Summary
The principles driving the organization of the ventral object-processing stream remain unknown. Here we show that stimulus-specific repetition suppression (RS) in one region of the ventral stream is biased according to motor-relevant properties of objects. Quantitative analysis confirmed that this result was not confounded with similarity in visual shape. A similar pattern of biases in RS according to motor-relevant properties of objects was observed in dorsal stream regions in the left hemisphere. These findings suggest that neural specificity for ‘tools’ in the ventral stream is driven by similarity metrics computed over motor-relevant information represented in dorsal structures. Support for this view is provided by converging results from functional connectivity analyses of the fMRI data and a separate neuropsychological study. More generally, these data suggest that a basic organizing principle giving rise to ‘category-specificity’ in the ventral stream may involve similarity metrics computed over information represented elsewhere in the brain.
Introduction
One principle of organization of the primate visual system is the division of labor between the ventral object-processing stream, mediating visual object recognition, and a dorsal object-processing stream, mediating online object-directed action and spatial analysis (Goodale and Milner, 1992; Ungerleider and Mishkin, 1982). The ventral stream projects from primary visual cortex to the lateral and ventral surfaces of occipital cortex, through to anterior ventral temporal cortex. The dorsal stream projects from primary visual cortex to dorsal occipital and lateral temporal cortex, through to parietal cortex (Ungerleider, 1995). Within the dorsal object-processing stream, a network of primarily left lateralized regions process object associated motion (left middle temporal gyrus), online visuo-motor transformations for grasping objects (posterior parietal cortex), and the motor commands associated with tool use (inferior parietal lobule) (e.g., Culham et al., 2003; Beauchamp et al., 2002; Johnson-Frey, 2004). Functional neuroimaging has shown that these dorsal regions are differentially activated when participants view manipulable objects compared to living things or large non-manipulable objects (e.g., Chao and Martin, 2000; Johnson-Frey et al., 2005; Okada et al., 2000; for review, see Lewis, 2006).
A second characteristic of the organization of the human visual system concerns the organization of high-order visual object recognition processes within the ventral stream, and in particular, within the fusiform gyrus. The fusiform gyrus processes visual properties of objects such as color and form (e.g., Martin et al., 1995; Miceli et al., 2001). A number of functional neuroimaging studies in humans have found that living things (e.g., faces, animals), compared to nonliving things, differentially activate the lateral portion of the fusiform gyrus (in the vicinity of the Fusiform Face Area – Kanwisher et al., 1999; Chao et al., 1999a). In contrast, manipulable objects such as tools and utensils, compared to living things, differentially activate the medial fusiform gyrus (e.g., Chao et al., 1999b; Noppeney et al., 2006; although the specificity of this claim has been challenged: Downing et al., 2006; Mechelli et al., 2006). Finally, stimuli that may be described as highly contextualized, such as large nonmanipulable objects, houses, and scenes, differentially activate the parahippocampal gyrus (Parahippocampal Place Area – Epstein et al., 1999; see also Avidan et al., 2002; Barr and Aminoff, 2003; Downing et al., 2006). These category-specific profiles of neural activation have also been observed at the neuronal level. Single cell recordings in humans have documented category-specificity in medial temporal lobe structures that receive input from ventral temporal-occipital cortex (Kreiman et al., 2000).
Thus, two broad properties of the organization of the human visual system can be distinguished. On the one hand, visually presented objects are processed in the ventral stream for recognition and in the dorsal stream for online guidance of action. On the other hand, there is articulated structure within the ventral object-processing stream in terms of the topography of category-specific neural responses. These two organizational characteristics of the visual system are generally viewed as functionally and physiologically independent. An important and as yet unresolved issue is the degree to which there exist functional interactions between the dorsal and ventral object-processing streams. This issue is particularly relevant in addressing the causes of neural specificity in the ventral stream for manipulable objects. To date, it has been argued that neural specificity in the ventral stream depends on similarity metrics that are computed over the information that is represented and processed internal to the ventral stream itself. For instance, it has been proposed that similarity in visual form (Haxby et al., 2001) or in the distribution of eccentricity preferences (Levy et al., 2001) explain the causes of category-specificity in the ventral stream.
An alternative conceptual framework that has not to date been explored is that neural specificity for objects in the ventral stream is determined, in part, by similarity metrics computed over information that is stored elsewhere. For example, neural specificity for manipulable objects in the ventral stream may depend on information represented in dorsal stream regions that directly mediate object-directed action. The left inferior parietal lobule processes motor commands associated with tool use (e.g., Heilman et al., 1982; Johnson-Frey et al., 2005; Rumiati et al., 2004) and the left middle temporal gyrus processes the rigid and unarticulated motion associated with nonliving objects (Beauchamp et al., 2002; 2003). In the course of manipulating and using objects, it is necessary to integrate the output of object recognition processes (ventral stream) with information about object motion (left middle temporal gyrus) and the motor commands necessary to realize the function of the objects (left inferior parietal lobule). The efficacy of such an information processing network would be increased if the organization of object recognition processes already anticipated the processing requirements of computations implemented ‘downstream.’
The physiology of the primate brain affords the possibility that inputs from neural structures beyond the ventral pathway determine, in part, neural specificity within the medial fusiform gyrus for manipulable objects. There are anatomical projections between ventral temporal cortex and the inferior parietal lobule (Rushworth et al., 2006; Webster et al., 1994; Zhong et al., 2003) as well as lateral temporal cortex (Saleem et al., 2000). There is also functional connectivity between ventral temporal and ventral prefrontal regions (Miller et al., 2003) which are involved in categorization and the determination of behavioral goals. Anterior motor areas such as premotor cortex in turn have substantial functional connectivity with these ventral prefrontal regions (Rizzolatti and Luppino, 2001). Finally, the basal ganglia, involved in motor control, and which receive input from frontal, parietal, and temporal structures also project to inferior temporal cortex (Middleton and Strick, 1996) (see also Ungerleider, 1995, and Pisella et al., 2006, for review).
Clear predictions follow from the view that the organization of the ventral stream is driven, in part, by functional connectivity with dorsal regions directly mediating object-directed action. The first prediction is that neural responses in the medial fusiform gyrus will be sensitive to the relationship between the physical structure of objects (in the visual modality, represented in the fusiform gyrus) and the motor movements associated with the use of those objects (represented in dorsal stream regions, including the left inferior parietal lobule). The second prediction is that perturbation of neural processes in the dorsal stream may disrupt the equilibrium of processes mediated by the ventral stream. We tested these predictions with a combination of functional neuroimaging and neuropsychological methods.
Rapid, event-related fMRI was used to study modulations in stimulus-specific repetition suppression (RS) in the medial fusiform gyrus as a function of motor-relevant properties of nonliving things. Stimulus-specific RS can serve as an index of neural specificity since it may be argued that only those sources of signal (i.e., populations of neurons) that are critically involved in the processing of a stimulus will show RS to repeated presentations of that stimulus (e.g., Avidan et al., 2002; Chao et al., 2002; Dobbins et al., 2004; Grill-Spector et al., 2006; James et al., 2002). We find that stimulus-specific RS in the left medial fusiform gyrus is observed only for manipulable objects with direct relationships between their physical structure and the motor movements associated with their use – ‘tools.’ A similar pattern of RS biased toward ‘tools’ was observed in the left middle temporal gyrus and in the left inferior parietal lobule.
The neuropsychological study evaluated regions of the brain in which lesions predict deficits for using and identifying objects. Converging with the results of the fMRI study, we find that lesions to the left middle temporal gyrus and the left inferior parietal lobule are associated with impairments for both using and identifying objects. We further show that when patients are separated on the anatomical criterion of having lesions involving parietal cortex, the distribution of performance of the patients for both identifying and using objects is modulated.
Finally, functional connectivity analyses demonstrated that the neural responses in the fMRI experiment in the left medial fusiform gyrus independently predicted neural responses in the left middle temporal gyrus and the left inferior parietal lobule. These dorsal stream regions identified by the functional connectivity analyses corresponded to regions of damaged tissue independently identified in the lesion overlap analyses.
