Abstract
Background
The chronic effects of interleukin 1-beta (IL-β) on vascular reactivity include augmentation of contraction and relaxation. Few studies have assessed the acute effects of IL-1β in vessels from hypertensive and normotensive rats. We hypothesized that IL-1β would enhance constriction in aorta from stroke-prone spontaneously hypertensive rats (SHRSP).
Methods
Endothelium denuded aortic rings from 12 week-old SHRSP and Wistar Kyoto (WKY) rats were mounted in a myograph and incubated with IL-1β (20ng/ml) for 1 hour before construction of a phenylephrine dose response curve. Indomethacin (1μM) and PP-2 (1μM) were utilized to inhibit cyclooxygenase (COX) and Src-kinase respectively.
Results
In aorta from SHRSP, IL-1β caused a significant increase in the force generated over the hour incubation; inhibition of COX or Src-kinase prevented this. The maximum phenylephrine-induced contraction was greater in aorta from SHRSP incubated with IL-1β than control. COX or Src-kinase inhibition prevented this. IL-1β had no effect on the vessels from WKY rats.
Conclusions
These novel data suggest that IL-1β has rapid effects on vascular smooth muscle from hypertensive rats to produce constriction and to enhance phenylephrine-induced constriction. The COX and Src-kinase pathways appear to be involved in this response.
Keywords: Inflammation, hypertension, vasoconstriction
1. Introduction
There is clear evidence that chronic inflammation plays an important role in the pathogenesis of hypertension (Bautista 2003). Hypertensive subjects have higher circulating levels of, and an enhanced capacity to produce proinflammatory cytokines (Dalekos et al. 1997; Peeters et al. 2001). Similarly, rodent models of hypertension exhibit an inflammatory phenotype. The levels of IL-1β and interleukin 6 are increased in the aorta and plasma of spontaneously hypertensive rats (SHR) compared to WKY rats (Sanz-Rosa et al. 2005). Similarly, kidneys from SHRSP have increased levels of inflammatory markers and inflammatory cell infiltration (Sironi et al. 2004).
Reports of the effects of IL-1β on vascular reactivity are conflicting. In vivo treatment with IL-1β increases the responsiveness of mesenteric arteries to vasoconstrictors (De Salvatore et al. 2003) and causes constrictive remodeling and vasospasm in coronary arteries (Morishige et al. 2001). Similarly, ex vivo incubation of temporal arteries with IL-1β for two days causes increased vessel responsiveness to endothelin B receptor agonists (White et al. 2000). In these studies, the duration of the incubation of IL-1β was sufficient for IL-1β to alter gene transcription and translation. However, studies have also shown that a short-term exposure of vessels to IL-1β increases the contractile response to angiotensin II (Vicaut et al. 1996).
Conversely, other studies suggest that IL-1β renders vessels hyporesponsive to vasoconstrictors. Overnight incubation of aortic rings with IL-1β reduces their ability to contract in response to phenylephrine (Soler et al. 2003); this may be due to increased nitric oxide (NO) availability in the IL-1β treated vessels (Dinarello 2002). This increase in NO availability may be caused by increased inducible NO synthase (NOS) expression (Yang et al. 2004) or increased levels of tetrahydrobiopterin, a co-factor for endothelial NOS (Shi et al. 2004).
Few studies have assessed the direct acute effects of IL-1β on vascular smooth muscle cell (VSMC) contractility, and none have compared its effects in hypertensive and normotensive rats. We hypothesized that acute IL-1β treatment would cause constriction in endothelium-denuded aorta from SHRSP and augment the phenylephrine-induced contraction. We also tested the involvement of the COX and Src-kinase pathways in the IL-1β-induced response; both of these pathways have previously been implicated in the pathogenesis of hypertension (Touyz et al. 2001; Touyz et al. 2002; Hermann et al. 2003; Wu et al. 2005).
2 Methods
2.1 Animals
Twelve-week-old male SHRSP were obtained from the breeding colony at the Medical College of Georgia. Age-matched male WKY rats were purchased from Harlan (Indianapolis IN). Rats were maintained on a 12-hour light dark cycle, housed two per cage and allowed access to normal chow and water ad libitum. These studies complied with the protocols for animal use outlined by the American Physiological Society.
