Abstract
The role of adenosine A3 receptors in synaptic transmission under severe (7 min) and shorter (2-5 min) ischemic conditions, obtained by oxygen and glucose deprivation (OGD), was investigated in rat hippocampal slices. The effects of selective A3 agonists or antagonists were examined on field excitatory postsynaptic potentials (fEPSPs) extracellularly recorded at the dendritic level of the CA1 pyramidal region. The novel, selective A3 antagonist LJ1251 ((2R,3R,4S)-2-(2-chloro-6-(3-iodobenzylamino)-9H-purin-9-yl)tetrahydrothiophene-3,4-diol, 0.1-10 nM) protected hippocampal slices from irreversible fEPSP depression induced by severe OGD and prevented or delayed the appearance of anoxic depolarization. Similar results were obtained when severe OGD was carried out with a long, receptor-desensitizing exposure to various selective A3 agonists: 5′-N-methylcarboxamidoadenosine derivatives Cl-IB-MECA (N6-(3-iodobenzyl)-2-chloro), VT72 (N6-methoxy-2-phenylethynyl), VT158 (N6-methoxy-2-phenylethynyl), VT160 (N6-methoxy-2-(2-pyridinyl)-ethynyl), and VT163 (N6-methoxy-2-p-acetylphenylethynyl) and AR132 (N6-methyl-2-phenylethynyladenosine).
The selective A3 antagonist MRS1523 (3-propyl-6-ethyl-5-[(ethylthio)carbonyl]-2-phenyl-4-propyl-3-pyridine carboxylate, 100 nM) reduced fEPSP depression evoked by 2-min OGD and induced a faster recovery of fEPSP amplitude after 5-min OGD. Similar results were obtained for 2- or 5-min OGD applied in the presence of each of the A3 agonists tested. Shorter exposure to A3 agonists significantly delayed the recovery of fEPSP amplitude after 5-min OGD.
This indicates that A3 receptors, stimulated by selective A3 agonists, undergo desensitization during OGD. It is inferred that CA1 hippocampal A3 receptors stimulated by adenosine released during brief ischemia (2 and 5 min) might exert A1-like protective effects on neurotransmission. Severe ischemia would transform the A3 receptor-mediated effects from protective to injurious.
Keywords: purines, G protein-coupled receptors, cerebral ischemia, hippocampal slices, field EPSP, desensitization
1. Introduction
Adenosine acts in the brain by activation of four receptor subtypes, A1, A2A, A2B, and A3, all coupled to their effector systems through heterotrimeric G proteins [1]. Of the four adenosine receptor subtypes, the adenosine A3 receptor is the most recently cloned [2]. A3 receptors have a widespread distribution in the rat brain [3] and in the hippocampus are preferentially expressed on nerve terminals [4,5]. The expression of A3 receptors in the brain is lower than that of other adenosine receptor subtypes [6], and the affinity for adenosine, calculated from binding experiments in rat brain membranes (6.5 μM [2]), is lower than that of A1 [7] and A2A [8] receptors. However, during ischemic conditions in vivo [9] and in vitro [10] adenosine extracellular concentrations may be sufficiently high to activate adenosine A3 receptors, which may play a role in brain ischemia (see [11,12]).
We recently demonstrated that selective antagonism of A3 receptors facilitates the recovery of synaptic activity induced by ischemic preconditioning in rat hippocampal slices [13]. A harmful role of A3 receptors during in vitro oxygen glucose deprivation (OGD) was confirmed by the observation that blocking the A3 adenosine receptor consistently abolishes or delays the occurrence of anoxic depolarization (AD) and significantly protects from the irreversible disruption of excitatory neurotransmission caused by a severe ischemic episode [14]. These results agree with the observation that acute administration of a selective adenosine A3 agonist exacerbates the damage elicited by global ischemia in the gerbil [15]. On the contrary, it was demonstrated that chronic preischemic administration of an A3 agonist protects against ischemic neuronal damage [15]. This effect may be attributable to desensitization of A3 receptors. In fact, both human and rat A3 receptors are desensitized within a few minutes after agonist exposure [16-21].
Contrary to the above information, Hentschel et al. [22] demonstrated that under hypoxic conditions, selective activation of A3 adenosine receptors brings about an inhibition of excitatory neurotransmission on cortical neurons, indicating that A3 receptors may sustain the neuroprotective action of adenosine induced by A1 receptors. Consistently with these reports, mice lacking A3 adenosine receptors showed increased neurodegeneration in response to repeated episodes of moderate hypoxia [23] or an increase in cerebral infarction after transient ligation of the middle cerebral artery [24]. Together, these data suggest that the outcome of A3 receptor stimulation on synaptic transmission during hypoxic/ischemic phenomena depends on the intensity and duration of stimulation.