Results
fMRI Stimulus characteristics
The experimental stimuli for the fMRI experiment consisted of grayscale photographs of animals, ‘tools’, arbitrarily manipulated objects, and nonmanipulable objects. ‘Tools’ refer to manipulable objects that have systematic relationships between their physical form and their manner of manipulation/function (e.g., hammer, scissors, wrench). Arbitrarily manipulated objects refer to equally manipulable objects that have variable or non-systematic relationships between their physical form and their manner of manipulation/function (e.g., book, wallet, envelope). The distinction between ‘tools’ and arbitrarily manipulated objects was confirmed by behavioral studies (see Figure 1 for details). The motor movements associated with the ‘tool’ stimuli were more central in determining their function and were more predictive of their identity than were the motor movements associated with arbitrarily manipulated objects. Critically, however, ‘tools’ and arbitrarily manipulated objects did not differ with respect to participants’ experience in physically manipulating the objects. Stimuli in the set ‘nonmanipulable objects’ were large objects that may be touched, but which are not ‘taken’ in the hands (e.g., anchor[of a ship], fence, desk). The four stimulus types – ‘tools’, arbitrarily manipulated objects, nonmanipulable objects, and animals – were matched on lexical frequency and concept familiarity. There was no difference in within-category similarity in visual shape between animals, tools, and nonmanipulable objects (see Figure 1D and Supplemental Online Experimental Procedures for details). Furthermore, there was no difference across the four stimulus types, in terms of similarity in visual shape between items from a given stimulus type, and all other items in the experiment from the other stimulus types (inter-category similarity) (ANOVA: F < 1).
Figure 1. fMRI Stimulus characteristics.
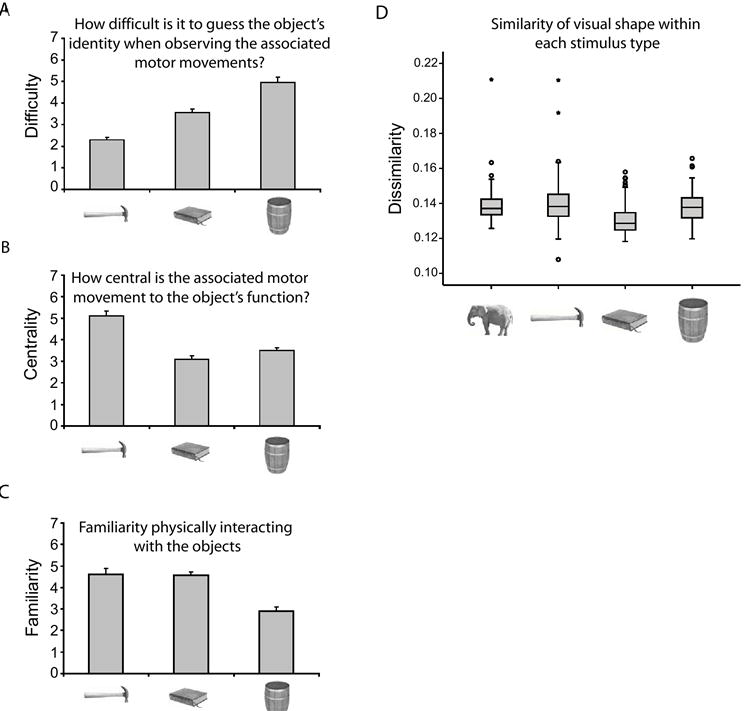
The motor-relevant distinctions between ‘tools’, arbitrarily manipulated objects, and nonmanipulable objects were confirmed by ratings (see Supplemental Online Experimental Procedures and Figure S6). Graphs (A-C) represent mean Likert rating ± SEM), with greater values indicating greater difficulty (A), more central (B), or more familiar (C). (A) ‘Tools,’ arbitrarily manipulated objects, and nonmanipulable objects differed monotonically (linear contrast analysis, p < .001; η2 = .92) in the degree to which their identity was predictable from their associated motor movements. (B) The motor movements associated with ‘tools’ were more central in determining their function than were the motor movements associated with arbitrarily manipulated objects and nonmanipulable objects (ps < .001). (C) ‘Tools’ and arbitrarily manipulated objects did not differ (p = .93) with respect to participants’ experience in physically interacting with the objects. (D) Previous research (Op de Beeck et al., 2001) demonstrates that visual shape similarity can determine the pattern of neuronal responses in the ventral stream. A quantitative analysis (see Belongie et al., 2002) of the experimental stimuli demonstrated that there was no difference in similarity of visual shape within stimulus type, among animals, ‘tools,’ and nonmanipulable objects (all ps > .05; see also Figure S7). Within category similarity in visual shape was greater for arbitrarily manipulated objects than for the other stimulus types (all ps < .05, Bonferroni correction). Larger values on the y-axis of the graph indicate greater dissimilarity in visual shape. Box plot represents medians ± inter-quartile ranges (IQRs). Outliers (circles) and extreme values (stars) are defined as values between 1.5 - 3 IQRs, and greater than 3 IQRs, respectively, from the tops and bottoms of the boxes. Not shown in this figure, a separate behavioral experiment measuring naming latencies demonstrated reliable repetition priming (one-way ANOVAs) for all stimulus types (all ps ≤ .05; see Supplemental Online Experimental Procedures for details).
Stimulus-specific RS according to motor-relevant properties of objects
Ventral Stream
A described in the introduction, the ventral object-processing stream is composed of a set of regions characterized by distinct profiles of category-specificity. Of interest in our study is the pattern of neural responses according to motor-relevant properties of nonliving objects in the medial fusiform gyrus, on the ventral surface of temporal-occipital cortex. This region is medial to the well-known Fusiform Face Area and posterior to the Parahippocampal Place Area. In this study, we did not use the localizer approach to identify voxels in the medial fusiform gyrus. Rather, we used functional contrasts internal to the experimental design which were orthogonal to the effect of interest (i.e., stimulus-specific RS for each object type).
In line with previously reports (e.g., Avidan et al., 2002; Chao et al., 1999b; Noppeney et al., 2006) we found increased activity in the medial fusiform gyri, bilaterally, when subjects named nonliving things (collapsed together), compared to naming animals (Figure 2A). In addition, although ‘tools’ failed to elicit more activity in the medial fusiform gyri than the other nonliving object types based on responses to the novel trials, there was a systematic bias in RS effects that followed motor-relevant properties of the stimuli (see histograms in Figure 2B). In the left medial fusiform gyrus, RS was observed for only ‘tools’ (p < .001; all other Fs < 1) while in the right medial fusiform gyrus, RS was biased toward ‘tools’ (p < .001) and arbitrarily manipulated objects (p = .067; all other Fs ≤ 1). These modulations in RS effects by Stimulus Type within the medial fusiform gyri were confirmed with ANOVAs. In the right medial fusiform gyrus, there was a marginal interaction between RS and Stimulus Type (p = .05); in the left medial fusiform gyrus, the interaction approached significance (p = .10) (for further analysis, see Supplemental Figure S1 and Table S2).
Figure 2. RS in the medial fusiform gyri modulated by motor-relevant properties of objects.
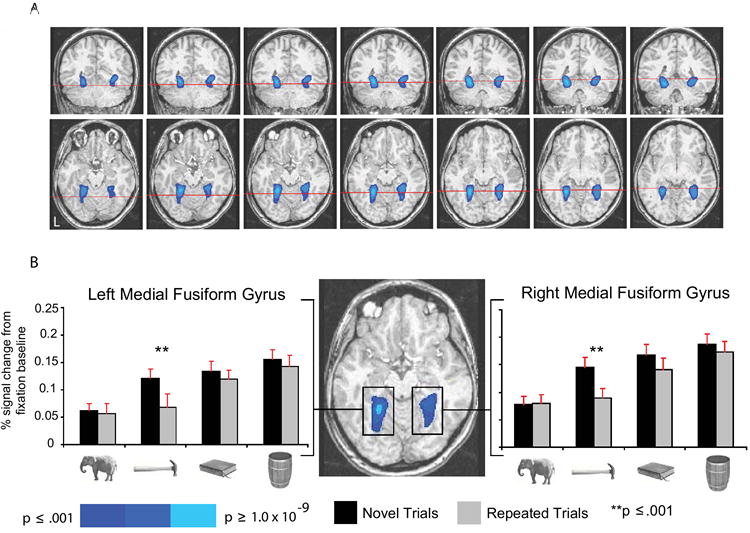
(A) The figure shows group averaged activity superimposed on the brain of an individual subject. Blue indicates regions in the medial fusiform gyri preferring nonliving things to animals (top row, left to right, y = -54 through y = -42, in steps of 2; bottom row, left to right, z = -16 through z = -4, in steps of 2). The red cross-sections in the top row indicate the plane of the axial slice directly below (and vice-versa). Voxels were defined at p < .001, corrected using family-wise error correction (Monte Carlo simulation), which takes into account cluster size and alpha level. (B) All histograms and statistical analyses of BOLD responses (here and elsewhere) were computed using mean BOLD responses by experimental condition, averaged across all voxels in the region. Error bars in all histograms of BOLD responses (here and elsewhere) represent the SEM. The peak differences for the contrast of nonliving things compared to animals were located (TT coordinates), in the left medial fusiform gyrus (8596mm3), at -24, -48, -8, and in the right medial fusiform gyrus (8264mm3), at 28, -41, -10 (figure shown at z = -12). There were main effects of RS bilaterally (ps < .005), collapsing across the four stimulus types. However, as depicted in the histograms, there was a systematic bias in RS toward ‘tools’ in the left medial fusiform gyrus, and toward ‘tools’ and arbitrarily manipulated objects in the right medial fusiform gyrus.