2.2 Measurement of Isometric Force Generation
Rats were euthanized with sodium pentobarbital (50mg/kg IP) and the thoracic aorta was removed and placed in ice cold PSS (in mmol/L; NaCl: 130, KCl: 4.7, KH2PO4: 1.18, MgSO4: 1.17, CaCl2: 1.6, NaHCO3: 14.9, dextrose: 5.5, EDTA: 0.03). The aorta was cleaned and cut into 3mm rings. To assess the direct effects of IL-1β on the vascular smooth muscle, the endothelium was removed by gentle rubbing of the luminal surface and the rings were mounted on stainless steel pins in a modified myograph system (DanishMyo Technologies, Aarhus, Denmark). The organ baths contained PSS warmed to 37°C and gassed with 95% O2-5% CO2. A passive tension of 36-37mN was placed on each ring and the rings were allowed to equilibrate for 45 minutes. The rings were then challenged with phenylephrine (10-7M) to ensure tissue viability and with acetylcholine (10-5M) to ensure the absence of an endothelium. A maximum contraction to phenylephrine (10-5M) was obtained, and then vessels were incubated with IL-1β (20ng/ml) (R&D Systems, Minneapolis MN) for 1 hour. When required, indomethacin (1μM) or PP-2 (1μM) (Biomol, Plymouth Meeting PA) were added to the bath 10 minutes prior to the addition of the IL-1β. After the incubation with IL-1β a cumulative phenylephrine does response curve was constructed (10-10-10-5M). Two rings were used per treatment for each experiment and the results obtained from these rings were averaged.
2.3 Statistics
Results are represented as the mean ± standard error of the mean. The contractions in response to IL-1β and phenylephrine are depicted as the increase in force generated from baseline. The baseline for the phenylephrine dose response was taken immediately prior to the addition of the first dose of phenylephrine. Dose response curves were compared by two-way repeated measures ANOVA. EC50 values were calculated using GraphPad Prism (GraphPad Software Inc. San Diego CA). The EC50 values and the IL-1β-induced contraction were compared by a Student’s T-test with a Bonferroni correction for multiple analyses were necessary. A p value of less than 0.05 was deemed to be significant.
2.4 Chemicals
Unless otherwise stated, all chemicals were purchased from Sigma Chemical Company (St. Louis MO).
3 Results
3.1 IL-1β causes contraction in aorta from SHRSP
IL-1β caused a marked increase in the force generated by the aorta from SHRSP but not WKY rats (Figure 1A). Indomethacin and PP-2 completely inhibited the IL-1β-induced contraction in the aorta from SHRSP (Figure 1B). While IL-1β did not increase the generation of tone in the vessels from WKY rats indomethacin and PP-2 both caused a significant reduction in the generation of tone in the vessels incubated with IL-1β (Figure 1C).
Figure 1.