In the present work we investigated the role of adenosine A3 receptors during ischemia by studying the effect of A3-selective agonists and antagonists under both prolonged (7-min) and brief (2- and 5-min) OGD episodes. Prolonged, severe episodes of OGD bring about the irreversible depression of neurotransmission and the appearance of AD [14,25], a phenomenon strictly correlated with the extent of brain damage during ischemia both in vivo and in vitro [26]. Brief periods of ischemia are followed by complete recovery of neurotransmission and imply shorter times of A3 receptor stimulation by endogenous adenosine released during the ischemic episode [10,13,27,28].
2. Materials and Methods
All animal procedures were carried out according to the European Community Guidelines for Animal Care, DL 116/92, application of the European Communities Council Directive (86/609/EEC). Experiments were carried out on rat hippocampal slices, prepared as previously described [13].
2.1. Slice preparation
Male Wistar rats (Harlan Italy, Udine, Italy; 150-200 g body weight) were killed with a guillotine while under anesthesia with ether, and their hippocampi were rapidly removed and placed in ice-cold oxygenated (95% O2, 5% CO2) artificial cerebral spinal fluid (aCSF) of the following composition (mM): NaCl 124, KCl 3.33, KH2PO4 1.25, MgSO4 2, CaCl2 2, NaHCO3 25, and D-glucose 10. Slices (400 μm thick) were cut with a McIlwain tissue chopper (Mickle Laboratory Engineering Co. Ltd., Gomshall, UK) and kept in oxygenated aCSF for at least 1 h at room temperature. A single slice was then placed on nylon mesh, completely submerged in a small chamber (0.8 ml), and superfused with oxygenated aCSF (30-32°C) at a constant flow rate of 2 ml · min−1. The treated solutions reached the preparation in 90 seconds and this delay was taken into account in our calculations.
2.2. Extracellular recording
Test pulses (80 μs, 0.066 Hz) were delivered through a bipolar nichrome electrode positioned in the stratum radiatum. Evoked extracellular potentials were recorded with glass microelectrodes (2-10 MΩ, Clark Electromedical Instruments, Panghourne, UK) filled with 150 mM NaCl and placed in the CA1 region of the stratum radiatum. Responses were amplified (BM 622, Mangoni, Pisa, Italy), digitized (sample rate, 33.33 kHz), and stored for later analysis with LTP (version 2.30D) Program [29]. Stimulus-response curves were obtained by gradual increases in stimulus strength at the beginning of each experiment, when a stable baseline of evoked response was reached. The test stimulus pulse was then adjusted to produce a field excitatory postsynaptic potential (fEPSP) whose slope and amplitude was 40% to 50% of the maximum and was kept constant throughout the experiment. The fEPSP amplitude was routinely measured and expressed as the percentage of the average amplitude of the potentials measured during the 5 min preceding exposure of the hippocampal slices to OGD. In some experiments both the amplitude and initial fEPSP slope were quantified, but because no appreciable differences between these two parameters were observed in drug effect and OGD, only the amplitude measurement is expressed in the figures.
2.3. Application of drugs and OGD
OGD was achieved by perfusing the slice for different times—brief (2 or 5 min) or prolonged (7 min)—with aCSF lacking glucose and gassed with nitrogen (95% N2, 5% CO2) [30]. This caused a drop in pO2 in the recording chamber from ∼500 mm Hg (normoxia) to a range of 120–150 mmHg (after 2-min OGD) and of 35-75 mm Hg (after 7-min OGD) [13], measured with an ISO2 and its associated OXEL-1 probe (WPI, Aston, UK). At the end of the ischemic period, the slice was again superfused with normal, glucose-containing, oxygenated aCSF. In the Results Section and figures, the term “untreated OGD slices” referred to hippocampal slices in which OGD episodes of different duration were applied in the absence of drugs.
Area under curve (AUC) analysis was conducted to evaluate the effects of various A3 agonists or A3 antagonists during 5-min OGD. Because 5-min OGD elicited a complete depression of synaptic activity in both the absence and the presence of A3 ligands, the AUC was measured and expressed as time (min), calculated from the beginning of fEPSP depression during OGD up to the recovery of fEPSP at baseline.
