | 0 | 0 | 0 | 0 | |
| hollandi | both | 54 | 18 | 9 | 16.7 | 69 | 8 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| hollandi | f | 27 | 4 | 3 | 11.1 | 15 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| hollandi | m | 25 | 14 | 6 | 24.0 | 69 | 5 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Euphaedra | kakamegae | both | 130 | 23 | 20 | 15.4 | 65 | 18 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| kakamegae | f | 7 | 0 | 0 | 0.0 | |||||||||||
| kakamegae | m | 83 | 11 | 10 | 12.0 | 65 | 8 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| medon | both | 2927 | 1036 | 624 | 21.3 | 293 | 456 | 90 | 52 | 11 | 5 | 4 | 1 | 0 | 4 | |
| medon | f | 830 | 195 | 117 | 14.1 | 250 | 87 | 17 | 9 | 1 | 0 | 2 | 0 | 0 | 1 | |
| medon | m | 2085 | 841 | 507 | 24.3 | 293 | 369 | 73 | 43 | 10 | 5 | 2 | 1 | 0 | 3 | |
| preussi | both | 620 | 278 | 166 | 26.8 | 185 | 119 | 27 | 14 | 2 | 2 | 1 | 1 | 0 | 0 | |
| preussi | f | 231 | 47 | 36 | 15.6 | 62 | 32 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| preussi | m | 392 | 231 | 130 | 33.2 | 185 | 87 | 24 | 13 | 2 | 2 | 1 | 1 | 0 | 0 | |
| uganda | both | 769 | 311 | 197 | 25.6 | 217 | 104 | 17 | 7 | 2 | 0 | 1 | 1 | 1 | 0 | |
| uganda | f | 279 | 102 | 63 | 22.6 | 166 | 52 | 8 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | |
| uganda | m | 485 | 208 | 133 | 27.4 | 217 | 104 | 17 | 7 | 2 | 0 | 1 | 1 | 1 | 0 | |
| zaddachi | both | 257 | 151 | 84 | 32.7 | 109 | 68 | 14 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | |
| zaddachi | f | 86 | 9 | 8 | 9.3 | 12 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| zaddachi | m | 176 | 134 | 72 | 40.9 | 109 | 56 | 14 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Euriphene | ribensis | both | 148 | 15 | 13 | 8.8 | 36 | 12 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ribensis | f | 51 | 6 | 4 | 7.8 | 5 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| ribensis | m | 97 | 9 | 9 | 9.3 | 36 | 8 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| saphirina | both | 12 | 0 | 0 | 0.0 | |||||||||||
| Euriphura | chalcis | both | 100 | 24 | 14 | 14.0 | 35 | 13 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eurytela | hiarbas | both | 414 | 4 | 4 | 1.0 | 22 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Harma | theobene | both | 1447 | 225 | 185 | 12.8 | 133 | 174 | 6 | 4 | 0 | 1 | 0 | 0 | 0 | 0 |
| theobene | f | 384 | 47 | 42 | 10.9 | 51 | 41 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| theobene | m | 1090 | 143 | 143 | 13.1 | 133 | 133 | 5 | 4 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Hypolimnas | salmacis | both | 10 | 0 | 0 | 0.0 | ||||||||||
| Junonia | stygia | both | 19 | 0 | 0 | 0.0 | ||||||||||
| Kallimoides | rumia | both | 945 | 197 | 151 | 16.0 | 188 | 134 | 14 | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| rumia | f | 436 | 95 | 68 | 15.6 | 55 | 66 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| rumia | m | 502 | 102 | 83 | 16.5 | 188 | 68 | 12 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | |
| Neptidopsis | ophione | both | 33 | 0 | 0 | 0.0 | ||||||||||
| Protogoniomorpha | parhassus | both | 9 | 0 | 0 | 0.0 | ||||||||||
| Pseudacraea | lucretia | both | 165 | 12 | 10 | 6.1 | 11 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Salamis | cacta | both | 231 | 2 | 2 | 0.9 | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| cacta | f | 1 | 0 | 0 | 0.0 | |||||||||||
| cacta | m | 26 | 0 | 0 | 0.0 | |||||||||||
| Salamis | temora | both | 10 | 0 | 0 | 0.0 | ||||||||||
| Satyrinae | ||||||||||||||||
| Bicyclus | auricruda | both | 753 | 94 | 83 | 11.0 | 171 | 67 | 12 | 2 | 1 | 0 | 1 | 0 | 0 | 0 |
| auricruda | f | 317 | 38 | 34 | 10.7 | 56 | 27 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| auricruda | m | 428 | 56 | 49 | 11.4 | 171 | 40 | 5 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | |
| dentatus | both | 20 | 1 | 1 | 5.0 | 22 | 1 | |||||||||
| graueri | both | 1490 | 586 | 381 | 25.6 | 209 | 275 | 67 | 21 | 8 | 5 | 3 | 2 | 0 | 0 | |
| graueri | f | 651 | 181 | 131 | 20.1 | 112 | 104 | 23 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | |
| graueri | m | 821 | 405 | 250 | 30.5 | 209 | 171 | 44 | 18 | 7 | 5 | 3 | 2 | 0 | 0 | |
| mandanes | both | 1619 | 256 | 211 | 13.0 | 92 | 198 | 11 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | |
| mandanes | f | 618 | 81 | 73 | 11.8 | 56 | 68 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| mandanes | m | 958 | 173 | 136 | 14.2 | 92 | 128 | 6 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | |
| mesogena | both | 8 | 1 | 1 | 12.5 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| mollitia | both | 3808 | 385 | 334 | 8.8 | 89 | 301 | 25 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | |
| mollitia | f | 1562 | 166 | 143 | 9.2 | 89 | 130 | 12 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| mollitia | m | 2239 | 218 | 190 | 8.5 | 85 | 170 | 13 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | |
| sambulos | both | 78 | 11 | 8 | 10.3 | 31 | 7 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| sambulos | f | 33 | 0 | 0 | 0.0 | |||||||||||
| sambulos | m | 45 | 11 | 8 | 17.8 | 31 | 7 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| sebetus | both | 237 | 102 | 65 | 27.4 | 172 | 43 | 13 | 4 | 3 | 1 | 1 | 0 | 0 | 0 | |
| sebetus | f | 87 | 10 | 10 | 11.5 | 17 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| sebetus | m | 165 | 76 | 45 | 27.3 | 147 | 26 | 12 | 4 | 2 | 1 | 0 | 0 | 0 | 0 | |
| Gnophodes | proposed name: Gnophodes arali | both | 303 | 39 | 32 | 10.6 | 119 | 27 | 3 | 2 | 1 | 2 | 0 | 0 | 0 | 0 |
| proposed name: Gnophodes arali | f | 274 | 38 | 31 | 11.3 | 119 | 26 | 3 | 2 | 1 | 2 | 0 | 0 | 0 | 0 | |
| proposed name: Gnophodes arali | m | 29 | 1 | 1 | 3.4 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| chelys | both | 1803 | 387 | 287 | 15.9 | 223 | 206 | 38 | 23 | 9 | 6 | 5 | 0 | 0 | 0 | |
| chelys | f | 442 | 59 | 47 | 10.6 | 48 | 43 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| chelys | m | 1361 | 328 | 240 | 17.6 | 175 | 163 | 34 | 23 | 9 | 6 | 5 | 0 | 0 | 0 | |
| grogani | both | 127 | 16 | 13 | 10.2 | 76 | 85 | 11 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | |
| grogani | f | 87 | 11 | 9 | 10.3 | 76 | 76 | 7 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | |
| grogani | m | 34 | 5 | 4 | 11.8 | 8 | 8 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Melanitis | ansorgei | both | 3 | 2 | 1 | 33.3 | 29 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| leda | both | 256 | 42 | 30 | 11.7 | 56 | 28 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
Life span in captivity
This experiment focused on species that were expected to do well in a large cage. Thus, species did not include small butterflies or those that were flying continuously against the mesh and had life spans in captivity of less than three days. Butterflies that had no or very little wing-damage were collected from the forest to represent young individuals and kept in a cage of 3 × 5 × 2 meters under a tin roof and with a concrete floor in a clearing close to the forest edge. They were provided with mashed banana on dishes on the ground and in containers hanging in the corner, where most butterflies spend the majority of their time. Water was sprayed on the floor daily to prevent butterflies from desiccating. All butterflies were marked individually and dead butterflies were collected daily. In addition, Charaxes fulvescens females collected from the forest were kept individually in small cylindrical cages (25 × 35 cm), with a diet of sugar water (10%), banana, or sugar water with amino acids, and host-plants as an egg-laying substrate. Deaths were recorded al least thrice a week.
RESULTS
Mark-recapture
Meaningful data for all species are summarized in table 1. Over 30 000 butterflies were marked, divided over 62 species. For 35 species, more than 100 individuals were marked, while for 11 species more than 1000 butterflies were marked (fig. 1 a). 4793 individuals were recaptured or resighted at least once (fig. 1 b). Recapture rates for species ranged from 0 to 33%, averaging 11.6 % of the marked individuals (fig. 1 c). The sexes often showed markedly different recapture probabilities (e.g.: Euphaedra zaddachi female 9.3%, male 40.9 %), with males usually having higher recapture rates (t-test for paired samples for species in which for both sexes at least 10 individuals were recaptured N=21 species, t=−2.66, p=0.0075; exceptions were Bebearia sophus, E. eusemoides, Bicyclus mollitia and Gnophodes betsimena).