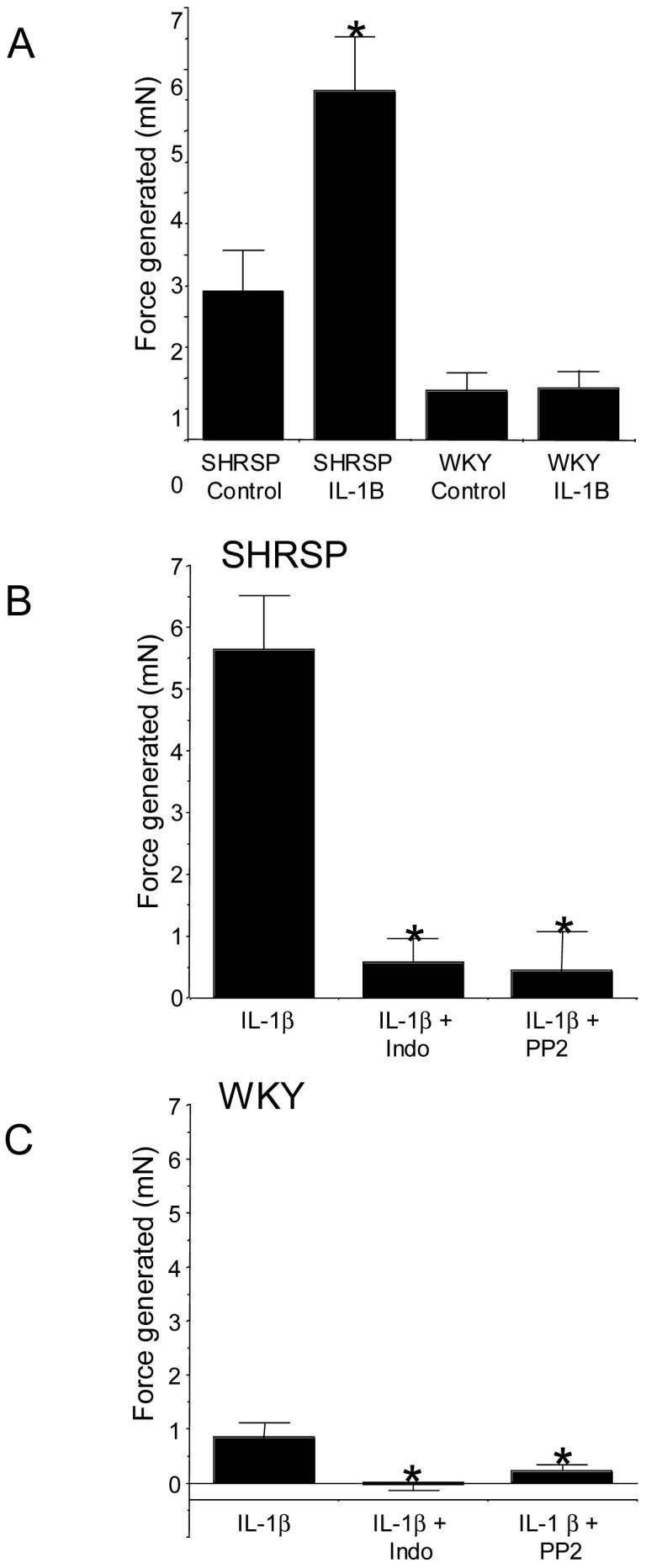
(A) IL-1β caused contraction in aorta from SHRSP (n=9) but not WKY (n=6) rats. Contraction was measured as the mN change in force generation from baseline. * Indicates a significant difference (p<0.05) from the appropriate strain control. (B) Indomethacin (n=5) and PP-2 (n=5) inhibit the IL-1β-induced contraction in aorta from SHRSP, * indicates a significant difference from the IL-1β treated vessels. C. Indomethacin (n=6) and PP-2 (n=6) reduced the force generated by the vessels from WKT rats incubated with IL-1β. WKY - Wistar Kyoto, SHRSP - Stroke prone spontaneously hypertensive rat, IL-1β - interleukin 1 beta, indo - indomethacin.