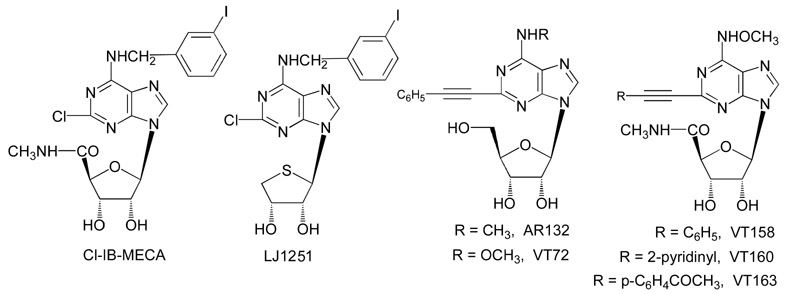
The selective A3 adenosine receptor antagonists LJ1251 and MRS1523 (3-propyl-6-ethyl-5-[(ethylthio)carbonyl]-2-phenyl-4-propyl-3-pyridine carboxylate), were applied for 10 min before and during OGD and 5 min after the ischemic episode. Concentrations of the selective adenosine A3 receptor antagonists were chosen on the basis of Ki values at the rat and human A3 receptors [31-33]. The binding affinities of LJ1251 at the human and rat adenosine receptors are shown in Table 1. Unlike MRS1523, which was effective at submicromolar concentrations on rat A3 receptors, LJ1251 was effective at nanomolar concentrations on both human and rat A3 receptors.
xTable 1.
Binding affinities of adenosine agonists and antagonists at three subtypes of ARs (human, unless noted; r = rat).a Nucleoside structures are shown:
The selective adenosine A3 receptor agonists Cl-IB-MECA (1-[2-chloro-6[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-β-D-ribofuranuronamide) [34], AR132 (N6-methyl-2-phenylethynyladenosine), VT72 (N6-methoxy-2-phenylethynyladenosine), VT158 (N6-methoxy-2-phenylethynyl-5′-N-methylcarboxamidoadenosine), VT160 (N6-methoxy-2-(2-pyridinyl)-ethynyl-5′-N-methylcarboxamidoadenosine), and VT163 (N6-methoxy-2-p-acetylphenylethynyl-5′-N-methylcarboxamidoadenosine) were each applied 5 min before, during, and 2 min after OGD (long application) or for only 2 min during OGD (short application). Cl-IB-MECA has been described as a highly selective agonist for both human and rat A3 adenosine receptors versus A1 and A2A receptors (Table 1) [32,35]. When Cl-IB-MECA and the A3 antagonist MRS1523 were coapplied, the antagonist was always superfused 5 min before combining the two drugs. Ki values for the A3 agonists AR132, VT72, VT158, VT160, and VT163 (synthesized at the University of Camerino, Italy, and shown in Table 1) were taken from binding experiments in CHO (Chinese hamster ovary) cells stably transfected with human recombinant adenosine receptors. The compounds have been reported to be highly selective for human A3 adenosine receptors versus human A1 receptors and A2A receptors [36-39].
When slices were subjected to 7 min of OGD, if the recovery of fEPSP amplitude after 15 min of reperfusion with glucose-containing and normally oxygenated aCSF was ≤15% of the preischemic value, a second slice from the same rat was submitted to a 7-min OGD insult in the presence of the A3 agonists or antagonists under investigation. To confirm the result obtained in the treated group, a third slice was taken from the same rat and another 7-min OGD was performed in control conditions, to verify that no difference between slices was caused by the time gap between the experiments.
2.4. Drugs
MRS1523 was purchased from Sigma (Milan, Italy). Cl-IB-MECA was from Tocris (Bristol, UK). The novel antagonist LJ1251 ((2R,3R,4S)-2-(2-chloro-6-(3-iodobenzylamino)-9H-purin-9-yl)tetrahydrothiophene-3,4-diol), structurally based on a truncated nucleoside template, was synthesized by L. Jeong, Ewha University, Seoul, Korea. AR132, VT72, VT158, VT160, and VT163 were provided by G. Cristalli, University of Camerino, Italy. Each drug was dissolved in dimethylsulfoxide (DMSO), and stock solutions were made to obtain concentrations in DMSO of 0.05% and 0.01% in aCSF, respectively. Control experiments, carried out in parallel, showed that this concentration of DMSO did not affect either fEPSP amplitude before OGD or the depression of synaptic potential induced by the subsequent OGD.
2.5. Statistical analysis
Data were analyzed with Prism 3.02 software (GraphPad Software, San Diego, CA, USA). All numerical data are expressed as the mean ± SEM. Data were tested for statistical significance with the paired two-tailed Student's t test or by analysis of variance (one-way ANOVA), as appropriate. When significant differences were observed, the Newman-Keuls multiple-comparison test (one-way ANOVA) was used. A value of P < 0.05 was considered significant.