As noted in the introduction, previous research indicates that contextualized visual stimuli (such as the large, nonmanipulable objects in our study) are differentially processed in parahippocampal cortex (Parahippocampal Place Area – Epstein et al., 1999; see also Barr and Aminoff, 2003), while living things such as animals are differentially processed in the lateral fusiform gyrus (e.g., Chao et al., 1999a,b), in the vicinity of the Fusiform Face Area (e.g., Kanwisher et al., 1999). Consistent with this body of research, we observed stimulus-specific RS only for nonmanipulable objects in parahippocampal cortex bilaterally and only for animals in the right lateral fusiform gyrus. These findings are depicted in Figure 3B.
Figure 3. RS by Stimulus Type in the ventral stream.

(A) Large bilateral regions encompassing medial and lateral regions of the fusiform gyrus were defined by the presence of a main effect of RS (both ps < .002), collapsing across the factor Stimulus Type (left to right, axial views at z = -17, -12, and -7). Consistent with the broad expanse of highlighted structures, there were main effects of Stimulus Type (ps < .0001) in both the left and right hemispheres. The interaction between RS and Stimulus Type was significant in the right hemisphere (p < .041), and there was a trend in the left hemisphere (p = .09). The peak TT coordinates for this contrast were -36, -57, -8 (left hemisphere) and 32, -46, -6 (right hemisphere). (B) The distinct colors represent RS (p < .01) biased toward each of the four Stimulus Types. We also coded voxels that showed RS biased toward both ‘tools’ and arbitrarily manipulated objects, in order to study potential overlap in RS effects for the two types of manipulable objects. As depicted in Figure 3B (left to right, axial views at z = -17, -12, and -7), the largest regions, located in the medial fusiform gyri bilaterally, showed RS biased toward ‘tools’ (blue). In addition, smaller clusters of voxels showing RS biased toward arbitrarily manipulated objects (yellow), and toward both ‘tools’ and arbitrarily manipulated objects (green), were also observed. RS was biased toward animals (red) in the right lateral fusiform gyrus. RS biased toward nonmanipulable objects (cyan) was restricted to parahippocampal cortex. This effect for nonmanipulable objects was present bilaterally (only right hemisphere activation shown). (C – E) Histograms represent RS effects (difference scores: novel – repeated) for each experimental condition, for the left (red bars) and right (green bars) medial fusiform gyrus. The corresponding functional group maps are shown (colored blue) next to each histogram (y = -42). As can be seen, RS was observed for ‘tools’ in the medial fusiform gyrus bilaterally, independently of how this region was defined. For further details of the analyses, see Supplemental Table S2; for histograms showing overall BOLD responses for the same contrasts, see Supplemental Figure S1. (C) Voxels in the medial fusiform gyrus showing greater activation for ‘tools’ than animals (p < .01). (D). Voxels in the medial fusiform gyrus showing greater activation for arbitrarily manipulated objects than animals (p < .01). (E). Voxels in the medial fusiform gyrus showing greater activation for nonmanipulable objects than animals (p < .01).
Importantly, stimulus-specific RS was biased toward ‘tools’ in the left medial fusiform gyrus independently of how voxels in this region were defined. For instance, as summarized in Figure 3 (panels C-E), RS was observed for only ‘tools’ in the left medial fusiform, independently of whether the region was defined by nonmanipulable objects versus animals, arbitrarily manipulated objects versus animals, or ‘tools’ versus animals. At the same time, the region in the medial fusiform gyrus showing an enhanced response to identifying non-manipulable objects included the region defined by arbitrarily manipulated objects versus animals. Similarly, the region showing enhanced activity for arbitrarily manipulated objects compared to animals included the region showing enhanced activity for ‘tools’ compared to animals (see Table S2 for details) . These relative differences in the sizes of object-responsive regions remained when bilateral medial fusiform regions were defined using only novel trials for each of the nonliving object types compared to animals (see Figure S1). This means that these relative size differences were not due to differential RS effects. In contrast, as depicted in Figure 3B, when voxels were defined in terms of the presence of RS effects for a single nonliving object type, RS for ‘tools’ was observed throughout the bilateral medial fusiform gyri, while RS for non-manipulable objects was restricted to parahippocampal cortex.
Dorsal Stream
In contrast to the pattern observed in the ventral stream, the contrast of all nonliving things versus animals failed to elicit activity in any of the dorsal stream regions. Moreover, and as expected, there was no RS for animals (all Fs < 1) or nonmanipulable objects (the lowest p = .22) in any of the dorsal stream regions identified by the contrasts of each of the nonliving object types compared to animals (see Figure 4, Supplemental Table S2 and Figure S3 for details). Here we focus on the pattern of findings within the regions showing greater activation for ‘tools’(novel + repeated) compared to animals(novel + repeated).
Figure 4. RS in the dorsal stream modulated by motor-relevant properties of objects.
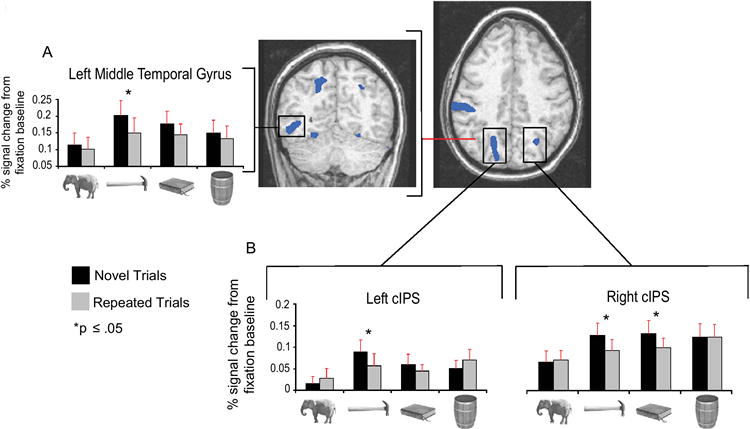
Histograms represent mean (+SEM) BOLD responses by experimental condition, averaged across all voxels (blue) showing a larger response (p < .01) for ‘tools’(novel + repeated) than for animals(novel + repeated). The red line in the axial view indicates the plane of the coronal view. (A) RS was observed for ‘tools’ in the left middle temporal gyrus (1,156mm3; TT: -49, -61, -7; shown at y = -61). (B) RS was restricted to ‘tools’ in the left caudal IPS (Culham et al., 2003) (3,416mm3; TT: -16, -67, 44) and was observed for both ‘tools’ and arbitrarily manipulated objects in right caudal IPS (223mm3; TT: 24, -64, 36) (axial view at z = 34). Caudal IPS is involved in visual analysis of object affordances for object-directed reaching (e.g., Culham et al., 2003).
In the left middle temporal gyrus, RS was observed for only ‘tools’ (p < .03) (see Figure 4A and Supplemental Table S2). As noted in the introduction, this region of lateral temporal cortex is just anterior to motion area MT (Beauchamp et al., 2002), and is involved in motion analysis of nonliving things (e.g., Beauchamp et al., 2003; Kable et al., 2002; Martin, 2007). One possible interpretation of the pattern of BOLD responses in the left posterior middle temporal gyrus is that there is a graded degree across the nonliving object types in the consistency of the movements that are associated with those objects (see Figure 1A for complimentary behavioral findings).
Of particular interest is the pattern of effects observed within the left inferior parietal lobule, known to process complex object-associated actions (e.g., Heilman et al., 1982; Johnson-Frey, 2004). Within the left inferior parietal lobule, there was a reliable BOLD response on novel trials only for ‘tools,’ and RS for only this stimulus type (p < .02) (Figure 5A). The BOLD responses to novel animals and novel arbitrarily manipulated objects did not differ from the fixation baseline (ts < 1; one-sample t-tests). For novel nonmanipulable objects, there was a trend toward deactivation (p = .09). These data suggest that the left inferior parietal lobule is a critical structure mediating between processing of object identity and object use. In particular, ‘tools’ were distinguished (psychophysically) from arbitrarily manipulated objects in that for the former, the motor movements associated with their use are instrumental in determining their function (see Figure 1B).