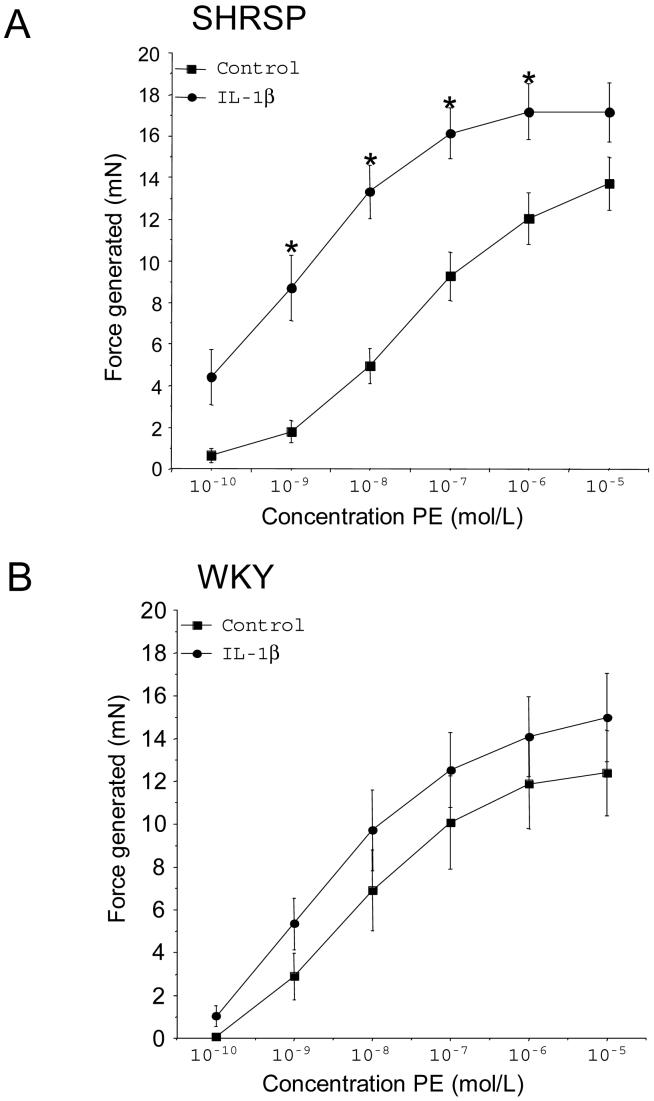
3.2 IL-1β enhances the phenylephrine-induced contraction in aorta from SHRSP
IL-1β caused an increase in the phenylephrine-induced contraction in aorta from SHRSP (Figure 2A). A similar trend was observed in the vessels from WKY rats but this did not reach statistical significance (Figure 2B). The EC50 values for the phenylephrine-induced contraction were lower in aorta from SHRSP incubated with IL-1β compared to control (Log EC50: -8.86±0.2.4 vs -7.59±0.21, IL-1β vs control, p<0.01, n=9). IL-1β did not affect the EC50 value for phenylephrine in aorta from WKY rats (Log EC50; -8.44±0.26 vs -7.91±0.26, IL-1β vs control, n=6).
Figure 2.
Prior incubation of vessels with IL-1β (20ng/ml) enhanced the phenylephrine-induced contraction in aorta from SHRSP (n=9) (A) but not WKY rats (n=6) (B). Results are represented as the increase in force generated from baseline, * indicates a significant difference (p<0.05) from control vessels. IL-1β - interleukin 1 beta, PE - phenylephrine, SHRSP - Stroke prone spontaneously hypertensive rat, WKY - Wistar Kyoto.
When PP-2 was added to the muscle bath prior to the IL-1β it prevented the IL-1β-induced increase in the responsiveness to phenylephrine observed in aorta from SHRSP (Figure 3A) and restored the EC50 value for the phenylephrine-induced contraction to control levels (Log EC50; -7.41±0.23 n=5). PP-2 also caused a reduction in the maximum response of the aorta from WKY rats to phenylephrine (Figure 3B) but did not alter the EC50 value (Log EC50: -6.9±0.22 n=6).
Figure 3.
PP-2 inhibits the IL-1β-induced enhancement of the phenylephrine-induced contraction in aorta from SHRSP (A) and WKY rats (B). Results are represented as the increase in force generated from baseline, * indicates a significant difference (p<0.05) from IL-1β treated vessels. N=9 for SHRSP vessels treated with IL-1β and n=5 for SHRSP vessels treated with IL-1β and PP-2. N=6 for both groups of WKY vessels. IL-1β - interleukin 1 beta, PE - phenylephrine, SHRSP - Stroke prone spontaneously hypertensive rat, WKY - Wistar Kyoto.
Indomethacin prevented the IL-1β-induced increase in the responsiveness to phenylephrine observed in aorta from SHRSP (Figure 4A) and returned the EC50 value for phenylephrine to control levels (Log EC50; -7.35±0.23 n=5). Indomethacin also caused a reduction in the maximum phenylephrine-induced contraction in aorta from WKY rats but did not change the sensitivity of the vessels to phenylephrine (Log EC50: -7.80±0.13 n=6).
Figure 4.