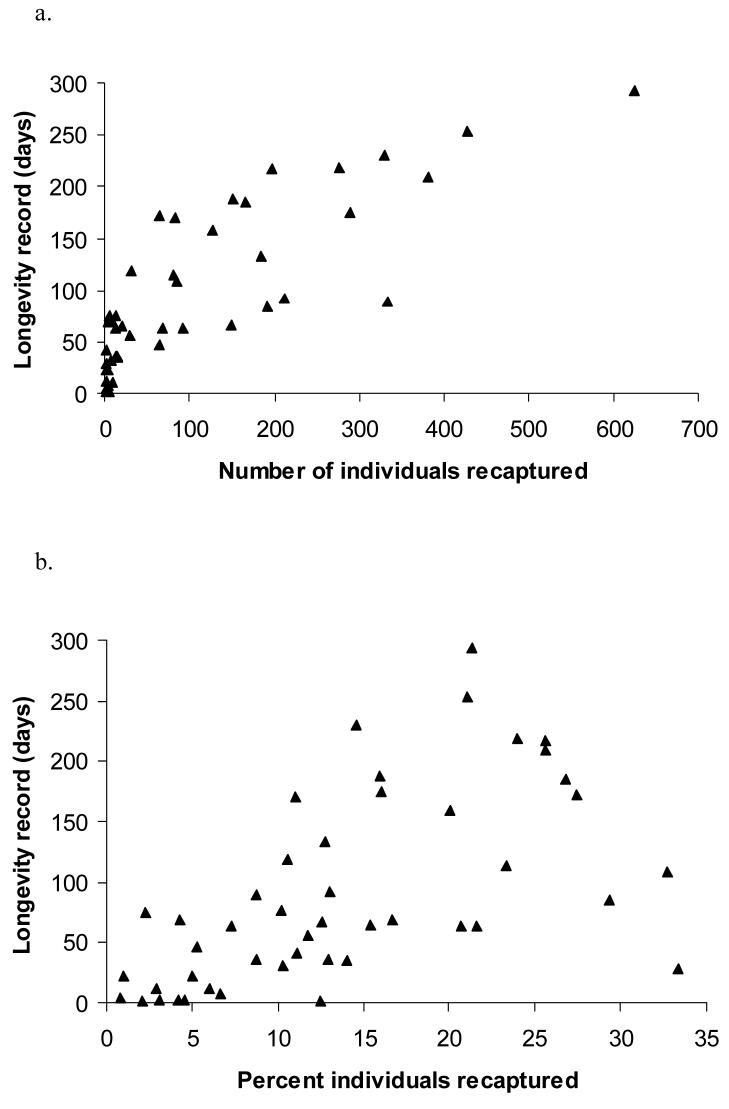
Figure 1.
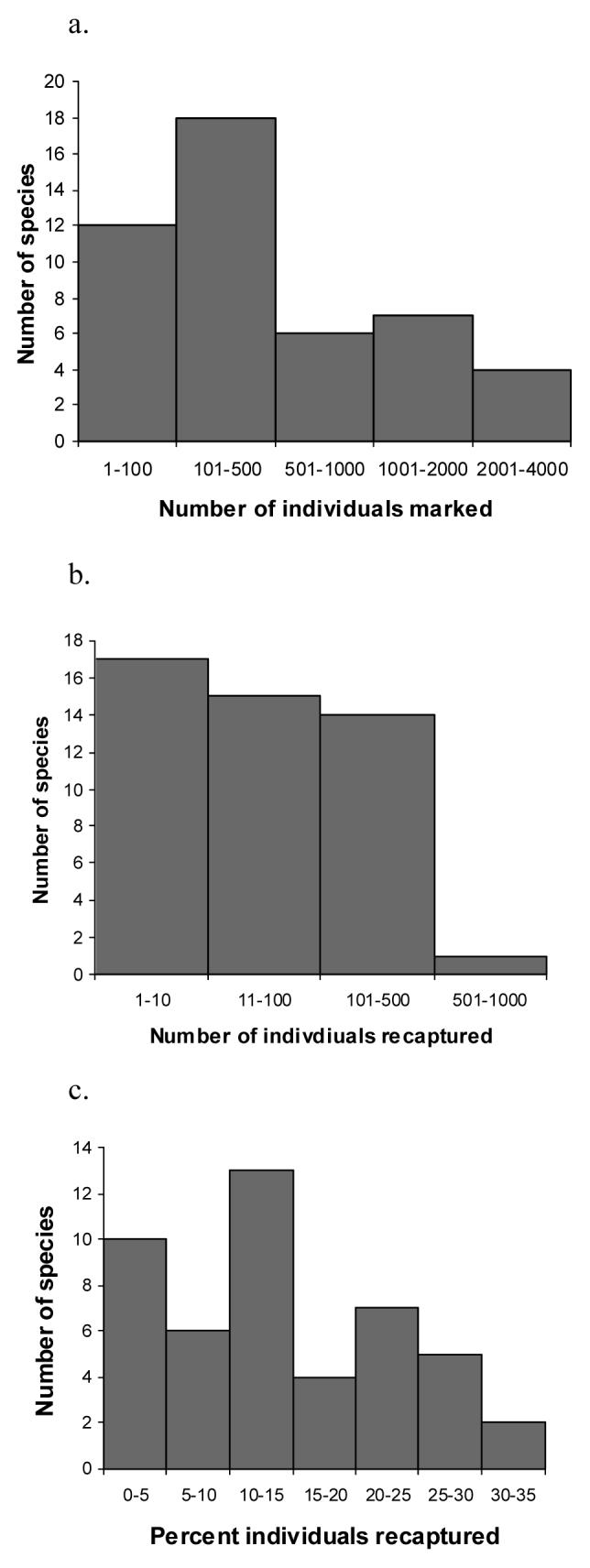
Summary of mark recapture dataset on 47 species of fruit-feeding butterflies in Kibale Forest, Uganda; a)the number of individuals marked per species: for most of the species less than 500 individuals were marked, for one species, Bicyclus mollitia 3808 were marked; b) number of individuals recaptured per species: for most species less than 100 individuals were recaptured, but for one species, Euphaedra medon, 1036; and c) percent recaptures per species ranged between 0 and 33, averaging 11.6%.
