Abstract
Alzheimer’s disease is characterized by the deposition of amyloid β-peptide (Aβ) plaques in the brain. Full-length amyloid-β precursor protein (APP) is processed by α- and β-secretases to yield soluble APP derivatives and membrane-bound C-terminal fragments, which are further processed by γ-secretase to a non-amyloidogenic 3 kDa product or to Aβ fragments. As different Aβ fragments contain different parts of the APP transmembrane helix, one may speculate that they are retained more or less efficiently in the membrane. Here, we use the translocon-mediated insertion of different APP-derived polypeptide segments into the endoplasmic reticulum membrane to assess the propensities for membrane retention of Aβ fragments. Our results show a strong correlation between the length of an Aβ-derived segment and its ability to integrate into the microsomal membrane.
Keywords: amyloid β-protein precursor, Aβ-peptide, translocon mediated, membrane insertion
1. Introduction
The aggregation of fibrillar amyloid β-peptide (Aβ) is thought to be the primary neuropathological event in Alzheimer’s disease (AD) [1]. Aβ is a 4 kDa peptide, primarily found as deposits (plaques) in the cerebral cortex and limbic system of the Alzheimer brain [2]. Aβ is an internal fragment of the 110–120 kDa amyloid-β precursor protein (APP). Full-length APP is processed by at least three proteases (α-, β- and γ-secretase), Fig. 1. α-Secretase cleaves APP within the Aβ domain to generate a soluble N-terminal and a membrane-bound C-terminal fragment (CTF) [4]. β-Secretase produces carboxyl-terminal fragments of APP by cleaving in its luminal domain [5]. Cleavage by α-secretase or β-secretase within the luminal/extracellular domain results in the formation of the large soluble APP domains, called APPsα and APPsβ, respectively, and a membrane-attached carboxyl-terminal fragment (αCTF or βCTF).
Figure 1.
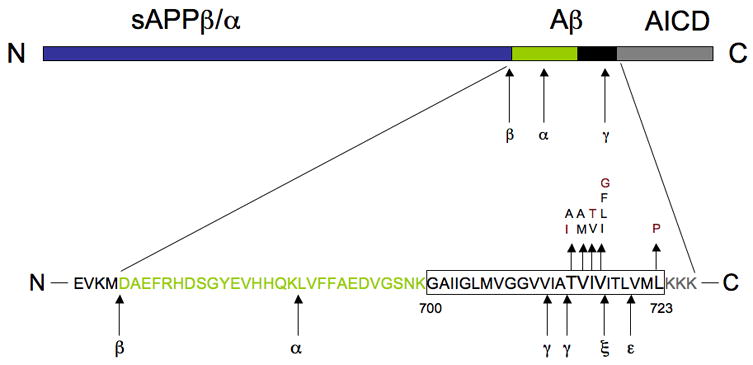
A schematic diagram of APP. The amyloid β peptide (Aβ) region contains part of the transmembrane domain (black box) and part of the extracellular domain of APP (green box). Cleavage by β-secretase generates the N terminus, and intramembranous cleavage by γ-secretase gives rise to the C terminus of Aβ. Cleavage by α-secretase precludes Aβ formation. Eleven missense mutations in the transmembrane region of APP are shown, together with the suggested cleavage sites for ε- and ξ-secretases. Red letters indicate mutations tested here.
Finally, γ-secretase cleaves βCTF in the middle of its transmembrane domain, leading to the release of Aβ, Fig. 1. Identification of multiple cleavage sites within the APP molecule has suggested that APP can also be cleaved by other proteases, namely ε- and ζ-secretases, that also generate an intracellular CTF domain (AICD) and longer Aβ fragments [6–8].
As different Aβ and CTF variants contain different portions of the APP transmembrane segment, one may suspect that they are released from the endoplasmic reticulum (ER) membrane to different degrees. Presumably, the formation of toxic aggregates will only take place after release of Aβ from the membrane, and inefficiently released fragments may hence be less toxic. In this study, we have measured the ability of different Aβ and CTF segments to insert into the membrane of microsomal vesicles derived from dog pancreas, using a system that allows precise measurement of the efficiency of membrane insertion of hydrophobic segments engineered into a model protein [9]. We find that association with the membrane is critically dependent on the length and the hydrophobicity of APP segment.