3. Results
The role of A3 adenosine receptors in synaptic transmission, either in normoxic conditions or during OGD episodes of different durations, was studied through extracellular recordings of fEPSPs in the CA1 region of rat hippocampal slices. Experiments were performed on a total of 208 slices taken from 121 rats.
3.1. The selective blocking of A3 receptors by LJ1251 or MRS 1523 protected hippocampal slices from irreversible depression of fEPSP induced by 7- min OGD
The data in Fig. 1B show that 7-min OGD was a duration of ischemic insult that induced an irreversible loss of hippocampal synaptic transmission and that both selective A3 adenosine receptor antagonists, the novel nucleoside derivative LJ1251 and the pyridine derivative MRS1523, were able to prevent this loss. LJ1251 (10 nM, n=5) did not change fEPSP amplitude under normoxic conditions (from 0.92 ± 0.1 mV in the absence to 0.94 ± 0.1 mV in the presence of the antagonist). However, it induced a significant recovery of fEPSP amplitude of 96.0 ± 3.3% (n = 5; P < 0.001, one-way ANOVA, Newman-Keuls multiple-comparison post hoc test) in comparison to that obtained in untreated OGD slices (4.1 ± 1.6 %, n = 13). Similar results were obtained in the presence of MRS1523. MRS1523 (100 nM, n=5) did not change fEPSP amplitude under normoxic conditions (from 0.94±0.06 mV in the absence to 0.92 ± 0.07 mV in the presence of the antagonist) but after 7-min OGD elicited a fEPSP recovery of 99.3 ± 5.9% (n = 5; P < 0.001, one-way ANOVA, Newman-Keuls multiple-comparison post hoc test versus untreated OGD slices). Fig. 1A (left panel) shows that 7-min OGD episodes always caused AD, recorded as negative d.c. shifts, with a mean peak latency of 6.6 ± 0.2 min from the beginning of ischemia and a peak amplitude of 7.5 ± 0.7 mV (n = 5). The duration of d.c. shifts was variable (range: 5-15 min) and was always accompanied by complete and irreversible disappearance of fEPSPs (Fig. 1B). In the presence of LJ1251 (10 nM, n = 5), AD was absent in all slices recorded (Fig. 1A, middle panel). Similarly, during 7-min OGD no AD appearance was recorded in the presence of MRS1523 (100 nM, n=5, Fig.1A, right panel). These results are in agreement with our previous results showing that MRS1523 and several chemically diverse A3 receptor antagonists prevented the irreversible failure of neurotransmission induced by 7-min OGD and induced a complete abolishment or delay of AD [14]. The protective effect of LJ1251 against OGD-evoked irreversible depression of fEPSPs was concentration dependent (Fig. 1C), with an apparent EC50 value of 0.037 nM (95% confidence interval (CI) 0.026-0.053 nM).
Fig. 1.
The selective A3 adenosine receptor antagonists, LJ1251 and MRS1523, minimized AD and protected CA1 hippocampus from irreversible fEPSP depression induced by 7-min OGD.
(A) AD, was recorded as the negative d.c. shift in response to 7-min OGD (solid bar) in the absence (n = 13, left panel), and in the presence of 10 nM LJ1251 (open bar, n = 5, middle panel) or 100 nM MRS1523 (open bar, n = 5, right panel). Note that both the A3 antagonists LJ1251 and MRS1583 completely prevented AD appearance. (B) Graphs show the time course of 7-min OGD (solid bar) effect on fEPSP amplitude, expressed as percentage of baseline, in untreated OGD slices (filled circles, mean ± S.E.M., n = 13), in the presence of 10 nM LJ1251 (open circles, mean ± S.E.M., n = 5) and 100 nM MRS1523 (filled triangles, mean ± S.E.M., n=5). Solid bars indicate the duration of OGD. (C) Concentration-response curves of LJ1251. The graph shows the fitting curve of concentration-dependent effects of LJ1251 on fEPSP recovery after 7-min OGD recorded in hippocampal slices at 15-min reperfusion in normal, oxygenated aCSF. Data (mean ± S.E.M., n = 3 in 0.001 nM LJ1251, n = 3 in 0.01 nM, n = 3 in 0.1 nM, n = 4 in 5 nM, n = 5 in 10 nM) are expressed as percentage of baseline values. *P < 0.01, one-way ANOVA, Newman-Keuls multiple-comparison post hoc test, versus 0.001 and 0.01 nM LJ1251-treated slices.