The maximum recorded longevity of a species was a function of the number of individuals of that species recaptured (fig 2 a) and the percentage recaptured (fig 2 b; GLM: N= 46 species R2= 71%, number of recaptured individuals F= 58.9, p<0.001, proportion recaptured F=6.3, p=0.016). The latter variable can be considered a proxy for dispersal or probability of moving outside the trapping site: most recaptures took place within a few days, and 80% within 30 days after marking, so most individuals that were not recaptured within two weeks are more likely to have moved from the study area than to have died, as most life span records exceeded 60 days. Maximum recorded life spans for species of which more than 100 individuals were recaptured ranged from 67 (B. auricruda) to 293 days (E. medon). Individuals of twelve species lived longer than five months, and of five species longer than seven months.
Figure 2.
Longevity records for 47 species of fruit-feeding butterflies in Uganda with a) number individuals recaptured; and b) percent of recaptured individuals. The percent recapture is a proxy for rate of dispersal from the study area. Higher recapture rates and larger sample size are both associated with higher longevity records (GLM: N=47 species, R2=71%, percent recaptured F=6.3, p=0.016, number of individuals recaptured F=58.9, p<0.001).
Life span in captivity
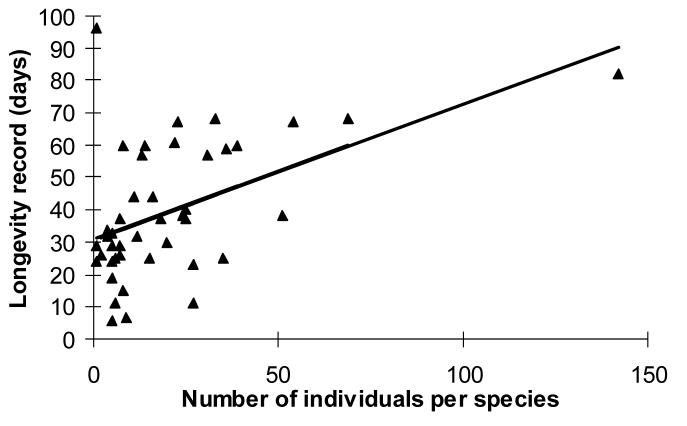
We collected 640 butterflies with little or no wing damage from the forest representing 31 species and kept them in a large cage. The maximum life span ranged from 7 to 96 days, the mean being 39 days (table 2). While the species with the longest-lived individual was represented by only a single individual (B. golo, 96 days), maximum life span per species per sex was positively correlated with sample size (Fig. 3; Regression: F=13.5, p=0.0007), while the median life span per species was not (Regression: F=0.48, p=0.49). The survival curves of E. alacris and C. fulvescens showed high mortality shortly after capture, followed by a steady decline and then few individuals surviving exceptionally well. Twenty out of twenty-six species had a lower maximum life span in captivity than in the mark-recapture study in the wild. Charaxes fulvescens females kept individually in small cages lived about 43 days on average and the maximum was 200 days (N=212). The mark-recapture data showed that this species can reach that age in the field (longevity record is 230 days), while in the large cage, the maximum life span was only 82 days (N=142).
Table 2.
Life span of 31 species of fruit-feeding butterflies collected from the field in a large group cage at veranda in Kibale NP. Sensoring refers to escapes and eaten by nocturnal animal.
| genus | species | sex | N | max | mean | median | sensored max: |
|---|---|---|---|---|---|---|---|
| Charaxes | brutus | 1 | 29 | 29 | 29 | ||
| candiope | 8 | 60 | 34 | 37 | Yes | ||
| cynthia | 4 | 32 | 16.25 | 15.5 | |||
| etheocles | 5 | 6 | 3.8 | 4 | |||
| fuvescens | 142 | 82 | 20.1 | 18.5 | |||
| pollux | 6 | 25 | 11.7 | 7.5 | |||
| bipunctatus | both | 25 | 37 | 11.7 | 7 | ||
| bipunctatus | f | 7 | 26 | 12.7 | 9 | ||
| bipunctatus | m | 18 | 37 | 11.3 | 6.5 | ||
| numenes | both | 16 | 44 | 22.5 | 25.5 | ||
| numenes | f | 5 | 33 | 14.2 | 7 | ||
| numenes | m | 11 | 44 | 26.7 | 26 | ||
| tiridates | 13 | 57 | 22.8 | 24 | |||
| Euxanthe | crossleyi | 5 | 24 | 19 | 19 | ||
| Euphaedra | alacris | both | 69 | 68 | 13.6 | 9 | |
| alacris | f | 36 | 59 | 11.7 | 7.5 | ||
| alacris | m | 33 | 68 | 15.7 | 10 | ||
| christyi | 9 | 7 | 4.1 | 4 | |||
| eusemoides | 4 | 34 | 13 | 9 | |||
| medon | both | 54 | 67 | 11.3 | 4 | ||
| medon | f | 23 | 67 | 10.2 | 4 | ||
| medon | m | 31 | 57 | 12 | 5 | ||
| preussi | 5 | 19 | 8.8 | 6 | |||
| spatiosa | 22 | 61 | 19.4 | 15.5 | |||
| uganda | 12 | 32 | 11.8 | 9.5 | |||
| edwardsi | 7 | 37 | 19 | 21 | |||
| Aterica | galene | 7 | 29 | 17.3 | 16 | ||
| Kallimoides | rumia | 20 | 30 | 6.9 | 4 | ||
| Cymothoe | herminia | 35 | 25 | 7.7 | 6 | ||
| hobarti | 6 | 11 | 5 | 5 | |||
| lurida | both | 51 | 38 | 6.2 | 5 | ||
| lurida | f | 24 | 38 | 7.9 | 5 | ||
| lurida | m | 27 | 11 | 4.6 | 5 | ||
| Harma | theobene | 15 | 25 | 7.7 | 5 | ||
| Pseudacraea | lucretia | 27 | 23 | 5.5 | 3 | ||
| Protogoniomorpha | parhassus | 1 | 24 | 24 | |||
| Gnophodes | chelys | both | 39 | 60 | 12.5 | 11 | Yes |
| chelys | f | 14 | 60 | 17.3 | 12 | Yes | |
| chelys | m | 25 | 40 | 9.7 | 4 | ||
| grogani | 8 | 15 | 6 | 4.5 | |||
| Bicyclus | golo | f | 1 | 96 | 96 | ||
| graueri | 5 | 29 | 18 | 25 | |||
| Melanitis | leda | 2 | 26 | 15.5 | |||
Figure 3.