Figure 5. RS restricted to ‘tools’ in the left inferior parietal lobule.
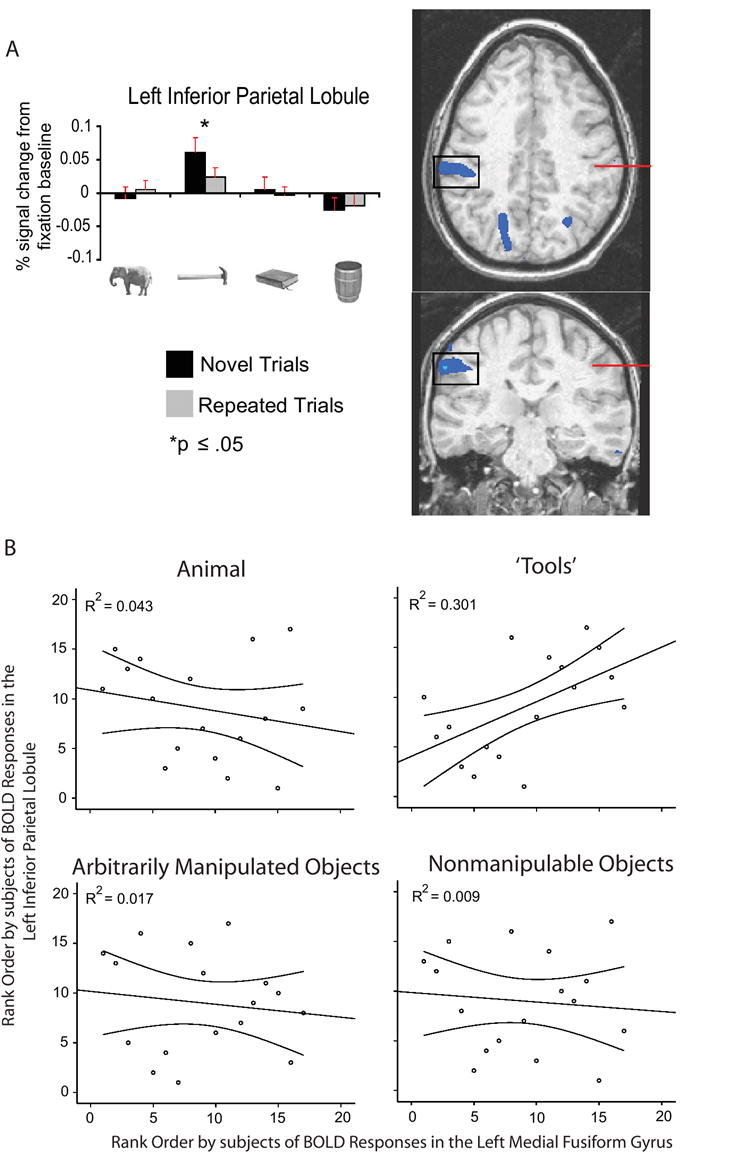
A. Histograms represent mean (+SEM) BOLD responses by experimental condition, averaged across all voxels (blue) showing a larger response (p < .01) for ‘tools’(novel + repeated) than for animals(novel + repeated). The red line in the axial view indicates the plane of the coronal view, and vice versa. The left inferior parietal lobule (Frey et al., 2005) (1,600mm3; TT: -57, -27, 34; axial view at z = 36) showed a reliable BOLD response on novel trials only for ‘tools’, and RS for only this stimulus type. B. The beta estimates for BOLD signal changes were extracted for all subjects from the left medial fusiform gyrus region (see also Supplementary Figure S1) and the left inferior parietal region described in (A) above (both regions defined by ‘tools’(novel + repeated) versus animals(novel + repeated)). Inter-subject rankings in β estimates of BOLD responses(novel + repeated) were reliably correlated (Spearman) between these two regions only for ‘tools.’ These data indicate a selective relationship in the distributions across subjects of BOLD responses in the left medial fusiform gyrus and the left inferior parietal lobule (see also Figure 8).
Interestingly, the similarity in neural responses between the left medial fusiform gyrus and the left inferior parietal lobule was not limited to analyses of RS. As depicted in Figure 5B, there was a reliable and positive correlation between overall BOLD responses(novel + repeated) in the left medial fusiform gyrus and the left inferior parietal lobule only for ‘tool’ stimuli (for the full correlation matrix, see Supplemental Figure S4). These data support the view that neural specificity for ‘tools’ in the left medial fusiform gyrus is related to processing in the left inferior parietal lobule.
Lesion overlap analysis
A large body of evidence (e.g., Goodale and Milner, 1992; James et al., 2002; Shmuelof and Zohary, 2005) indicates that the ventral and dorsal object-processing streams can operate relatively autonomously with respect to one another, and that object processing in the two streams occur largely in parallel (e.g., Fang and He, 2005). Damage to the ventral stream can lead to an inability to identify visually presented objects, despite normal object-directed action (visual-agnosia-without-optic-ataxia). Damage to dorsal occipital and posterior parietal regions can lead to an impairment for object-directed grasping despite intact object identification (optic-ataxia-without-visual-agnosia) (for review see Goodale and Milner, 1992). It is also known that damage to the left inferior parietal lobule can lead to impairments for using objects, despite intact object identification (for reviews see Johnson-Frey, 2004; Mahon and Caramazza, 2005). These neuropsychological dissociations mean that the integrity of neural processes mediating object grasping and use are not necessary in order for successful object identification to occur. However, it remains an open possibility, whether inputs from dorsal structures mediating object-directed action modulate the efficacy of ventral stream processing.
To address these issues, we studied the lesion correlates in a group of 42 patients associated with impairments for using and identifying objects. All patients were administered object identification and object use tasks, always using the same set of objects within each patient. The objects consisted primarily of ‘tools’, as defined herein (see Experimental Procedures for a list of the materials). Patients were asked to demonstrate the use of, and to name, each real object (for details on the neuropsychological background of the patients, see Negri et al., under revision). Lesioned voxels for each patient were mapped in standard space (Tzourio-Mazoyer et al., 2002), and the lesions of all patients were overlaid on the same template. We then determined which lesioned voxels predicted (p < .05; Liebermeister analysis) impairments for identifying objects, and in a separate analysis, using objects. Critically, lesion overlap was calculated for each task separately (e.g., object identification), independently of performance on the other task (e.g., object use). The results of this analysis are depicted in Figure 6A. Damaged voxels associated with impairments for using objects are colored red, while damaged voxels associated with impairments for identifying objects are colored blue. Regions that were found to be lesioned in both analyses are colored purple. Lesion overlap between the two tasks was concentrated in left parietal cortex, the left middle temporal gyrus, and the left inferior frontal gyrus. Notably, a similar region of the left inferior parietal lobule that showed RS biased toward ‘tools’ (Figure 5A) in the fMRI experiment was associated with impaired performance in both object use and object identification.
Figure 6. Lesion overlap analysis for object use, object identification, and object decision.