2. Material and methods
Enzymes and chemicals
All enzymes, plasmid pGEM1, DTT, and the TnT coupled transcription/translation system were from Promega (Madison, WI). [35S]-Met and deoxyribonucleotides were from GE-Healthcare (Uppsala, Sweden). Oligonucleotides were from Cybergene (Stockholm, Sweden).
Plasmid construction
Site-specific mutagenesis was performed using the QuikChange™ Site-Directed Mutagenesis kit from Stratagene (La Jolla, USA). All mutants were confirmed by sequencing of plasmid DNA at BM labbet AB (Furulund, Sweden). All cloning steps were done according to standard procedures using restriction enzymes from Promega (Madison, USA).
DNA manipulations
H-segment-containing Lep constructs carrying acceptor sites for N-linked glycosylation in positions 96–98 (Asn-Ser-Thr; G1) and 258–260 (Asn-Ala-Thr; G2) [9] were expressed from the pGEM1 plasmid.
Oligonucleotides encoding the different H-segment APP fragments (Aβ peptides and CTF fragments) were introduced as previously described [9]. To mimic the natural Aβ peptide, tandem translation stop codons (TAG TAA) were introduced at the end of the APP fragment, together with an extra acceptor site N-terminal of the natural APP TM segment at position 27–29 (Asn-Lys-Thr) in the Aβ peptide using mutagenesis. To examine known human mutations in the APP domain and to insert proline residues in the Aβ TM segment, the desired amino acid was altered by site-specific mutagenesis. All APP inserts were confirmed by sequencing of plasmid DNA.
Expression in vitro
Constructs in pGEM1 were transcribed and translated in the TnT Quick systems from Promega. 1μg DNA template, 1 μl [35S]-Met (5 mCi) and 2 equivalents of dog pancreas rough microsomes [10] were added at the start of the reaction, and samples were incubated for 90 min at 30°C. Translation products were analyzed by SDS-PAGE and gels were visualized in a Fuji FLA-3000 phosphoimager and analyzed using the Image Reader 8.1j software.
The degree of membrane release of each APP fragment was quantified from SDS-PAGE gels (Fig. 3) by calculating the quotient between the intensity of the doubly glycosylated band divided by the summed intensities of the singly glycosylated and doubly glycosylated bands. On average, glycosylation levels vary by no more than ±2% between repeat experiments and all data points are mean values from at least two independent experiments.
Figure 3.
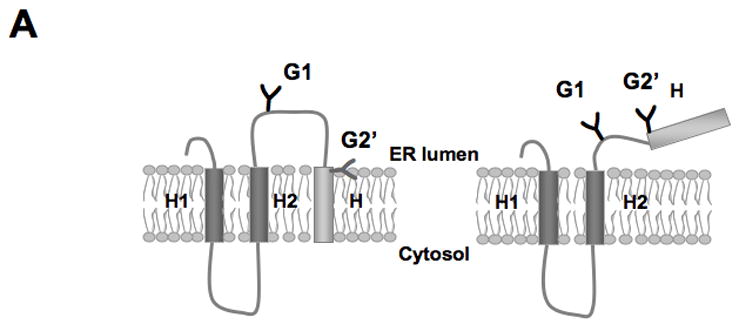
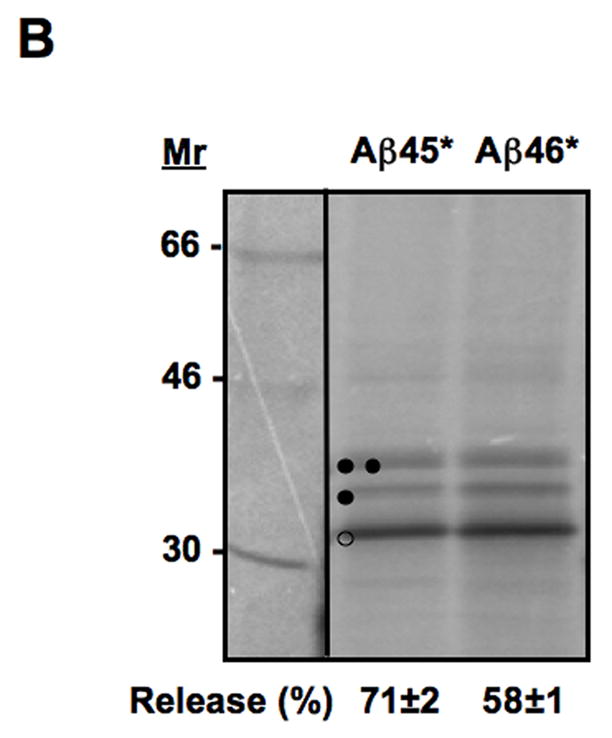