Maximum life span in captivity for 31 species of fruit-feeding butterflies collected from a tropical forest in Uganda, was correlated with the number of individuals tested (Regression: F=13.5, p<0.001).
DISCUSSION
We have presented adult life span data from a large mark-recapture study covering 62 species of fruit-feeding butterflies collected over a five year period in a tropical forest. Examples of long life spans were recorded for many species even though mark-recapture studies are associated with many caveats including loss of individuals due to dispersal, and part of the actual life span being before marking and after recapturing. For some species we were also able to compare data for individuals held in captivity.
To provide accurate estimates of mean life expectancy, mark-recapture data have to take into account dispersal rates and chances of capture in the absence of mortality and dispersal. Longevity records are a problematic demographic concept because it is dependent on sample size and environment as is also demonstrated by our data (fig 2 and 3.) and it is difficult to determine some form of confidence interval for it. With large enough sample sizes, intensive trapping and reasonably limited dispersal as in our field study, longevity records can be expected to be less sensitive to these caveats.
We found remarkable differences among the sexes within species in numbers captured, percentage recaptured, and longevity records. These differences may be explained by differences in movement patterns, and the number of feeding bouts, as well as genuine differences in life span. Fermon (2003) showed that among fruit-feeding butterflies in a tropical forest in Benin, the sexes differ in movement patterns: in all surveyed species of Nymphalinae and Satyrinae the males tended to move shorter distances, while in species of Charaxinae no trend or the opposite was found. Longer flights and time intervals between captures as well as lower numbers captured might also indicate that females spend less time foraging than males.
Insight into differences between life span patterns in captive environments and in the wild can provide insight into the proportion of mortality that is due to aging in the wild (Ricklefs, 2000). Captive life spans in our study were long for several species compared to species reported on in the literature (Jervis, et al., 2005), but tended to be shorter than those obtained from our mark-recapture data (20 out of 26 species). This is striking because it concentrated on fresh looking individuals and exact date of death was noted rather than day last seen. In addition, life span of C. fulvescens was longer when kept individually in small caged than in the large cage. The pattern of mortality in the large cage for the two most intensively sampled species indicates that mortality there was not age related, but rather represented extrinsic conditions: the cage being a harsh environment. Therefore, it appears that captive conditions can modify butterfly longevity to a large extent and usually towards shorter life spans than are possible in the wild. That life spans in the field can be at least as long as in captivity indicates that butterflies live long enough in the wild to senesce. However, more extensive data are needed to confirm this because samples sizes are generally lower for the captivity experiments, and longevity measurements strongly depend on the number of observations (figure 2).
Heliconius butterflies are known for their exceptional long reproductively active life spans (Boggs, 1979, Erhardt and Baker, 1990, Jervis, et al., 2005). However, the longevity records reported here were up to two-fold higher than those reported for Heliconius species (i.e.: Heliconius charitonius >120 days). That our data show life spans that are exceptionally long compared to the literature suggests that for butterflies, fruit-feeding in tropical forests is associated with selection pressures that favor the evolution of long life spans. However, systematic data on life spans of butterflies that do not feed as adults on fruit from the same tropical forests, as well as for species from other tropical biotopes, are scarce. Fruit-feeding butterflies in neo-tropical forests can have similarly long life spans (P. J. DeVries unpublished data). Bicyclus butterflies in more open and seasonal forests in Malawi can survive for many months in the field during the dry season in reproductive diapause (Brakefield and Reitsma, 1991), while wet-season forms of B. anynana from that location can survive up to three months in the laboratory (Brakefield and Kesbeke, 1995). These life spans are within the range of those found among Bicyclus at our study site in Uganda that has a much less seasonal environment. However, Swedish satyrine butterflies living in forest habitats were shown to live shorter than species from open habitats (Karlsson and Wiklund, 2005), and it would be interesting to know if this trend is consistent among butterfly taxa and climates, and what selection pressures could be responsible.