3.2. Selective A3 adenosine receptor agonist Cl-IB-MECA also protects from the irreversible depression of neurotransmission induced by 7-min OGD
The effects of 10 nM Cl-IB-MECA on fEPSP amplitude before and after 7-min OGD are shown in Fig. 2. Seven-min OGD induced an irreversible block of neurotransmission, as indicated by the absence of fEPSP amplitude recovery (4.2 ± 3.3%, n = 16) when the slices were superfused in normal oxygenated aCSF. In eight slices in which we also measured the appearance of AD, 7-min OGD episodes always caused AD, with a mean peak latency of ∼5.7 ± 0.3 min from the beginning of OGD and a peak amplitude of 7.1 ± 0.5 mV (n = 8, Fig. 2A). Cl-IB-MECA (10 nM, 5 min before, during, and 2 min after OGD) did not modify fEPSP amplitude under normoxic conditions (from 1.02 ± 0.12 mV in the absence and 1.02 ± 0.14 mV in the presence of the A3 agonist, n=15) but induced a significant fEPSP recovery after 7-min OGD (81.5 ± 10.5%, P < 0.001, one-way ANOVA, Newman-Keuls multiple-comparison post hoc test versus untreated OGD slices, n = 15, Fig. 2B). AD was absent in five slices out of eight tested, and it was significantly delayed in the remaining three slices (peak latency of 7.8 ± 0.2 min, peak amplitude 6.6 ± 2.2, Fig. 2A). Similar effects on fEPSP recovery were obtained by using different A3 agonists: AR132 (10 nM, n = 5), VT72 (10 nM, n = 8), VT158 (5 nM, n = 3), VT160 (5 nM, n = 4), and VT163 (5 nM, n = 3, Fig. 2C). No changes in fEPSP amplitude were observed during the 5 min of drug superfusion before OGD application (from 0.83±0.06 mV in the absence to 0.82 ± 0.07 mV in the presence AR132; from 0.81±0.06 mV in the absence to 0.78 ± 0.07 mV in the presence VT72; from 0.80 ± 0.10 mV in the absence to 0.81 ± 0.10 mV in the presence VT158; from 0.92 ± 0.11 mV in the absence to 0.92 ± 0.11 mV in the presence VT160; from 0.82 ± 0.04 mV in the absence to 0.80 ± 0.05 mV in the presence VT163). In addition, all the A3 agonists tested prevented or significantly delayed AD after 7-min OGD (not shown).
Fig. 2.
The selective A3 adenosine agonist Cl-IB-MECA protects hippocampal slices from the irreversible fEPSP depression induced by 7-min OGD.
(A) AD was recorded as the negative d.c. shift in response to 7-min OGD (solid bars) in the absence (n = 8) and in the presence of 10 nM Cl-IB-MECA (open bar, n = 8). The agonist prevented AD appearance in 5 slices and delayed it in the remaining 3 slices. (B) Time course of fEPSP amplitude before, during 7-min OGD, and after reperfusion in normal oxygenated aCSF in the absence and in the presence of 10 nM Cl-IB-MECA (open bar). Data (mean ± S.E.M., n = 16 in untreated OGD slices, n = 15 in Cl-IB-MECA) are expressed as percentage of baseline values. Cl-IB-MECA was applied 5 min before, during, and 2 min after OGD. (C) Columns in the graph summarize the effects of different A3 agonists on fEPSP recovery after 7- min OGD. *P < 0.05, one-way ANOVA followed by Newman-Keuls multiple-comparison post hoc test versus untreated OGD slices (D) Each graph shows the time course of fEPSP amplitude before anf after 7-min OGD. Data (mean ± S.E.M., n = 3 for each experimental group) are expressed as percentage of baseline values. Note that in a group of slices OGD was applied 20 min after the end of Cl-IB-MECA superfusion (open bar, 10 nM, 15 min). A complete recovery of fEPSP amplitude was obtained only in the slices in which OGD was applied after Cl-IB-MECA application. Solid bars indicate the duration of OGD.
In a different experimental protocol, 10 nM Cl-IB-MECA was superfused for 15 min and up to 20 min before application of 7-min OGD. As shown in Fig. 2D, 10-nM Cl-IB-MECA did not change the fEPSP amplitude under normoxia (from 0.90 ± 0.09 mV before to 0.95 ± 0.10 in the presence of Cl-IB-MECA, n=3), but allowed a significant fEPSP recovery after 7-min OGD (103.0 ± 3.6%, n = 3, versus 4.1 ± 3.6%, n = 3, recorded in non pretreated Cl-IB-MECA slices).