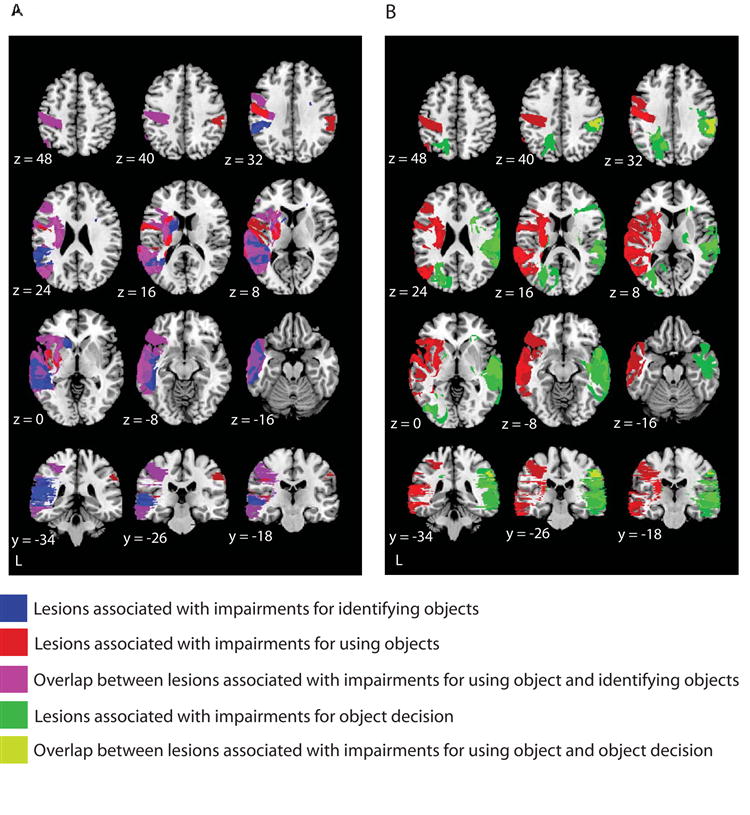
Liebermeister analysis (MRIcron; http://www.sph.sc.edu/comd/rorden/mricron/) was used to detect reliable lesion overlap in a group of 42 patients, all of whom completed the same three tasks: object use, object identification, and object decision (deciding whether drawings of objects depicted real or unrelated objects: VOSP, Warrington and James, 1991). The statistical threshold was set at p < .05 (z = 1.65). (A) Lesioned voxels associated with impairments for object use are shown in red, and impairments in object identification are shown in blue; overlap between the two tasks is shown in purple. Of particular interest, there was overlap in the left parietal cortex, the left inferior frontal gyrus, and the left middle temporal gyrus. (B) Lesioned voxels associated with impairments for object use are shown in red, and with impairments for object decision are shown in green; overlap in lesions associated with the two tasks is shown in yellow. Consistent with previous analyses (Warrington and James, 1991), impairments for object decision were associated with primarily right hemisphere lesions. Overlap between lesions associated with deficits for object use and object decision was observed in the right parietal cortex. This shows that the critical region of left parietal cortex (see A above) does not show overlap between impairments for object use and any task requiring fine grained visual analysis of objects.
In a second analysis, the 42 patients were separated into two groups according to whether or not their lesions involved the parietal cortex (in either the left or the right hemispheres). We then computed correlations (Pearson), separately within each group, between performance in using objects and performance in identifying objects. The results of this analysis are depicted in Figure 7. There was a reliable correlation (R2 = .595, p < .001) only in the group of patients with lesions involving the parietal cortex (for the group not having lesions involving the parietal cortex, R2 = .013; p = .62; see also Figures 7 and S5 for further analyses).
Figure 7. Behavioral performance of patients according to anatomy of lesions.
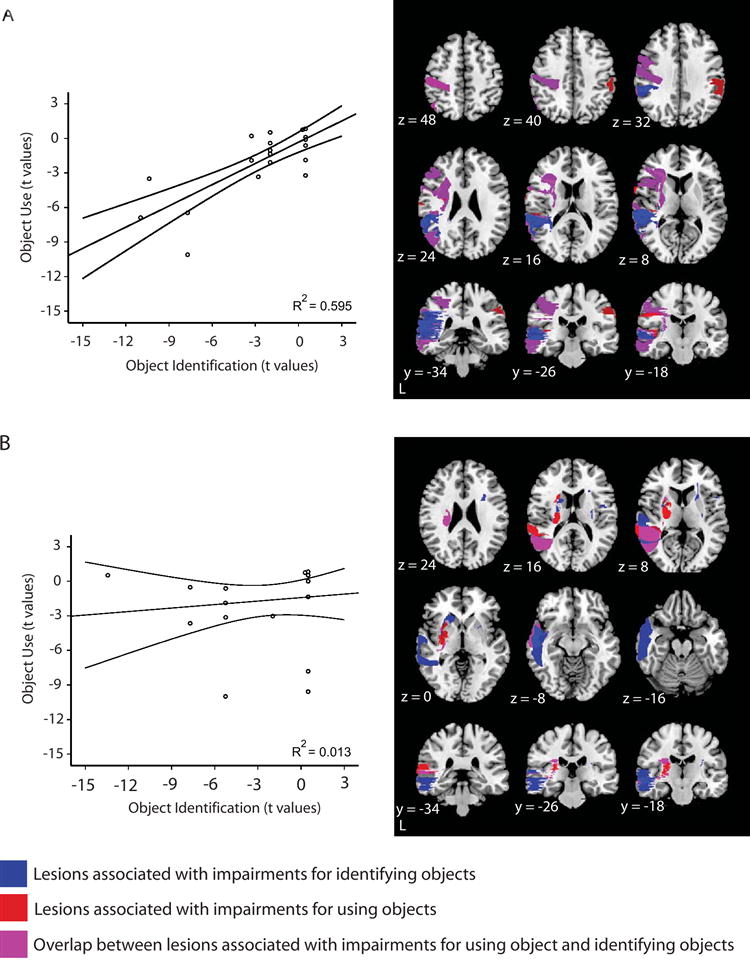
In a second level analysis, the 42 patients were separated into two groups according to whether or not their lesions involved parietal cortex (either the left or right hemispheres). There were 21 patients with lesions involving the parietal cortex (shown in A) and 21 whose lesions did not involve the parietal cortex (shown in B). The lesion overlap analyses were recomputed, as described in Figure 6, and we also studied the relationship between performance in object use and object identification in the two groups. (A) The behavioral performance in object identification and object use for the group of patients with lesions involving the parietal cortex was correlated at the group level. As can be seen, there was a strong relationship at the group level between performance profiles in these two tasks (R2 = .595, p < .001). For comparison, we carried out the parallel correlational analysis relating performance in object use and object decision; there was no relationship (R2 = .036; p = .41). (B) There was no relationship between performance in object use and object identification at the group level for patients with lesions that did not involve parietal cortex (R2 = .013; p = .62). There was also no relationship between performance in object use and object decision (R2 = .016; p = .59). As can be seen in the axial slices, there remained reliable lesion overlap in the left middle temporal gyrus that was associated with joint impairments for object use and object identification.
It is important to note that in both groups of patients (those with, and those without parietal cortex lesions) lesions to the left middle temporal gyrus were associated with impairments for object identification and object use. This is in accord with previous research (e.g., Damasio et al., 2004; Tranel et al., 1997) that has documented that lesions to the left middle temporal gyrus are associated with ‘conceptual’ impairments for manipulable objects. The correlational analyses between performance in object use and object identification indicate that lesions to the left middle temporal gyrus do not, in and of themselves, result in modulation of performance in both tasks. This is because, while both groups of patients had reliable lesion overlap in the left middle temporal gyrus, only in the group of patients with lesions involving the parietal cortex was there a reliable relation between performance in object use and object identification The implication is that damage to parietal cortex, in the context of damage to the left middle temporal gyrus, modulates the relationship between object use and object identification at the group level.
Functional connectivity analyses of fMRI data and their relation to regions of lesion overlap
As noted in the introduction, previous research has described anatomical connections between ventral temporal cortex and both lateral temporal cortex and the inferior parietal lobule (Rushworth et al., 2006; Saleem et al., 2000; Webster et al., 1994; Zhong et al., 2003). In order to provide a more stringent test of the view that neural specificity in the ventral stream is tied to neural specificity in the dorsal stream, we carried out functional connectivity analyses over the BOLD responses in the fMRI experiment (Gitelman et al., 2003) (see Supplemental Online Experimental Procedures for details). The seed voxel for these analyses was the peak response in the left medial fusiform gyrus for the contrast of all nonliving object types compared to animals (see Figure 2). In this way, the seed voxel for the analysis was not defined either in terms of the stimulus type of interest (‘tools’) or the dependent measure of interest (stimulus-specific RS). We then correlated RS effects, within stimulus-type, between all voxels in the brain and the seed voxel. This analysis defined regions in the left middle temporal gyrus and the left inferior parietal lobule that showed RS for only ‘tools’ (see histograms in Figure 8 for a representation of RS effects by stimulus type in these regions). This analysis demonstrates functional connectivity between the left medial fusiform gyrus and the left middle temporal gyrus, as well as between the left medial fusiform gyrus and the left inferior parietal lobule.
Figure 8. Overlay of connectivity analyses and lesion overlap analyses.