Insertion of truncated Aβ (stop) constructs into microsomal membranes. (A) The truncated Lep construct used. In addition to the G1 glycosylation site, a second Asn-X-Thr site (G2’) has been introduced just upstream of the Aβ (stop) segment (see Table S1). Constructs in which the Aβ (stop) segment is released from the membrane become glycosylated on both the G1 and G2’ sites (right), whereas constructs in which the Aβ (stop) segment is retained in the membrane are glycosylated only on the G1 site (left; see text). (B) In vitro translation in the presence of dog pancreas rough microsomes of constructs containing two different Aβ (stop) segments. Unglycosylated, singly glycosylated and doubly glycosylated forms of the protein are indicated by one open circle, one filled circle and two filled circles, respectively. The percentage of molecules not retained in the membrane is given below the lanes (average and standard deviation of at least three independent experiments).
3. Results
Model protein and membrane insertion assay
To estimate the degree of membrane retention of different Aβ fragments, we used a previously developed experimental system for measuring the efficiency of translocon-mediated insertion into the ER membrane of hydrophobic segments in membrane proteins [9]. Although the translocon is a complicated molecular machine [11,12], the available data suggests that translocon-mediated membrane insertion of hydrophobic segments is driven by peptide-lipid interactions [9,13–15]. Therefore, Aβ fragments should behave similarly in the membrane-insertion assay as they do after presenilin processing.
As in our earlier studies of protein insertion into the ER membrane, we used the well-characterized Escherichia coli inner membrane protein leader peptidase (Lep) as a model protein [9,16] to study the translocon-mediated membrane insertion of different APP-derived polypeptide segments. Lep consists of two transmembrane segments (H1, H2) connected by a short cytoplasmic loop (P1) and a large C-terminal periplasmic domain (P2). When expressed in vitro in the presence of dog pancreas rough microsomes, Lep adopts the same topology as in E. coli, with both the N terminus and the C-terminal P2 domain located in the lumen of the microsome [17]. The microsomes used in the assay are prepared from pancreas and not brain but, since the Sec61 translocon is the same in both tissues, the source of the preparation should not matter to the results.
We have constructed an engineered version of Lep [9] that allows quantitative measurements of the efficiency of membrane insertion of short polypeptide segments such as the Aβ fragments, Fig. 2A. Briefly, polypeptide segments corresponding to different Aβ segments are engineered into the P2 domain, 150 residues downstream of the H2 transmembrane segment. In addition, two Asn-X-Thr acceptor sites for N-linked glycosylation are present in the P2 domain: one (G1) between the H2 segment and the Aβ segment, and the other (G2) just downstream of the Aβ segment. After in vitro translation in the presence of microsomes, the degree of insertion into the membrane of a given Aβ segment can be quantified by comparing the fractions of singly glycosylated (i.e., membrane-integrated) and doubly glycosylated (i.e., released) molecules.
Figure 2.