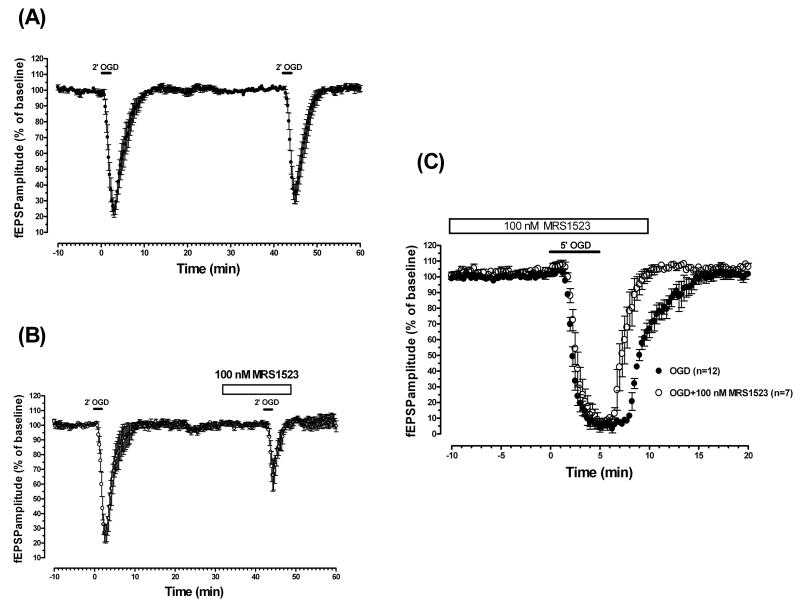
3.3. Selective block of A3 adenosine receptors by MRS1523 reduces fEPSP depression evoked by 2-min OGD and induces a faster recovery of fEPSP amplitude after 5-min OGD
The data in Fig. 3 show that periods of 2- or 5-min OGD induced, respectively, a partial or complete reduction of synaptic potential amplitude that was always reversible upon reperfusion with normal oxygenated aCSF. These results are in agreement with our previous studies [13,27,28].
Fig. 3.
The selective A3 adenosine receptor antagonist MRS1523 reduces fEPSP depression induced by 2-min OGD and induced a faster recovery of fEPSP amplitude after 5-min OGD.
(A) Time course of fEPSP amplitude before, during, and after the application of two consecutive ischemic insults of 2-min duration. The second 2-min OGD, administered 40 min after the end of the first period, elicited a comparable depression of synaptic potentials (P > 0.05, evaluated by t-paired two-tailed Student's t test). Data (mean ± S.E.M., n = 13) are expressed as a percentage of baseline values recorded before the first OGD period. (B) Time course of fEPSP amplitude changes elicited by 2-min OGD in the absence and in the presence of MRS1523. Data (mean ± S.E.M., n = 10) are expressed as a percentage of baseline values. MRS1523 (100 nM) was applied 10 min before, during, and 5 min after OGD, as indicated by the open bar. Note that in the presence of MRS1523, the fEPSP depression induced by OGD was significantly lower than that obtained in the absence of the drug (P < 0.05, paired two-tailed Student's t test). (C) Time course of fEPSP amplitude changes elicited by 5-min OGD in the absence (n = 12) and in the presence of MRS1523 (n = 7). Data (mean ± S.E.M.) are expressed as a percentage of baseline values recorded before the respective OGD periods. MRS1523 (100 nM), applied 10 minutes before, during, and 5 minutes after OGD application induced a faster fEPSP recovery after OGD application. Solid bars indicate the duration of OGD.
The application of 2-min OGD resulted in a partial but consistent depression of synaptic potentials, reaching a maximal inhibition of 76.3 ± 4.2% (n = 13, P < 0.001, paired two-tailed Student's t test versus preischemic baseline) a few seconds after reperfusion with normal oxygenated aCSF (Fig. 3A). After 2 min of reperfusion in normal, glucose-containing aCSF, fEPSPs progressively reappeared and reached a complete recovery within 5 min after the beginning of reperfusion. The application of a second period of 2-min OGD, 40 min after the end of the first one, resulted in a similar depression (66.4 ± 6.2%) of fEPSP amplitude, with the same time course. No significant differences were found by comparing synaptic potential amplitude at any time during the first and second OGD (Fig. 3A). Therefore, the effect of the A3 selective antagonist MRS1523 on fEPSPs was evaluated before and during the second period of 2-min OGD in comparison with the first OGD insult (Fig. 3B). MRS1523 (100 nM, n = 10) applied 10 min before, during, and 5 min after OGD did not modify fEPSP amplitude in normoxic conditions (from 0.90 ± 0.04 mV in the absence to 0.92 ± 0.04 in the presence of 100 nM MRS1523) but significantly inhibited fEPSP amplitude depression evoked by 2-min OGD (74.1 ± 6.1% in the absence and 34.3 ± 10.0% in the presence of the antagonist, P < 0.05, paired two-tailed Student's t test, Fig. 3B). The effect of 100 nM MRS1523 was maximal, because the increase of the antagonist concentration to 1 μM did not potentiate the effect (n = 3, data not shown).