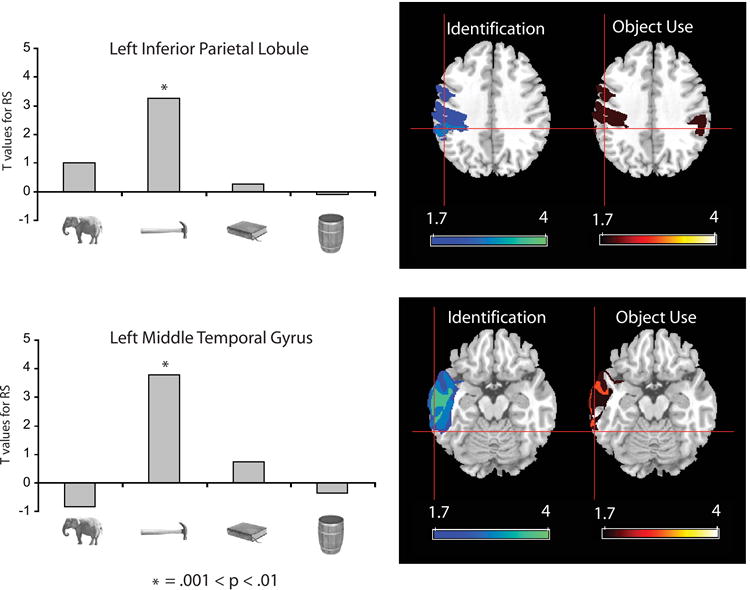
Functional connectivity analyses were conducted on the event related fMRI data. The seed voxel for this analysis was the peak response in the left medial fusiform gyrus showing greater activation for All Nonliving objects compared to animals. Voxels throughout the brain were then identified that showed a reliable correlation with the seed voxel in terms of stimulus-specific RS, separately for each of the stimulus types. Regions identified by this analysis included the left middle temporal gyrus (TT: -64, -47, -12) and the left inferior parietal cortex (TT: -51, -34, 36), both of which showed RS only for ‘tools’ (see Supplemental Table S8 for all regions identified by this analysis). The histograms represent the strength (t-values) of the RS effects in these regions for each stimulus type. Negative t-values indicate that the difference between novel and repeated trials (in e.g., the left middle temporal gyrus) was negatively correlated with the corresponding difference in the seed voxel in the left medial fusiform gyrus. The results of the functional connectivity analysis were then overlaid in Talairach space with the results of the lesion overlap analysis (see text and Figures 6 and 7). The axial slices (z = 36) in the top row show voxels in parietal cortex associated with impairments for identifying objects (left) and using objects (right). The axial slices (z = -12) in the bottom row show voxels in temporal cortex associated with impairments for identifying objects (left) and using objects (right). Color bars indicate z scores for lesion overlap calculated separately for each task. Cross-hairs on all slices show the locations in inferior parietal cortex (top) and lateral temporal cortex (bottom) that were identified through the functional connectivity analysis on the fMRI data. There is anatomical convergence between the two analyses. Together with the results displayed in Figure 7, these data demonstrate functional interactions between the left inferior parietal lobule, the left middle temporal gyrus, and the left medial fusiform gyrus.
We then overlaid in standard space the results of this functional connectivity analysis with the results of the lesion analysis described above. As can be seen in Figure 8, the regions of the left middle temporal gyrus and the left inferior parietal lobule identified by the functional connectivity analysis corresponded to regions of reliable lesion overlap independently defined in the neuropsychological study.
Discussion
Three new findings have been reported:
(1) Using stimulus-specific RS, we found that neural specificity in the left medial fusiform gyrus tracks motor-relevant properties of objects. Importantly, the pattern of RS in the left medial fusiform gyrus according to motor-relevant properties of objects cannot be explained by differential similarity in visual shape between the items within the different stimulus types (see Figure 1D). A similar pattern of RS restricted to ‘tools’ was observed in the left middle temporal gyrus and the left inferior parietal lobule.
(2) Using the neuropsychological approach, we found that lesions to the left inferior parietal lobule, together with lesions to the left middle temporal gyrus, modulated the relationship between performance on object identification and object use at the group level.
(3) Functional connectivity analyses of the fMRI data demonstrated that the RS effect for ‘tools’ in the left medial fusiform gyrus predicted RS effects for ‘tools’ in the left inferior parietal lobule and the left middle temporal gyrus. The regions of the left middle temporal gyrus and the left inferior parietal lobule identified by the functional connectivity analysis corresponded to the regions independently identified in the lesion analysis.
On the basis of these data we suggest that neural specificity in the left medial fusiform gyrus for ‘tools’ is determined, in part, by similarity metrics that are computed over motor-relevant properties of objects. The convergence of the fMRI and neuropsychological data indicate that the left middle temporal gyrus and the left inferior parietal lobule represent, at least in part, the similarity metrics that determine neural specificity for ‘tools’ in ventral temporal-occipital cortex. The neural circuit that is composed of the left medial fusiform gyrus, the left middle temporal gyrus, and the left inferior parietal lobule is ‘domain-specific’. By ‘domain-specific’, it is meant that the circuit can be defined with respect to the content of the object class that is processed, independently of the different types of information (i.e., form, motion, action) that are processed by different components of the circuit (for discussion, see Caramazza and Mahon, 2006; Martin, 2007).
Relation between overall BOLD responses and stimulus-specific RS
An important aspect of the pattern of BOLD responses in the ventral stream is that they demonstrate a dissociation between category preferences, as indexed by the amplitude of BOLD responses on novel trials, and stimulus-specific RS. In the medial fusiform gyri, RS was strongest for ‘tools’ despite the fact that there was either no difference on novel trials among the nonliving object types, or novel nonmanipulable objects elicited greater activation than ‘tools.’ These findings indicate that the overall amplitude of BOLD responses cannot be taken, in and of itself, as an indication of neural specificity.
Previous studies have used overall BOLD responses to test for dissociations within the medial fusiform gyrus between different types of nonliving things. In particular, Downing and colleagues (2006) tested a wide range of different classes of nonliving objects and found that the amplitude of BOLD responses did not distinguish between the different nonliving stimulus types. On the basis of those data, the authors concluded that there is neural specificity in the ventral stream for only faces, places, and body parts. The pattern of BOLD responses on novel trials that we have reported (see Figure 2) essentially replicates what Downing and colleagues reported. However, and as discussed above, overall BOLD responses cannot be taken, in and of themselves, as an index of neural specificity. Thus, contrary to recent arguments (Downing et al., 2006; Mechelli et al., 2006; Tyler et al., 2003) the findings reported herein demonstrate neural specificity in the left medial fusiform gyrus for ‘tools’.
The observation that initial BOLD responses to nonmanipulable objects were large in amplitude throughout the medial fusiform gyrus bilaterally may indicate a high density of neurons that respond to nonmanipulable objects. The fact that the same medial fusiform regions show very little, if any, stimulus-specific RS for nonmanipulable objects may suggest that there is substantial adaptation of the BOLD response across different novel presentations of the nonmanipulable objects. Following this reasoning, it would follow that neurons in the medial fusiform gyrus are ‘broadly tuned’ to nonmanipulable objects, whereas neurons in parahippocampal cortex are ‘finely tuned’ to nonmanipulable (cf spatially contextualized) objects. Similarly, the strong stimulus-specific RS observed for ‘tools’ throughout the left medial fusiform gyrus indicates a high density of neurons that are ‘finely tuned’ to this object type.
Two previous studies used RS to study neural specificity for different types of objects in the ventral stream. Avidan and colleagues (2002) studied the categories of houses and faces, while Chao and colleagues (2002) studied the categories of ‘tools’ and animals. Both studies observed category-preferences, as measured through overall BOLD responses, with nonliving and living things differentially activating distinct regions of ventral temporal-occipital cortex. However, in each study, equivalent RS effects were observed for the preferred as well as the non-preferred category. One possible reason why those studies did not observe modulation of RS effects by stimulus type is because both studies used designs in which either all repeated or all novel stimuli were presented within a block. Such a design may lead to stimulus non-specific modulations in attention (e.g., Wojciulik et al., 1998) that mask biases in RS by stimulus type.
Conclusion
Clearly, a single dimension is not sufficient to explain all aspects of the organization of the ventral object-processing stream (e.g., Haxby et al., 2001; Levy et al., 2001). However, given the pattern of findings reported herein, one may speculate as to whether neural specificity in the ventral stream for other types of stimuli, such as faces and animals, words, and places, may also depend in part on functional connectivity with neural systems outside (or ‘downstream’) from ventral temporal-occipital cortex (Caramazza and Mahon, 2006). For instance, neural specificity for faces in the lateral fusiform gyrus (Fusiform Face Area) may be driven, in part, by functional connectivity between this region and regions of the brain mediating affective reactions, such as the amygdala (e.g., Kreiman et al., 2000). Similarly, it may be reasonable to speculate that neural specificity for written language in the visual word form area may be driven in part by functional connectivity with regions of the brain mediating language processing (Martin, 2006). An important issue for future research is thus whether functional connectivity between the ventral stream and structures outside of the ventral stream is a general organizing principle giving rise to category-specificity in the primate visual system.