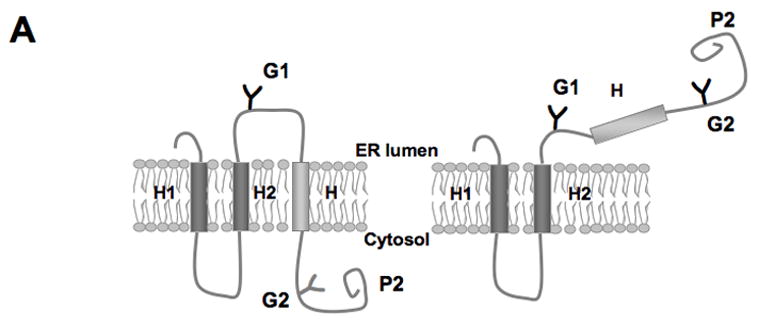
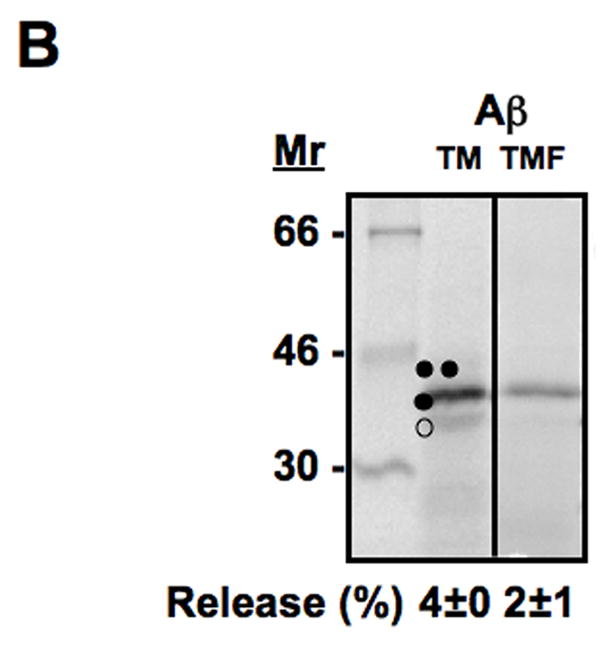
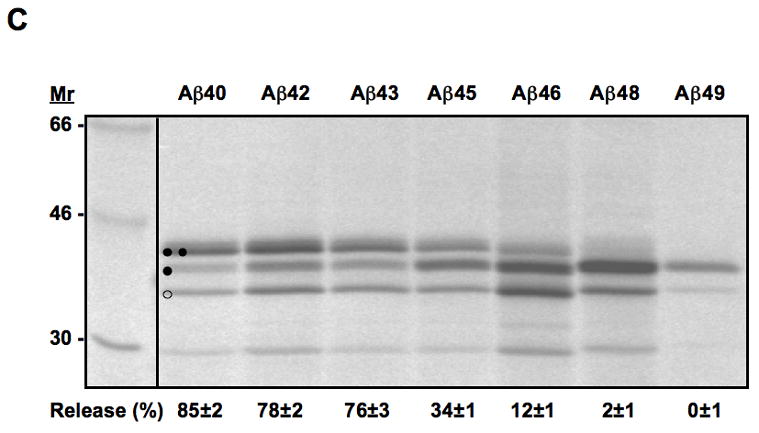
Translocon-mediated membrane insertion of different Aβ and CTF constructs. (A) The leader peptidase model protein. Wild-type leader peptidase (Lep) has two transmembrane helices (H1, H2) and a large lumenal domain (P2). It inserts into rough microsomes in an Nlum–Clum orientation. In the constructs reported here, Aβ 40–49 and CTF C50–59 segments (see Table S1) were inserted into the P2 domain in the position indicated (H, gray rectangle), and Asn-X-Thr glycosylation acceptor sites (G1, G2) were introduced on both sides of the Aβ and CTF segments. Constructs in which the Aβ or CTF segment is integrated into the endoplasmic reticulum (ER) membrane become glycosylated only on the G1 site (left), whereas those in which the segment is translocated across the ER membrane become glycosylated on both the G1 and G2 sites (right). (B) In vitro translation in the presence of dog pancreas rough microsomes (RMs) of constructs containing the Aβ transmembrane segment with (lane TMF) and without (lane TM) additional flanking residues from APP (see Table S1). (C) In vitro translation in the presence of dog pancreas rough microsomes of constructs containing the indicated Aβ segments. Unglycosylated, singly glycosylated, and doubly glycosylated forms of the protein are indicated by one open circle, one filled circle and two filled circles, respectively. The percentage of molecules not retained in the membrane is given below the lanes (average and standard deviation of at least three independent experiments).
We have also used a different version of Lep in which the Aβ segment is present at the very C terminus of the molecule and where the G2 glycosylation site is placed immediately upstream of the Aβ segment. With this construct we can measure the membrane integration efficiency of Aβ segment with a free C terminus, more closely mimicking the situation after cleavage of the APP transmembrane segment by presenilin.