Insight into seasonal reproduction and adult diapause is important for the understanding of aging (Tatar and Yin, 2001). At least some of the remarkably long-lived butterflies in our study also appear to be reproductively active throughout their lives (Molleman, et al., 2005b), and therefore their life spans are not extended due to any adult diapause or reproductive dormancy. However, this is not the case for Gnophodes chelys of which the egg-laying of this species has been monitored since 2001. The adults appear unmated throughout the dry season (Molleman, et al., 2005b), and almost all eggs are laid within a four-week period at the beginning of the next rainy season (there are 2 rainy seasons per year, F. Molleman, M. van Dijk, P. Boons unpublished data).
Some of the fore-mentioned Heliconius butterflies are also tropical forest dwellers and their long life spans have been linked to their ability to use nutrients derived from pollen for egg-production (Dunlap-Pianka et al., 1977, Gilbert, 1972, O'Brien et al., 2003). Feeding ecology may also play a similar role in life span evolution in fruit-feeding butterflies. Fruits can be enriched in amino acids compared to nectar (Molleman, et al., 2005d), but see reference to fruit nutrient content in Fischer et al. (2004). Although earlier studies failed to provide evidence for a role of amino acids in the adult diet in the fruit-feeding butterfly B. anynana (Bauerfeind and Fischer, 2005, Molleman, 2004), unpublished data (F. Molleman) on C. fulvescens show that some species can use amino acids in the adult diet for egg production. Fruit-feeding also supported the conservation of egg weight with age in the Australian satyrine Mycalesis terminus (Braby and Jones, 1995). In addition, wild caught male fruit-feeding butterflies in a Bornean rainforest tended to live longer on an amino acid enriched diet than on sugar only (Beck, In Press). Therefore, it is likely that some fruit-feeding butterfly species use nitrogenous compounds in the adult diet in a similar manner to some nectar and pollen feeders (Boggs and Dau, 2004, Mevi-Schutz and Erhardt, 2005), which may partly explain long active life spans in this group.
Feeding efficiency could also be associated with life span. Based on variation in proboscis morphology and feeding behavior, fruit-feeding butterflies can be divided in two groups: 1) piercing butterflies that are efficient at foraging on soft substrates (all Charaxinae), and 2) sweeping butterflies that can use a wide range of substrates, but have lower intake rates (all other fruit-feeding butterflies; (Molleman et al., 2005c). However, Charaxinae did not have longer longevity records than sweeping butterflies in our study, which may be attributed to low population densities and high dispersal rates in Charaxinae (Fermon, et al., 2003).
While nutritional ecology may be important for long reproductively-active life spans, the distribution of suitable hosts (for females) or receptive females (for males; (Gotthard et al., 2000), and the ability to avoid or escape predators must also shape life spans. While much is known about predator avoidance strategies in butterflies (e.g. aposematism, mimicry, eyespots, crypsis, flight allometry), systematic quantification that could provide a link with life span is lacking.
Our data on fruit-feeding butterflies in Uganda include the longest published active life spans of butterflies. Because the majority of Lepidoptera (butterflies and moths) are short-lived, these butterfly species with remarkably long life spans can help to identify factors that affect life span evolution and aging. We view life span evolution in the light of investment in survival (including strategies to avoid predation) and resulting fitness (depending on temporal patterns in reproduction that in turn can evolve in response to host-plant traits and nutritional ecology of the adult). Because for butterflies there is a long tradition of field-research on nutritional ecology and predator avoidance strategies, studies on fruit-feeding butterflies have much potential for gaining insight into the evolution of life span and aging.
Acknowledgements
We thank Patrick Kagoro, Boniface Balyeganira, Dennis Sebugwawo, and Moses Musana for their invaluable assistance with the fieldwork. We are grateful to Easter Mugurusi and Christina Galey for data entry and Vincent Tu, Luyang Zhang, and Erick Loomis for data entry and processing. Arjan Kop, K.D. Dijkstra and Rob de Vos have been helpful with butterfly identifications. This study was conducted with kind permission of the Uganda Wildlife Authority (U.W.A.) and the Ugandan National Council for Science and Technology (U.N.C.S.T.). The funding for this project was provided by the Netherlands Foundation for the Advancement of Tropical Research (WOTRO) (W80-82-238 to PMB), the Schure-Beijerinck-Popping foundation (FM), Prof. Russ Lande, and the National Institute of Aging (NIA) (PO1 AG022500-01 and PO1 AG608761-10 to JRC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Austad SN. Retarded senescence in an insular population of Virginia opossums (Didelphis virginiana) J. Zool. 1993;229:695–708. [Google Scholar]