Experimental Procedures
fMRI Experimental Stimuli and Design
There were twenty distinct items within each of the four stimulus types, and four exemplars of each item (total n = 320). The twenty items within each stimulus type were divided into two sets, A and B. Thus, each set within a stimulus type had 40 different pictures (4 exemplars each of 10 different items). A given participant (both for the fMRI study and the behavioral study on naming latencies) either saw set A three times and Set B once, or the reverse. The factor Set (A and B) was counterbalanced across participants, so that across all participants, the same materials appeared as novel and repeated trials. This design resulted in 640 trials for each subject (e.g., [Set A: 40 X 3 Presentations X 4 Stimulus Types] + [Set B: 40 X 1 Presentation X 4 Stimulus types]). After collapsing the first presentations of stimuli from sets A and B into the ‘novel’ condition, and the second and third presentations into the ‘repeated condition’, there were 80 novel trials per stimulus type and 80 repeated trials per stimulus type. Stimuli from all four stimulus types, for both novel and repeated events, were intermixed throughout the entire experiment (see Supplemental Online Experimental Procedures).
MR Data Collection Parameters
Seventeen subjects (10 female) were recruited from the NIH Healthy Volunteer pool and paid for their participation in the study. Informed consent was obtained in writing under an approved National Institute of Mental Health protocol. fMRI data were collected at the NIH Clinical Center NMR Research Facility on a GE Signa 3 Tesla scanner using standard imaging procedures. Before collecting experimental data, a high-resolution SPGR anatomical sequence (124 axial slices, 1.2 mm thickness, FOV=24cm, Acquisition matrix = 256×256) was performed. Data were collected using an echo-planar series, with a TR of 2000 ms, and a TE of 30 ms with 3.75 mm in-plane resolution. Volumes were collected in the axial plane in 24 contiguous, interleaved slices.
fMRI Data Analysis
All MR data were analyzed using the AFNI software package (Cox, 1996). To account for motion artifacts, all scan series were registered to the volume acquired closest to the high-resolution anatomy. Then a spatial filter with a 4.5 mm full width half-maximum Gaussian filter was applied to each volume. For each individual subject, echo-planar and anatomical volumes were transformed into the standardized Talairach and Tournoux (TT) volume (Talairach and Tournoux, 1988).
A random effects approach within the general linear model in AFNI was used to analyze the data. The response to each stimulus type and repetition compared to the fixation baseline was calculated using multiple regression. There were eight regressors of interest: two for each of the four stimulus types to represent the novel and repeated stimuli, and six regressors of no interest (6 outputs from volume registration to account for residual variance from subject motion not corrected by registration). Within each regressor of interest, delta functions representing the response following stimulus presentation at each full volume echo-planar acquisition (2 s) in a 12 s window were fit to the MR signal, resulting in an estimate of the response to a single stimulus of each stimulus type and repetition with no assumptions about the shape of the hemodynamic response. This resulted in a 12 s time series for each stimulus type in each voxel.
The amplitude of the response to the stimulus was estimated by summing the β weights of the regressors representing TRs 2 through 4. The regression model of the estimated amplitude provided a single estimate of the response to each stimulus type in each voxel for each subject. A two-way mixed effects ANOVA was performed on each voxel in standardized space. Fixed effects contrasts of the stimulus and repetition variables were performed, with subjects acting as the random effect repeated measure.
Analysis of RS effects biased toward each stimulus type
Analyses were performed to isolate voxels showing RS biased toward each stimulus type (Figures 3B & S3). First, mask files of the RS effect (novel > repeated) were created for each of the four stimulus types. Next, the four masks were overlaid to create a color map illustrating areas where RS was significant for only a single stimulus type, as well as regions of overlap for ‘tools’ and arbitrarily manipulated objects.
All TT coordinates reported in the manuscript refer to the location of the voxel showing the greatest difference for that given contrast.
Lesion overlap study
Forty-two consecutive patients with unilateral strokes were recruited from the rehabilitation ward of the Ospedali Riuniti in Trieste (age: mean = 64.2; Standard Deviation (SD) = 11.0 years; education: mean = 9.4; SD = 3.7 years). Only patients with no previous neurological history, and who had CT- or MRI-scans available were included. The lesions in each patient were mapped into standard space using MRIcro by a neuroradiologist (M.U.). Twenty-five neurologically healthy individuals matched for age and education (age: mean = 66; SD = 11; education: mean = 8.96; SD = 4.1) with the patient group were recruited from patients’ and staff’s relatives, as well as from the rehabilitation ward of the Ospedali Riuniti in Trieste, where they were treated following orthopedic surgery. The Revised Standardized Difference Test (RSDT) was used to detect impaired performance for single patients against controls (Crawford & Garthwaite, 2006). The scatter plots in Figure 7 plot modified t-values as calculated with the procedure described in Crawford and Garthwaite (2006). Thirty-five of the patients completed the object identification and object use task using the same set of 29 real objects (bottle, cigarette, coffee mug, comb, dust cloth, eraser, fork, glass, gun, hammer, iron, jug, key, knife, ladle, lemon squeezer, light bulb, lipstick, match stick, paintbrush, pen, razor, saw, scissors, screwdriver, wrench, spoon, tennis racket, tooth brush). Seven patients completed the same tasks with a subset (n = 20) of those objects (excluding: bottle, dust cloth, fork, glass, knife, ladle, match stick, racket, tooth brush). Patients were compared to controls taking into account only the items in common to patients and controls (control performance for all 29 items: naming: mean = 99.2; standard deviation (SD) = 1.5; object use: mean = 96.3; SD = 4.4; control performance for 20 items: naming: mean = 99.6; SD = 1.4; object use: mean = 96.5; SD = 4.6). No feedback (either positive or negative) was given to either patients or controls during testing. For further details of the testing procedures, see Supplemental Online Experimental Procedures, and Negri, Rumiati, Zadini, Ukmar, Mahon and Caramazza (under revision).