Membrane insertion of Aβ segments
To examine the membrane integration of different Aβ and C-terminal APP segments, we first introduced the chosen segments into the P2 domain of full-length Lep and measured the insertion into the ER membrane of Aβ 40, 42, 43, 45, 46, 48, 49 and the C-terminal segments C50, C57, C59 [6,18–21], Table SI. As expected, the natural APP transmembrane segment (Aβ TM from Gly700 to Leu723) is efficiently retained into the membrane (96–97% singly glycosylated molecules, both without and with additional flanking residues from APP), Fig. 2B. The shorter Aβ 40–43 segments, in contrast, are not retained in the ER membrane. Aβ 45 is released to about 34%, while little or no release is seen for Aβ 46–49, Fig. 2C and 4A.
Figure 4.
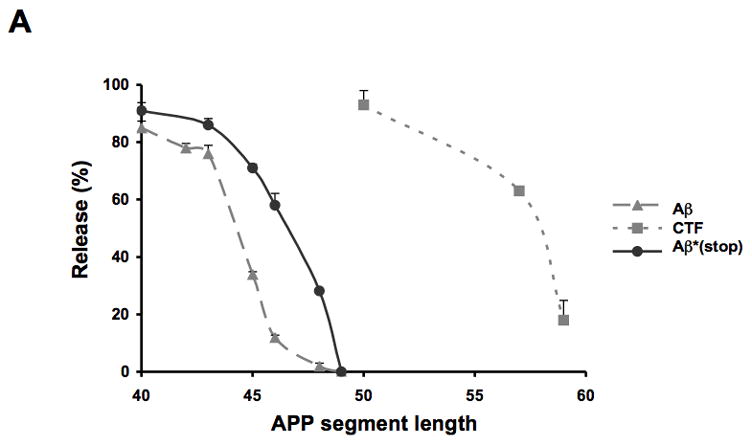
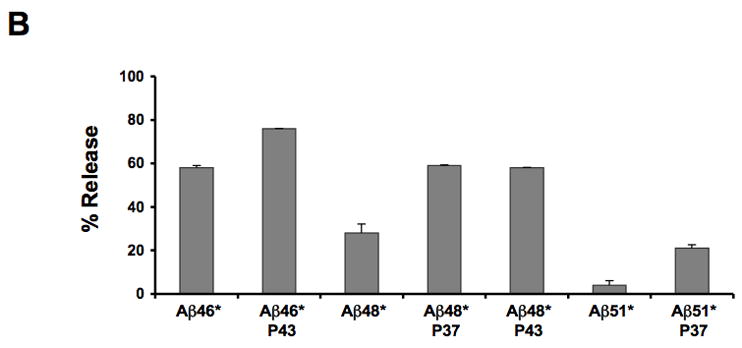
(A) Insertion of Aβ segments (grey dashed line, filled triangle), Aβ (stop) segments (black line, filled circles) and CTF segments (grey dashed line, filled squares) into the microsomal membrane. The percentage of molecules not integrated into the membrane (average and standard deviation of at least three independent experiments) is shown as a function of segment length. (B) Membrane integration of Aβ (stop) segments containing a proline residue in position 37 or 43. The percentage of molecules not retained in the membrane (average and standard deviation of at least two independent experiments) is shown.
The corresponding C-terminal segments show a similar behavior, with shorter segments being less efficiently integrated. The CTF 50 segment is efficiently released from the ER membrane (88% doubly glycosylated molecules) while CTF 57 is released to ~60% and CTF 59 hardly at all, Fig. 4A.
Membrane insertion of Aβ (stop) segments
In the above constructs, the Aβ segments are embedded within the larger full-length model protein. To more closely mimic the context of the hydrophobic segment in the natural Aβ peptides which, after cleavage by γ-, ζ- or ε-secretase, is located at the very C terminus of the peptide, we added stop codons after the Aβ segments and measured the efficiency of integration of these truncated constructs into the microsomal membrane. To this end, we introduced a new glycosylation acceptor site (G2’) in the Aβ-peptide portion, just N-terminal to the natural APP TM segment, Fig. 3A and Table SI. As an Asn-X-Thr glycosylation acceptor site must be located at least 14–15 residues from the membrane for efficient glycosylation [17], the G2’ site will only be glycosylated if the Aβ segment is released from the membrane, whereas the G1 site will be glycosylated regardless of whether the segment is released or not. For the truncated Aβ constructs, ~60% release was seen for the Aβ 46* construct (Fig. 3B and 4A), demonstrating that the C-terminal Aβ (stop) segments are slightly less efficiently integrated into the membrane than when they are embedded within the Lep P2 domain.