- Bauerfeind SS, Fischer K. Effects of adult-derived carbohydrates, amino acids and micronutrients on female reproduction in a fruit-feeding butterfly. J. Insect Physiol. 2005;51:545–554. doi: 10.1016/j.jinsphys.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Beck J. The importance of amino acids in the adult diet of male tropical rainforest butterflies. Oecologia. doi: 10.1007/s00442-006-0613-y. In Press. [DOI] [PubMed] [Google Scholar]
- Boggs CL. Resource allocation and reproductive strategies in several heliconiine butterfly species. University of Texas; Austin: 1979. [Google Scholar]
- Boggs CL. Ecology of nectar and pollen feeding in Lepidoptera. Nutritional ecology of insects, mites and spiders. In: Slansky FJR, editor. Nutritional ecology of insects, mites and spiders. John Wiley and sons; New York: 1987. pp. 369–391. J.G. New York. [Google Scholar]
- Boggs CL, Dau B. Resource specialization in puddling Lepidoptera. Environ. Entomol. 2004;33:1020–1024. [Google Scholar]
- Boggs CL, Freeman KD. Larval food limitation in butterflies: effects on adult resource allocation and fitness. Oecologia. 2005;144:353–361. doi: 10.1007/s00442-005-0076-6. [DOI] [PubMed] [Google Scholar]
- Braby MF, Jones RE. Reproductive patterns and resource-allocation in tropical butterflies: influence of adult diet and seasonal phenotype on fecundity, longevity and egg size. Oikos. 1995;72:189–204. [Google Scholar]
- Brakefield PM, Kesbeke F. Raised adult lifespan and female fecundity in tropical fruit-feeding Bicyclus butterflies. Proceedings in Experimental and Applied Entomology. 1995;6:93–98. [Google Scholar]
- Brakefield PM, Reitsma N. Phenotypic plasticity, seasonal climate and the population biology of Bicyclus butterflies (Satyridae) in Malawi. Ecological Entomology. 1991;16:291–303. [Google Scholar]
- Bronikowski AM, Promislow DEL. Testing evolutionary theories of aging in wild populations. Trends Ecol. Evol. 2005;20:271–273. doi: 10.1016/j.tree.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Bryant MJ, Reznick D. Comparative studies of senescence in natural populations of guppies. Am. Nat. 2004;163:55–68. doi: 10.1086/380650. [DOI] [PubMed] [Google Scholar]
- Carey JR. Insect biodemography. Annu. Rev. Entomol. 2001;46:79–110. doi: 10.1146/annurev.ento.46.1.79. [DOI] [PubMed] [Google Scholar]
- Carey JR, Judge DS. Longevity records: life spans of mammals, birds, amphibians, reptiles and fish. Odense University Press; Odense: 2000. [Google Scholar]
- Carey JR, Liedo P. Sex mortality differentials and selective survival in large medfly cohorts: implications for human sex mortality differentials. Gerontologist. 1995;35:588–596. doi: 10.1093/geront/35.5.588. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Chapman LJ, Struhsaker TT, Zanne AE, Clark CJ, Poulsen JR. A long-term evaluation of fruiting phenology: importance of climate change. Journal of Tropical Ecology. 2005;21:31–45. [Google Scholar]
- DeVries PJ. Papilionidae, Pieridae and Nymphalidae. Princeton University Press; New Jersey: 1987. The butterflies of Costa Rica and their natural history. [Google Scholar]
- Dunlap-Pianka HL, Boggs CL, Gilbert LE. Ovarian dynamics in Heliconiine butterflies: programmed senescence versus eternal youth. Science. 1977;197:487–490. doi: 10.1126/science.197.4302.487. [DOI] [PubMed] [Google Scholar]
- Erhardt A, Baker I. Pollen amino-acids - an additional diet for a nectar feeding butterfly. Plant Syst. Evol. 1990;169:111–121. [Google Scholar]
- Fermon H, Waltert M, Muhlenberg M. Movement and vertical stratification of fruit-feeding butterflies in a managed West African rainforest. Journal of Insect Conservation. 2003;7:7–19. [Google Scholar]
- Fischer K, O'Brien DM, Boggs CL. Allocation of larval and adult resources to reproduction in a fruit-feeding butterfly. Functional Ecology. 2004;18:656–663. [Google Scholar]
- Gaillard JM, Viallefont A, Loison A, Festa-Bianchet M. Assessing senescence patterns in populations of large mammals. Animal Biodiversity and Conservation. 2004;27:47–58. [Google Scholar]
- Gilbert LE. Pollen feeding and reproductive biology of Heliconius butterflies. Proceedings of the National Academy of Science, USA. 1972;69:1403–1407. doi: 10.1073/pnas.69.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotthard K, Nylin S, Wiklund C. Mating opportunity and the evolution of sex-specific mortality rates in a butterfly. Oecologia. 2000;8:36–43. doi: 10.1007/PL00008833. [DOI] [PubMed] [Google Scholar]
- Hill RI, Vaca JF. Differential wing strength in Pierella butterflies (Nymphalidae, Satyrinae) supports the deflection hypothesis. Biotropica. 2004;36:362–370. [Google Scholar]
- Horiuchi S. Interspecies comparison of life table patterns: humans versus invertebrates. Gerontologist. 2002;42:2–2. [Google Scholar]