Supplementary Material
Acknowledgments
The fMRI study was supported by the National Institute of Mental Health, Division of Intramural Research. Preparation of this manuscript was supported by an NSF Graduate Research Fellowship to BZM and NIH grant DC0542 to AC. We would like to thank Jessica Gilbert and Kim Vargas for their help in collecting data, Maja Ukmar for assistance in mapping the lesions of the patients, Sabrina Aristei for assistance with the analysis of shape similarity, and Gabriele Miceli, Jens Schwarzbach, and Massimo Turatto for their comments on earlier drafts of this manuscript. We are grateful to Antonella Zadini for referring the patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avidan G, Hasson R, Hendler T, Zohary E, Malach R. Analysis of the neuronal selectivity underlying low fMRI signals. Curr Biol. 2002;12:964–972. doi: 10.1016/s0960-9822(02)00872-2. [DOI] [PubMed] [Google Scholar]
- Barr M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. Parallel Visual Motion Processing Streams for Manipulable Objects and Human Movements. Neuron. 2002;34:149–159. doi: 10.1016/s0896-6273(02)00642-6. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. fMRI Responses to Video and Point-Light Displays of Moving Humans and Manipulable Objects. J Cognit Neurosci. 2003;15:991–1001. doi: 10.1162/089892903770007380. [DOI] [PubMed] [Google Scholar]
- Belongie S, Malik J, Puzicha J. Shape matching and object recognition using shape contexts. Transactions on pattern analysis and machine intelligence. 2002;24:509–522. doi: 10.1109/TPAMI.2005.220. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Mahon BZ. The organisation of conceptual knowledge in the brain: The future’s past and some future directions. Cognit Neuropsychol. 2006;23:13–38. doi: 10.1080/02643290542000021. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A, Haxby JV. Are face-responsive regions selective only for faces? Neuroreport. 1999a;10:2945–2950. doi: 10.1097/00001756-199909290-00013. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in posterior temporal cortex for perceiving and knowing about objects. Nat Neurosci. 1999b;2:913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A. Representation of manipulable man-made objects in the dorsal stream. Neuroimage. 2000;12:478–484. doi: 10.1006/nimg.2000.0635. [DOI] [PubMed] [Google Scholar]
- Chao LL, Weisberg J, Martin A. Experience dependent modulation of category-related cortical activity. Cereb Cortex. 2002;12:545–551. doi: 10.1093/cercor/12.5.545. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Garthwaite PH. Methods of testing for a deficit in single case studies: Evaluation of statistical power by Monte Carlo simulation. Cognit Neuropsych. 2006;23:877–904. doi: 10.1080/02643290500538372. [DOI] [PubMed] [Google Scholar]
- Culham JC, Danckert SL, DeSouza JF, Gati JS, Menon RS, Goodale MA. Visually guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Exp Brain Res. 2003;153:180–189. doi: 10.1007/s00221-003-1591-5. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognit. 2004:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Schnyer DM, Verfaellie M, Schacter DL. Cortical activity reductions during repetition priming can result from rapid response learning. Nature. 2004;428:316–319. doi: 10.1038/nature02400. [DOI] [PubMed] [Google Scholar]
- Downing PE, Chan AW-Y, Peelen MV, Dodds CM, Kanwisher N. Domain specificity in visual cortex. Cereb Cortex. 2006;16:1453–1461. doi: 10.1093/cercor/bhj086. [DOI] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. The Parahippocampal Place Area: Recognition, Navigation, or Encoding? Neuron. 1999;23:115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Fang F, He S. Cortical responses to invisible objects in the human dorsal and ventral pathways. Nat Neurosci. 2005;8:1380–1385. doi: 10.1038/nn1537. [DOI] [PubMed] [Google Scholar]
- Frey SH, Vinton D, Norlund R, Grafton ST. Cortical topography of human anterior intraparietal cortex active during visually guided grasping. Cogn Brain Res. 2005;23:397–405. doi: 10.1016/j.cogbrainres.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. NeuroImage. 2003:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Rothi LJ, Valenstein E. Two forms of ideomotor apraxia. Neurology. 1982;32:342–346. doi: 10.1212/wnl.32.4.342. [DOI] [PubMed] [Google Scholar]
- James TW, Humphrey GK, Gati JS, Menon RS, Goodale MA. Differential effects of viewpoint on object-driven activation in dorsal and ventral streams. Neuron. 2002;35:793–801. doi: 10.1016/s0896-6273(02)00803-6. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH. The neural basis of complex tool use in humans. Trends Cogn Sci. 2004;8:71–78. doi: 10.1016/j.tics.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH, Newman-Norlund R, Grafton ST. A distributed left hemisphere network active during planning of everyday tool use skills. Cereb Cortex. 2005;15:681–695. doi: 10.1093/cercor/bhh169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Kan IP, Wilson A, Thompson-Schill SL, Chatterjee A. Conceptual Representations of Action in the Lateral Temporal Cortex. J Cognit Neurosci. 2002;17:1855–1870. doi: 10.1162/089892905775008625. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Stanley D, Harris A. The fusiform face area is selective for faces not animals. Neuroreport. 1999;10:183–187. doi: 10.1097/00001756-199901180-00035. [DOI] [PubMed] [Google Scholar]
- Kreiman G, Koch C, Fried I. Category-specific visual responses of single neurons in the human medial temporal lobe. Nat Neurosci. 2000;3:946–953. doi: 10.1038/78868. [DOI] [PubMed] [Google Scholar]
- Levy I, Hasson U, Avidan G, Hendler T, Malach R. Center-periphery organization of human object areas. Nat Neurosci. 2001;4:533–539. doi: 10.1038/87490. [DOI] [PubMed] [Google Scholar]
- Lewis JW. Cortical Networks Related to Human Use of Tools. Neuroscientist. 2006;12:211–231. doi: 10.1177/1073858406288327. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. The orchestration of the sensory-motor systems: Clues from neuropsychology. Cogn Neuropsychol. 2005;22:480–494. doi: 10.1080/02643290442000446. [DOI] [PubMed] [Google Scholar]
- Martin A. Shades of Déjerine—Forging a Causal Link between the Visual Word Form Area and Reading. Neuron. 2006;50:173–175. doi: 10.1016/j.neuron.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG. Discrete cortical regions associated with knowledge of color and knowledge of action. Science. 1995;270:102–105. doi: 10.1126/science.270.5233.102. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Sartori G, Orlandi P, Price CJ. Semantic Relevance explains category effects in medial fusiform gyri. Neuroimage. 2006;3:992–1002. doi: 10.1016/j.neuroimage.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Miceli G, Fouch E, Capasso R, Shelton JR, Tomaiuolo F, Caramazza A. The dissociation of color from form and function knowledge. Nat Neurosci. 2001;4:662–667. doi: 10.1038/88497. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. The temporal lobe is a target of output from the basal ganglia. Proc Natl Acad Sci. 1996;93:8683–8687. doi: 10.1073/pnas.93.16.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Nieder A, Freedman DJ, Wallis JD. Neural correlates of categories and concepts. Curr Opin in Neurobio. 2003;13:198–203. doi: 10.1016/s0959-4388(03)00037-0. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ, Penny WD, Friston KJ. Two distinct neural mechanisms for category-selective responses. Cereb Cortex. 2006;16:437–445. doi: 10.1093/cercor/bhi123. [DOI] [PubMed] [Google Scholar]
- Okada T, Tanakac S, Nakaid T, Nishizawa S, Inuic T, Sadatob N, Yonekurae Y, Konishia J. Naming of animals and tools: a functional magnetic resonance imaging study of categorical differences in the human brain areas commonly used for naming visually presented objects. Neurosci Lett. 2000;296:33–36. doi: 10.1016/s0304-3940(00)01612-8. [DOI] [PubMed] [Google Scholar]
- Op de Beeck H, Wagemans J, Vogels R. Inferotemporal neurons represent low-dimensional configurations of parameterized shapes. Nat Neurosci. 2001;4:1244–1252. doi: 10.1038/nn767. [DOI] [PubMed] [Google Scholar]
- Pisella L, Binkofski F, Lasek K, Toni I, Rossetti Y. No double-dissociation between optic ataxia and visual agnosia: Multiple sub-streams for multiple visuo-manual integrations. Neuropsychologia. 2006;44:2734–2748. doi: 10.1016/j.neuropsychologia.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G. The cortical motor system. Neuron. 2001;31:889–901. doi: 10.1016/s0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- Rumiati RI, Weiss PH, Shallice T, Ottoboni G, Noth J, Zilles K, Fink GR. Neural basis of pantomiming the use of visually presented objects. NeuroImage. 2004;21:1224–1231. doi: 10.1016/j.neuroimage.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Behrens TEJ, Johansen-Berg H. Connection patterns distinguish 3 regions of human parietal cortex. Cereb Cortex. 2006;16:1418–1430. doi: 10.1093/cercor/bhj079. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Suzuki W, Tanaka K, Hashikawa T. Connections between Anterior Inferotemporal Cortex and Superior Temporal Sulcus Regions in the Macaque Monkey. J of Neurosc. 2000;20:5083–5101. doi: 10.1523/JNEUROSCI.20-13-05083.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuelof L, Zohary E. Dissociation between ventral and dorsal fMRI activation during object action recognition. Neuron. 2005;47:457–470. doi: 10.1016/j.neuron.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Tranel D, Damasio H, Damasio AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997;35:1319–1327. doi: 10.1016/s0028-3932(97)00085-7. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Bright P, Dick E, Tavares P, Pilgrim L, Fletcher P, Greer M, Moss H. Do semantic categories activate distinct cortical regions? Evidence for a distributed neural semantic system. Cogn Neuropsychol. 2003;20:541–559. doi: 10.1080/02643290244000211. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG. Functional Brain Imaging Studies of Cortical Mechanisms for Memory. 1995;270:769–775. doi: 10.1126/science.270.5237.769. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. Cambridge, Ma: The MIT Press; 1982. pp. 549–586. [Google Scholar]
- Warrington EK, James M. The Visual Object and Space Perception Battery. Bury St. Edmunds, UK: Thames Valley Test Company; 1991. [Google Scholar]
- Webster MJ, Bachevalier J, Ungerleider LG. Connections of inferior temporal areas TEO and TE with parietal and frontal cortex in macaque monkeys. Cereb Cortex. 1994;4:470–483. doi: 10.1093/cercor/4.5.470. [DOI] [PubMed] [Google Scholar]
- Wojciulik E, Kanwisher N, Driver J. Covert visual attention modulates face-specific activity in the human fusiform gyrus: fMRI study. J Neurophysiol. 1998;79:1574–1578. doi: 10.1152/jn.1998.79.3.1574. [DOI] [PubMed] [Google Scholar]
- Zhong Y-M, Rockland KS. Inferior parietal lobule projections to anterior inferiortemporal cortex (area TE) in Macaque monkey. Cereb Cortex. 2003;13:527–540. doi: 10.1093/cercor/13.5.527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