A number of known mutations associated with AD are located in the Aβ hydrophobic domain [22–25], Fig. 1. We selected four in which a hydrophobic residue is replaced by a more polar residue or vice versa for analysis: Thr714Ile, Ile716Thr, Val717Gly, and Leu723Pro (Fig. 1, indicated in red). The degree of membrane integration was measured for the first three in different Aβ (stop) constructs (Table S1); in no case did the mutation change the fraction of integrated molecules, Table S2. The toxicity of these mutant Aβ peptides therefore seems not to be associated with their degree of membrane retention but rather must reflect their aggregation propensity per se. The Leu723Pro mutation was tested in the CTF segment C57, Table S1. For this mutation the fraction of integrated CTF decreased from 37% to 5%, Table S2.
As our previous studies on membrane integration of transmembrane segments have suggested that efficient integration depends on the formation of an α-helical structure [9], we also made a set of constructs where a helix-breaking proline residue was inserted in the Aβ (stop) segments. We measured the membrane insertion of three Aβ (stop) constructs (Aβ 46*, 48* and 51*) with a proline residue either at position 37 or 43, Table SI. A significant decrease in insertion efficiency was seen for all constructs (Fig. 4B), suggesting that the membrane-integrated forms of Aβ must adopt a helical conformation.
4. Discussion
How efficiently are different Aβ peptides released from the membrane upon cleavage by γ-, ζ- or ε-secretase? To address this question, we have carried out a detailed study of the translocon-mediated insertion of different APP segments into microsomal membranes. Two different series of Aβ segments – one truncated at the C terminus and one with a long C-terminal tail – yield similar results: shorter Aβ segments (Aβ 40–45) are not integrated into the membrane, while longer ones (Aβ 46–49) are efficiently retained in the membrane, Fig. 5. Membrane integration of the different CTFs shows a similar dependency on the length of the remaining part of the APP transmembrane segment.
Figure 5.
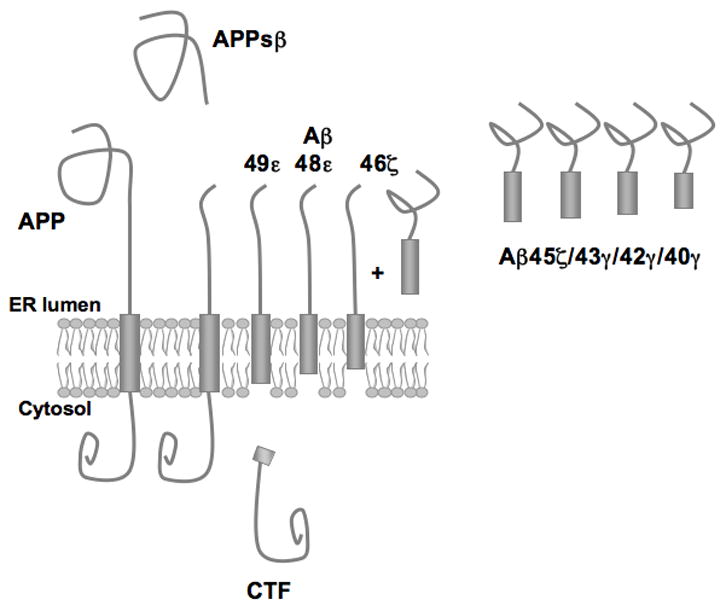
Model for Aβ-fragment release from the membrane. Mature APP is cleaved by β-secretase, releasing a soluble N-terminal fragment (APPsβ). The C-terminal fragment (CTF) is produced by ε-secretase cleavage together with Aβ 48–49 membrane-bound fragments. Longer Aβ fragments are further processed by ζ/γ-secretases, leaving Aβ 46 partly in the membrane while shorter, less hydrophobic fragments (Aβ 40–45) are released into the lumen of the ER.