Indomethacin inhibits the IL-1β-induced increase in the vessel response to phenylephrine in aorta from SHRSP (A) and WKY rats (B). Results are represented as the increase in force generated from baseline, * indicates a significant difference (p<0.05) from IL-1β treated vessels. N=9 for SHRSP vessels treated with IL-1β and n=5 for SHRSP vessels treated with IL-1β and indomethacin. N=6 for both groups of WKY vessels. IL-1β - interleukin 1 beta, indo - indomethacin, PE - phenylephrine, SHRSP - Stroke prone spontaneously hypertensive rat, WKY - Wistar Kyoto.
4. Discussion
There were two novel findings from this study. Firstly, IL-1β causes constriction in endothelium-denuded aorta from SHRSP and enhances the responsiveness of aorta to phenylephrine as evidenced by an increase in the maximal phenylephrine-induced contraction and a reduction in the EC50 value for phenylephrine. Secondly, the COX and Src-kinase pathways are involved in the rapid response to IL-1β in aorta from SHRSP. Because endothelium-denuded vessels were used these appear to be VSMC related phenomena. These findings may be of physiological relevance considering the levels of IL-1β are elevated in hypertensive rats (Sironi et al. 2004; Sanz-Rosa et al. 2005). One might anticipate that an increase in the levels of circulating cytokines would result in a down-regulation of their receptor and a reduction in the response to exogenous cytokines. Clearly this was not the case here as the vessels from the SHRSP still respond to the IL-1β.
To the best of our knowledge this is the first study showing that IL-1β directly causes vessel contraction. The contractile response was relatively rapid, the contraction began approximately 20 minutes after the addition of IL-1β and after 1 hour it had reached its plateau. Because of the rapid nature of this response, it is unlikely that it requires de novo protein synthesis, unlike many of the other reported effects of IL-1β which require long incubations or in vivo treatments (White et al. 2000; Morishige et al. 2001; De Salvatore et al. 2003). It should be noted, however that although the term rapid is being used to describe the response to IL-1β these responses take several minutes to be generated. Rapid has, in this case, been used to describe the contraction to make it clear that we do not believe that gene transcription and translation are required for the response to occur. However, in terms of regulation of vascular tone, the effects of IL-1β are likely to be more long-term than the second to second regulation that occurs with other vasoconstrictor systems. IL-1β also caused the vessels from the SHRSP to be more sensitive to the constrictor effects of phenylephrine. The baseline for the phenylephrine dose response curve was taken after the one-hour incubation with IL-1β indicating the potentiation of the phenylephrine response does not just reflect the higher tension on the vessels at the beginning of the dose response curve.
One of the interesting findings of this study was that the IL-1β-induced contraction occurred in aorta from SHRSP but not WKY rats. As mentioned previously, SHRSP exhibit signs of systemic inflammation (Sironi et al. 2004; Sanz-Rosa et al. 2005) and it is possible that this background inflammation is required for IL-1β to cause contraction. It is also possible that hypertension per se affects the ability of the vessels to contract. While the aorta from the WKY rats did not contract in response to IL-1β, there was a trend toward an increase in the sensitivity of the vessels to phenylephrine. It is not clear if a longer incubation with IL-1β would further increase the sensitivity of the vessels to phenylephrine. While IL-1β did not significantly increase the tone in the WKY rats, both indomethacin and PP-2 causes a significant reduction in the tone generated by the vessels. This suggests that COX and Src-kinase may be important for the regulation of basal tone in the aorta from WKY rats.