- Hughes JB, Daily GC, Ehrlich PR. Use of fruit bait traps for monitoring of butterflies (Lepidoptera : Nymphalidae) Revista De Biologia Tropical. 1998;46:697–704. [Google Scholar]
- Jervis MA, Boggs CL, Ferns PN. Egg maturation strategy and its associated trade-offs: a synthesis focusing on Lepidoptera. Ecological Entomology. 2005;30:359–375. [Google Scholar]
- Karlsson B, Wiklund C. Butterfly life history and temperature adaptations; dry open habitats select for increased fecundity and longevity. J. Anim. Ecol. 2005;74:99–104. [Google Scholar]
- Kassarov L. Are birds the primary selective force leading to evolution of mimicry and aposematism in butterflies? An opposing point of view. Behaviour. 2003;140:433–451. [Google Scholar]
- Kirkwood TBL, Feder M, Finch CE, Franceschi C, Globerson A, Klingenberg CP, LaMarco K, Omholt S, Westendorp RGJ. What accounts for the wide variation in life span of genetically identical organisms reared in a constant environment? Mechanisms of Ageing and Development. 2005;126:439–443. doi: 10.1016/j.mad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Larsen TB. The butterflies of Kenya and their natural history. Oxford University Press; Oxford: 1991. [Google Scholar]
- Livingstone D, Livingstone C. Expedition to the Zambesi river and its tributaries and the discovery of lakes Shirwa and Nyassa 1858-1864. The Narrative Press; Santa Barbara, CA: 2001. [Google Scholar]
- Loison A, Festa-Bianchet M, Gaillard JM, Jorgenson JT, Jullien JM. Age-specific survival in five populations of ungulates: Evidence of senescence. Ecology. 1999;80:2539–2554. [Google Scholar]
- Mevi-Schutz J, Erhardt A. Amino acids in nectar enhance butterfly fecundity: a long-awaited link. Am. Nat. 2005;165:411–419. doi: 10.1086/429150. [DOI] [PubMed] [Google Scholar]
- Molleman F. Evolutionary Biology. Leiden University; Leiden: 2004. Patterns of biodiversity and life history in fruit-feeding butterflies. [Google Scholar]
- Molleman F, Alphen MEV, Brakefield PM, DeVries PJ, Zwaan BJ. Food intake of fruit-feeding butterflies: evidence for adaptive variation in proboscis morphology. Biol. J. Linnean Soc. 2005a;86:333–343. [Google Scholar]
- Molleman F, Grunsven RHA, Liefting M, Zwaan BJ, Brakefield PM. Is male puddling behaviour of tropical butterflies targeted at sodium for nuptial gifts or activity? Biol. J. Linnean Soc. 2005b;86:345–361. [Google Scholar]
- Molleman F, Kop A, Brakefield PM, De Vries PJ, Zwaan BJ. Vertical and temporal patterns of biodiversity of fruit-feeding butterflies in a tropical forest in Uganda. Biodiversity and Conservation. 2006;15:107–121. [Google Scholar]
- Molleman F, Krenn HW, Van Alphen ME, Brakefleld PM, Devries PJ, Zwaan BJ. Food intake of fruit-feeding butterflies: evidence for adaptive variation in proboscis morphology. Biol. J. Linnean Soc. 2005c;86:333–343. [Google Scholar]
- Molleman F, van Alphen ME, Brakefield PM, Zwaan BJ. Preferences and food quality of fruit-feeding butterflies in Kibale Forest. Uganda. Biotropica. 2005d;37:657–663. [Google Scholar]
- Norris MJ. The feeding-habits of the adult Lepidoptera Heteroneura. Transactions of the Royal Entomological Society of London. 1936;85:61–90. [Google Scholar]
- O'Brien DM, Boggs CL, Fogel ML. Pollen feeding in the butterfly Heliconius charitonia: isotopic evidence for essential amino acid transfer from pollen to eggs. Proc. R. Soc. Lond. Ser. B-Biol. Sci. 2003;270:2631–2636. doi: 10.1098/rspb.2003.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro CEG. Palatability and escaping ability in neotropical butterflies: tests with wild kingbirds (Tyrannus melancholicus, Tyrannidae) Biol. J. Linnean Soc. 1996;59:351–365. [Google Scholar]
- Pinheiro CEG. Does Mullerian mimicry work in nature? Experiments with butterflies and birds (Tyrannidae) Biotropica. 2003;35:356–364. [Google Scholar]
- Promislow DEL. Mate choice, sexual conflict, and evolution of senescence. Behavior Genetics. 2003;33:191–201. doi: 10.1023/a:1022562103669. [DOI] [PubMed] [Google Scholar]
- Promislow DEL, Tatar M, Pletcher S, Carey JR. Below threshold mortality: implications for studies in evolution, ecology and demography. J. Evol. Biol. 1999;12:314–328. [Google Scholar]
- Ricklefs RE. Intrinsic aging-related mortality in birds. Journal of Avian Biology. 2000;31:103–111. [Google Scholar]
- Tatar M, Gray DW, Carey JR. Altitudinal variation for senescence in Melanoplus grasshoppers. Oecologia. 1997;111:357–364. doi: 10.1007/s004420050246. [DOI] [PubMed] [Google Scholar]
- Tatar M, Yin C-M. Slow aging during insect diapause: why butterflies, grasshoppers and flies are like worms. Experimental Gerontology. 2001;36:723–738. doi: 10.1016/s0531-5565(00)00238-2. [DOI] [PubMed] [Google Scholar]
- Windig JJ, Brakefield PM, Reitsma N, Wilson JGM. Seasonal polyphenism in the wild: survey of wing patterns in 5 species of Bicyclus butterflies in Malawi. Ecological Entomology. 1994;19:285–298. [Google Scholar]