Application of 5-min OGD resulted in a complete depression of synaptic potentials, reaching a maximal inhibition ∼4 min after OGD application (n = 12, Fig. 3C). After 3.5 min of reperfusion in normal, glucose-containing aCSF, fEPSPs progressively reappeared and reached a complete recovery within 10 min from the beginning of reperfusion. Because it has been demonstrated that a second 5-min OGD episode, 50 min after the end of the first one, is followed by an appreciably faster recovery of fEPSPs [27], the effect of the selective A3 antagonist was studied in a separate group of slices. MRS1523 (100 nM, n = 7), applied 10 min before, during, and 5 min after OGD application. The A3 antagonist did not modify fEPSP amplitude in normoxic conditions (from 0.9 ± 0.04 mV in the absence to 1.0 ± 0.04 in the presence of 100 nM MRS1523) but induced a faster fEPSPs recovery (AUC: 10.1 ± 0.5 min in untreated OGD slices and 7.1 ± 0.7 min in the presence of the antagonist, P < 0.05, one-way ANOVA, Newman-Keuls multiple-comparison post hoc test), measured from the beginning of fEPSP depression during OGD up to the recovery of fEPSP at baseline (Fig. 3C).
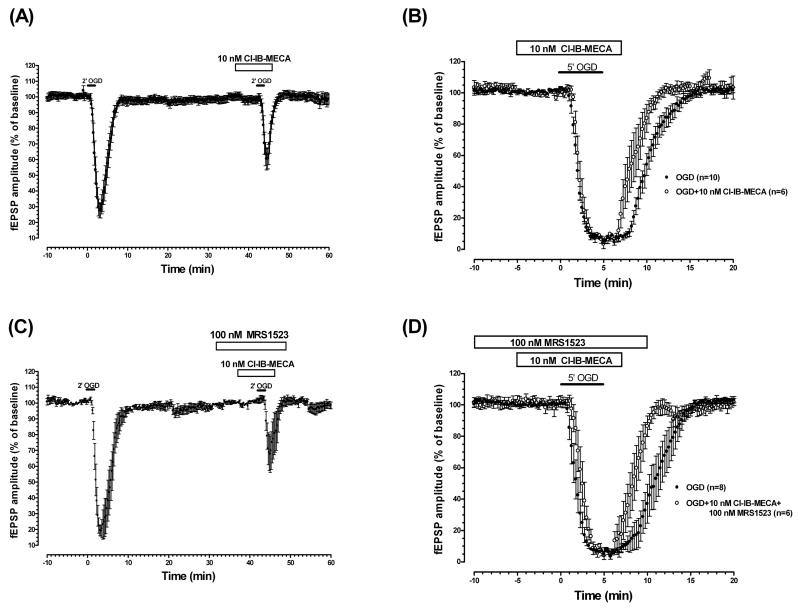
3.4. Selective stimulation of adenosine A3 receptors by a long application of Cl-IB-MECA reduces fEPSP depression evoked by 2-min OGD and induces a faster recovery of fEPSP amplitude after 5-min OGD
The effect of Cl-IB-MECA (10 nM) on fEPSP amplitude after 2-min OGD is shown in Fig. 4A. The selective A3 agonist did not affect the amplitude of fEPSPs under normoxic conditions (from 0.93 ± 0.07 mV in the absence to 0.96 ± 0.07 mV in the presence of 10 nM Cl-IB-MECA, n=13), but induced a significant decrease in fEPSP depression evoked by 2-min OGD (72.5 ± 4.5% in control and 39.5 ± 7.1% in the presence of the agonist, n = 13, P < 0.05, paired two-tailed Student's t test). The effect of 10 nM Cl-IB-MECA was maximal, because the increase of the agonist concentration to 100 nM did not potentiate the effect (75.1 ± 7.3% in control and 41.2 ± 10.0% in the presence of the agonist, n = 6, P < 0.05, paired two-tailed Student's t test, data not shown). The effect of 10 nM Cl-IB-MECA was not antagonized by the selective A2A receptor antagonist 4-(2-[7-amino-2-(2-furyl)(1,2,4)triazolo(2,3-a)(1,3,5,)triazin-5-ylamino]ethyl)phenol (ZM241385), superfused 15 min before Cl-IB-MECA and then coapplied with Cl-IB-MECA (73.4 ± 9.1% in the absence and 38.0 ± 14.2% in the presence of 100 nM ZM241385 in combination with Cl-IB-MECA, n = 3, data not shown).