Different models for Aβ processing have been proposed in the literature: either γ-, ζ- and ε-cleavage are correlated with each other [6,19], or the γ- and ε-cleavage are independent processes [8,22]. Ihara and coworkers identified only two species of AICDs (C50 and C51), despite detection of various Aβ species (Aβ 40, 42, 43, 45, 46, 48, and 49) [18,19,21]. They, therefore, proposed a model in which longer Aβs are processed stepwise at every third residue by γ-secretase. In the context of this model, we would suggest that the stepwise processing occurs only for those Aβ species that are hydrophobic enough to be retained in the membrane. The intermediate products Aβ 48/49 would be generated by ε-secretase cleavage, the Aβ 48 and Aβ 49 products would be further processed by ζ- and γ-secretase to generate Aβ 45/46, and finally be released only when further cleaved to yield Aβ 40/42 [6].
Three known human mutations, Thr714Ile, Ile716Thr, and Val717Gly, which are close to the γ-secretase site and therefore suggested to change the pattern of APP processing, had no effect on the efficiency of Aβ segment insertion. The Leu723Pro mutation did, however, decrease the insertion of the CTF segment C57 into the membrane. Results for Aβ (stop) segments containing proline residues further suggest that the formation of an α-helical structure in the membrane-embedded part of Aβ is critical for efficient retention in the membrane.
In conclusion, we have demonstrated that different Aβ segments are integrated into the ER membrane to different degrees, depending on their overall length, hydrophobicity and helix potential. We speculate that this might also affect their access to the secretases in the ER membrane and their ability to partake in the formation of toxic aggregates.
Supplementary Material
Acknowledgments
We gratefully thank Yiwei Miao and Yuanlong Shao for technical assistance. This work was supported by grants from the Swedish Cancer Foundation to I.N and GvH, from Magnus Bergvalls Stiftelse, Henrik Granholms Stiftelse and Carl Tryggers Stiftelse to I.N, from the Swedish Research Council to J.N, by NIH grant GM26494 to A.E.J and support from the Robert A. Welch Chair grant BE-0017 to A.E.J.
The abbreviations used are
- AD
Alzheimer’s disease
- Aβ
amyloid β-peptide
- APP
amyloid β-protein precursor
- AICD
APP intracellular domain
- CTF
C-terminal fragment
- TM
transmembrane
- p3
3 kDa product
- ER
endoplasmic reticulum
- Lep
leader peptidase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–12. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 2.Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–90. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 3.Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–66. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 4.Buxbaum JD, et al. Evidence that tumor necrosis factor alpha converting enzyme is involved in regulated alpha-secretase cleavage of the Alzheimer amyloid protein precursor. J Biol Chem. 1998;273:27765–7. doi: 10.1074/jbc.273.43.27765. [DOI] [PubMed] [Google Scholar]
- 5.Vassar R, et al. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease. BACE Science. 1999;286:735–41. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 6.Zhao G, Cui MZ, Mao G, Dong Y, Tan J, Sun L, Xu X. gamma-Cleavage is dependent on zeta-cleavage during the proteolytic processing of amyloid precursor protein within its transmembrane domain. J Biol Chem. 2005;280:37689–97. doi: 10.1074/jbc.M507993200. [DOI] [PubMed] [Google Scholar]
- 7.Zhao G, Mao G, Tan J, Dong Y, Cui MZ, Kim SH, Xu X. Identification of a new presenilin-dependent zeta-cleavage site within the transmembrane domain of amyloid precursor protein. J Biol Chem. 2004;279:50647–50. doi: 10.1074/jbc.C400473200. [DOI] [PubMed] [Google Scholar]
- 8.Kume H, Kametani F. Abeta 11-40/42 production without gamma-secretase epsilon-site cleavage. Biochem Biophys Res Commun. 2006;349:1356–60. doi: 10.1016/j.bbrc.2006.08.181. [DOI] [PubMed] [Google Scholar]