The mechanism for the IL-1β-induced contraction and increase in vessel sensitivity to phenylephrine has not been completely defined. It is however possible to suggest that Src-kinase and COX are involved in the process because both effects could be inhibited with PP-2 or indomethacin. c-Src is the most prominent Src-kinase in the vasculature and it has been implicated in the pathogenesis of hypertension (Touyz et al. 2001; Touyz et al. 2002). PP-2 caused the phenylephrine-induced contraction to be reduced to less than the baseline levels. Previous studies have shown that Src-kinase is involved in mediating the contractile response to phenylephrine, 5-HT and angiotensin II (Banes et al. 1999; Touyz et al. 2001; Alioua et al. 2002). Therefore, it is possible that in the current studies PP-2 is also inhibiting the phenylephrine-induced contractions. That said, the fact that PP-2 inhibits the contraction mediated by IL-1β alone suggests that Src-kinase is involved in the response of the vessels to IL-1β. Similarly, studies show that COX, in particular COX-2, is involved in the pathogenesis of hypertension (Hermann et al. 2003; Wu et al. 2005). Interestingly, COX-2 inhibition prevents the enhanced contractile response to endothelin-B agonists observed in vessels treated with IL-1β (White et al. 2000). However, this effect occurred over a much longer time frame than the studies presented here suggesting that de novo protein synthesis was required. The COX enzymes (COX 1 and 2) metabolize arachidonic acid to produce both constrictor and dilator prostanoids. It is possible that an increase in the activity of one or both of the COX enzymes causes an increase in the production of constrictor prostanoids and that these are responsible for the vessel contraction observed.
The involvement of the COX enzymes in the contractile response is not entirely surprising. Other studies have shown that COX, in particular COX-2, is increased in smooth muscle cells in response to inflammatory mediators (Bartlett et al. 1999; Rikitake et al. 2001). However, in these studies the induction of mRNA required an approximately 2 hour incubation with IL-1β and 2-4 hour incubation times were needed for increased COX-2 protein synthesis. In our studies indomethacin inhibited the rapid effects of IL-1β, while this suggests the effects are COX dependent, the time course of the effects suggests that de novo protein synthesis was not required for the IL-1β mediated contraction to occur. It is however possible that IL-1β activates pre-existing COX enzymes.
Our results also suggest that Src-kinase is involved in the IL-1β induced contraction and increased sensitivity to phenylephrine. IL-1β increases COX-2 expression in a Src-kinase dependent manner in gastric endothelial cells and osteoplasts (Chang et al. 2004; Park et al. 2004). Others have shown that IL-1β activates Src-kinase in vascular smooth muscle cells (Gui et al. 2000). Again, it is unlikely that the response observed here required protein synthesis, it is however possible to hypothesize that through Src-kinase, IL-1β activates COX to produce vasoconstrictor prostanoids. As mentioned previously, Src-kinase is implicated in the pathogenesis of hypertension (Touyz et al. 2001; Touyz et al. 2002), making this another possible explanation why we observe a response to IL-1β in the vessels from SHRSP but not WKY rats.
5 Conclusion
We have shown that IL-1β causes contraction in endothelium denuded aorta from SHRSP but not WKY rats and that it also enhances the contractile response to phenylephrine in the SHRSP. Both these responses are rapid and therefore most probably do not require de novo protein synthesis. The IL-1β-induced responses were inhibited by PP-2 and indomethacin, suggesting the involvement of Src-kinase and COX. These studies present a novel effect of IL-1β in the vasculature and add to the growing list of deleterious actions of inflammatory cytokines in the cardiovascular system.
6 Acknowledgements
This work was supported by grants from the National Institutes for Health (HL077385) and American Heart Association (0130364N). The author thanks Dr. Jennifer Waller for her advice regarding statistics.