- 9.Hessa T, et al. Recognition of transmembrane helices by the endoplasmic reticulum translocon. Nature. 2005;433:377–81. doi: 10.1038/nature03216. [DOI] [PubMed] [Google Scholar]
- 10.Walter P, Blobel G. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 1983;96:84–93. doi: 10.1016/s0076-6879(83)96010-x. [DOI] [PubMed] [Google Scholar]
- 11.Rapoport TA, Goder V, Heinrich SU, Matlack KE. Membrane-protein integration and the role of the translocation channel. Trends Cell Biol. 2004;14:568–75. doi: 10.1016/j.tcb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Alder NN, Johnson AE. Cotranslational membrane protein biogenesis at the endoplasmic reticulum. J Biol Chem. 2004;279:22787–90. doi: 10.1074/jbc.R400002200. [DOI] [PubMed] [Google Scholar]
- 13.Meindl-Beinker NM, Lundin C, Nilsson I, White SH, von Heijne G. Asn- and Asp-mediated interactions between transmembrane helices during translocon-mediated membrane protein assembly. EMBO Rep. 2006;7:1111–6. doi: 10.1038/sj.embor.7400818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nilsson I, Saaf A, Whitley P, Gafvelin G, Waller C, von Heijne G. Proline-induced disruption of a transmembrane alpha-helix in its natural environment. J Mol Biol. 1998;284:1165–75. doi: 10.1006/jmbi.1998.2217. [DOI] [PubMed] [Google Scholar]
- 15.Monne M, Nilsson I, Elofsson A, von Heijne G. Turns in transmembrane helices: determination of the minimal length of a “helical hairpin” and derivation of a fine-grained turn propensity scale. J Mol Biol. 1999;293:807–14. doi: 10.1006/jmbi.1999.3183. [DOI] [PubMed] [Google Scholar]
- 16.Hermansson M, von Heijne G. Inter-helical hydrogen bond formation during membrane protein integration into the ER membrane. J Mol Biol. 2003;334:803–9. doi: 10.1016/j.jmb.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson I, von Heijne G. Determination of the Distance Between the Oligosaccharyltransferase Active Site and the Endoplasmic Reticulum Membrane. J Biol Chem. 1993;268:5798–5801. [PubMed] [Google Scholar]
- 18.Qi-Takahara Y, et al. Longer forms of amyloid beta protein: implications for the mechanism of intramembrane cleavage by gamma-secretase. J Neurosci. 2005;25:436–45. doi: 10.1523/JNEUROSCI.1575-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yagishita S, Morishima-Kawashima M, Tanimura Y, Ishiura S, Ihara Y. DAPT-induced intracellular accumulations of longer amyloid beta-proteins: further implications for the mechanism of intramembrane cleavage by gamma-secretase. Biochemistry. 2006;45:3952–60. doi: 10.1021/bi0521846. [DOI] [PubMed] [Google Scholar]
- 20.Funamoto S, Morishima-Kawashima M, Tanimura Y, Hirotani N, Saido TC, Ihara Y. Truncated carboxyl-terminal fragments of beta-amyloid precursor protein are processed to amyloid beta-proteins 40 and 42. Biochemistry. 2004;43:13532–40. doi: 10.1021/bi049399k. [DOI] [PubMed] [Google Scholar]
- 21.Kakuda N, Funamoto S, Yagishita S, Takami M, Osawa S, Dohmae N, Ihara Y. Equimolar production of amyloid beta-protein and amyloid precursor protein intracellular domain from beta-carboxyl-terminal fragment by gamma-secretase. J Biol Chem. 2006;281:14776–86. doi: 10.1074/jbc.M513453200. [DOI] [PubMed] [Google Scholar]
- 22.Hecimovic S, Wang J, Dolios G, Martinez M, Wang R, Goate AM. Mutations in APP have independent effects on Abeta and CTFgamma generation. Neurobiol Dis. 2004;17:205–18. doi: 10.1016/j.nbd.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki N, Cheung TT, Cai XD, Odaka A, Otvos L, Jr, Eckman C, Golde TE, Younkin SG. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994;264:1336–40. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 24.Hardy J. Amyloid, the presenilins and Alzheimer's disease. Trends Neurosci. 1997;20:154–9. doi: 10.1016/s0166-2236(96)01030-2. [DOI] [PubMed] [Google Scholar]
- 25.Goate A. Segregation of a missense mutation in the amyloid beta-protein precursor gene with familial Alzheimer's disease. J Alzheimers Dis. 2006;9:341–7. doi: 10.3233/jad-2006-9s338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