Abbreviations
- COX
cylcooxygenase
- IL-1β
interleukin 1 beta
- SHR
spontaneously hypertensive rat
- SHRSP
stroke prone spontaneously hypertensive rat
- VSMC
vascular smooth muscle cell
- WKY
Wistar Kyoto
- PP-2
4-Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7 References
- Alioua A, Mahajan A, Nishimaru K, Zarei MM, Stefani E, Toro L. Coupling of c-Src to large conductance voltage- and Ca2+-activated K+ channels as a new mechanism of agonist-induced vasoconstriction. Proc Natl Acad Sci U S A. 2002;99(22):14560–5. doi: 10.1073/pnas.222348099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banes A, Florian JA, Watts SW. Mechanisms of 5-hydroxytryptamine(2A) receptor activation of the mitogen-activated protein kinase pathway in vascular smooth muscle. J Pharmacol Exp Ther. 1999;291(3):1179–87. [PubMed] [Google Scholar]
- Bartlett SR, Sawdy R, Mann GE. Induction of cyclooxygenase-2 expression in human myometrial smooth muscle cells by interleukin-1beta: involvement of p38 mitogen-activated protein kinase. J Physiol. 1999;520(2):399–406. doi: 10.1111/j.1469-7793.1999.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista LE. Inflammation, endothelial dysfunction, and the risk of high blood pressure: epidemiologic and biological evidence. J Hum Hypertens. 2003;17(4):223–30. doi: 10.1038/sj.jhh.1001537. [DOI] [PubMed] [Google Scholar]
- Chang YJ, Wu MS, Lin JT, Sheu BS, Muta T, Inoue H, Chen CC. Induction of cyclooxygenase-2 overexpression in human gastric epithelial cells by Helicobacter pylori involves TLR2/TLR9 and c-Src-dependent nuclear factor-kappaB activation. Mol Pharmacol. 2004;66(6):1465–77. doi: 10.1124/mol.104.005199. [DOI] [PubMed] [Google Scholar]
- Dalekos GN, Elisaf M, Bairaktari E, Tsolas O, Siamopoulos KC. Increased serum levels of interleukin-1beta in the systemic circulation of patients with essential hypertension: additional risk factor for atherogenesis in hypertensive patients? J Lab Clin Med. 1997;129(3):300–8. doi: 10.1016/s0022-2143(97)90178-5. [DOI] [PubMed] [Google Scholar]
- De Salvatore G, De Salvia MA, Piepoli AL, Natale L, Porro C, Nacci C, Mitolo CI, Mitolo-Chieppa D. Effects of in vivo treatment with interleukins 1beta and 6 on rat mesenteric vascular bed reactivity. Auton Autacoid Pharmacol. 2003;23(2):125–31. doi: 10.1046/j.1474-8673.2003.00286.x. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20(5 Suppl 27):S1–13. [PubMed] [Google Scholar]
- Gui Y, Zheng XL, Hollenberg MD. Interleukin-1beta, Src- and non-Src tyrosine kinases, and nitric oxide synthase induction in rat aorta in vitro. Am J Physiol Heart Circ Physiol. 2000;279(2):H566–76. doi: 10.1152/ajpheart.2000.279.2.H566. [DOI] [PubMed] [Google Scholar]
- Hermann M, Camici G, Fratton A, Hurlimann D, Tanner FC, Hellermann JP, Fiedler M, Thiery J, Neidhart M, Gay RE, Gay S, Luscher TF, Ruschitzka F. Differential effects of selective cyclooxygenase-2 inhibitors on endothelial function in salt-induced hypertension. Circulation. 2003;108(19):2308–11. doi: 10.1161/01.CIR.0000101683.30157.0B. [DOI] [PubMed] [Google Scholar]
- Morishige K, Shimokawa H, Eto Y, Kandabashi T, Miyata K, Matsumoto Y, Hoshijima M, Kaibuchi K, Takeshita A. Adenovirus-mediated transfer of dominant-negative rho-kinase induces a regression of coronary arteriosclerosis in pigs in vivo. Arterioscler Thromb Vasc Biol. 2001;21(4):548–54. doi: 10.1161/01.atv.21.4.548. [DOI] [PubMed] [Google Scholar]
- Park YG, Kang SK, Kim WJ, Lee YC, Kim CH. Effects of TGF-beta, TNF-alpha, IL-beta and IL-6 alone or in combination, and tyrosine kinase inhibitor on cyclooxygenase expression, prostaglandin E2 production and bone resorption in mouse calvarial bone cells. Int J Biochem Cell Biol. 2004;36(11):2270–80. doi: 10.1016/j.biocel.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Peeters AC, Netea MG, Janssen MC, Kullberg BJ, Van der Meer JW, Thien T. Pro-inflammatory cytokines in patients with essential hypertension. Eur J Clin Invest. 2001;31(1):31–6. doi: 10.1046/j.1365-2362.2001.00743.x. [DOI] [PubMed] [Google Scholar]
- Rikitake Y, Hirata K, Kawashima S, Takeuchi S, Shimokawa Y, Kojima Y, Inoue N, Yokoyama M. Signaling mechanism underlying COX-2 induction by lysophosphatidylcholine. Biochem Biophys Res Commun. 2001;281(5):1291–7. doi: 10.1006/bbrc.2001.4510. [DOI] [PubMed] [Google Scholar]
- Sanz-Rosa D, Oubina MP, Cediel E, de Las Heras N, Vegazo O, Jimenez J, Lahera V, Cachofeiro V. Effect of AT1 receptor antagonism on vascular and circulating inflammatory mediators in SHR: role of NF-kappaB/IkappaB system. Am J Physiol Heart Circ Physiol. 2005;288(1):H111–5. doi: 10.1152/ajpheart.01061.2003. [DOI] [PubMed] [Google Scholar]
- Shi W, Meininger CJ, Haynes TE, Hatakeyama K, Wu G. Regulation of tetrahydrobiopterin synthesis and bioavailability in endothelial cells. Cell Biochem Biophys. 2004;41(3):415–34. doi: 10.1385/CBB:41:3:415. [DOI] [PubMed] [Google Scholar]
- Sironi L, Gelosa P, Guerrini U, Banfi C, Crippa V, Brioschi M, Gianazza E, Nobili E, Gianella A, de Gasparo M, Tremoli E. Anti-inflammatory effects of AT1 receptor blockade provide end-organ protection in stroke-prone rats independently from blood pressure fall. J Pharmacol Exp Ther. 2004;311(3):989–95. doi: 10.1124/jpet.104.072066. [DOI] [PubMed] [Google Scholar]
- Soler M, Camacho M, Vila L. Imidazolineoxyl N-oxide prevents the impairment of vascular contraction caused by interleukin-1beta through several mechanisms. J Infect Dis. 2003;188(6):927–37. doi: 10.1086/377586. [DOI] [PubMed] [Google Scholar]
- Touyz RM, He G, Wu XH, Park JB, Mabrouk ME, Schiffrin EL. Src is an important mediator of extracellular signal-regulated kinase 1/2-dependent growth signaling by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients. Hypertension. 2001;38(1):56–64. doi: 10.1161/01.hyp.38.1.56. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Wu XH, He G, Park JB, Chen X, Vacher J, Rajapurohitam V, Schiffrin EL. Role of c-Src in the regulation of vascular contraction and Ca2+ signaling by angiotensin II in human vascular smooth muscle cells. J Hypertens. 2001;19(3):441–9. doi: 10.1097/00004872-200103000-00012. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Wu XH, He G, Salomon S, Schiffrin EL. Increased angiotensin II-mediated Src signaling via epidermal growth factor receptor transactivation is associated with decreased C-terminal Src kinase activity in vascular smooth muscle cells from spontaneously hypertensive rats. Hypertension. 2002;39(2 Pt 2):479–85. doi: 10.1161/hy02t2.102909. [DOI] [PubMed] [Google Scholar]
- Vicaut E, Rasetti C, Baudry N. Effects of tumor necrosis factor and interleukin-1 on the constriction induced by angiotensin II in rat aorta. J Appl Physiol. 1996;80(6):1891–7. doi: 10.1152/jappl.1996.80.6.1891. [DOI] [PubMed] [Google Scholar]
- White LR, Juul R, Skaanes KO, Aasly J. Cytokine enhancement of endothelin ET(B) receptor-mediated contraction in human temporal artery. Eur J Pharmacol. 2000;406(1):117–22. doi: 10.1016/s0014-2999(00)00642-7. [DOI] [PubMed] [Google Scholar]
- Wu R, Laplante MA, de Champlain J. Cyclooxygenase-2 inhibitors attenuate angiotensin II-induced oxidative stress, hypertension, and cardiac hypertrophy in rats. Hypertension. 2005;45(6):1139–44. doi: 10.1161/01.HYP.0000164572.92049.29. [DOI] [PubMed] [Google Scholar]
- Yang NC, Lu LH, Kao YH, Chau LY. Heme oxygenase-1 attenuates interleukin-1beta-induced nitric oxide synthase expression in vascular smooth muscle cells. J Biomed Sci. 2004;11(6):799–809. doi: 10.1007/BF02254365. [DOI] [PubMed] [Google Scholar]