Fig. 4.
The selective A3 adenosine receptor agonist Cl-IB-MECA decreased fEPSP depression induced by 2-min OGD and induced a faster recovery of fEPSP amplitude after 5-min OGD.
(A) Time-course of fEPSP amplitude changes elicited by 2-min OGD in the absence and in the presence of Cl-IB-MECA (10 nM; n = 13). Data (mean ± S.E.M.) are expressed as a percentage of respective baseline values recorded before the respective OGD periods. The solid bar indicates the duration of OGD. As indicated by the open bar, the A3 agonist was applied 5 min before, during, and 2 min after OGD. Note that in the presence of Cl-IB-MECA, the fEPSP depression induced by 2-min OGD was significantly lower than that obtained in the absence of the drug (P < 0.05, paired two-tailed Student's t test). (B) Time course of fEPSP amplitude changes elicited by 5 min OGD in the absence (n = 10) and presence of Cl-IB-MECA (10 nM, n = 6). Data (mean ± S.E.M.) are expressed as a percentage of baseline values recorded before the respective OGD periods. The A3 agonist, applied 5 min before, during, and 2 min after OGD (open bar) induced a faster fEPSP recovery after 5 min OGD. (C) Time course of fEPSP amplitude changes elicited by 2-min OGD in the absence and presence of Cl-IB-MECA (10 nM) in combination with MRS1523 (100 nM). The antagonist was always superfused 5 min before the agonist; coapplied with the agonist 5 min before, during, and 2 min after OGD; and then superfused alone for 3 min. Data (mean ± S.E.M., n = 7) are expressed as a percentage of the respective baseline values. Note that in the presence of Cl-IB-MECA and MRS1523, the fEPSP depression induced by 2-min OGD was significantly lower than (P < 0.05, paired two-tailed Student's t test) that obtained in the absence of the drugs. (D) Time course of fEPSP amplitude changes elicited by 5 min OGD in the absence (n = 8) and presence of Cl-IB-MECA (10 nM) in combination with MRS1523 (100 nM) (n = 6). As indicated by open bars, the antagonist was always superfused 5 min before the agonist; coapplied with the agonist 5 min before, during and 2-min after OGD; and then superfused alone for 3 min. Data (mean ± S.E.M.) are expressed as a percentage of respective baseline values. Solid bars indicate the duration of OGD.
The effect of 10 nM Cl-IB-MECA during 5-min OGD is shown in Fig. 4B. Cl-IB-MECA did not change the fEPSP time course before (from 1.0 ± 0.06 mV in the absence to 1.1 ± 0.07 in the presence of Cl-IB-MECA) and during OGD but reduced the time of fEPSP recovery (AUC: 9.5 ± 0.6 min in untreated OGD slices, n = 10, and 7.7 ± 0.6 min in the presence of the agonist, n = 6, P < 0.05, one-way ANOVA, Newman-Keuls multiple-comparison post hoc test).
Fig. 4C and D show the effect of coapplication of Cl-IB-MECA and MRS1523 on 2- and 5-min OGD, respectively. The antagonist was always superfused 5 min before the agonist. The effect of the coapplication of the two drugs on fEPSP depression induced by 2-min OGD (81.0 ± 3.3% in the absence and 32.3 ± 11.5% in the presence of the two drugs, n = 7, P < 0.05, paired two-tailed Student's t test, Fig. 4C) was not significantly different from those observed in the presence either of MRS1523 alone (Fig. 3B) or Cl-IB-MECA alone (Fig. 4A). Similarly, the effect induced by the coapplication of the two drugs on the time of fEPSP recovery after 5-min OGD (AUC: 10.6 ± 1 min in untreated OGD slices, n = 8, and 7.8 ± 0.5 in the presence of the two drugs, n = 6, P < 0.05, one-way ANOVA, Newman-Keuls multiple-comparison post hoc test) was not different from those observed in the presence of either MRS1523 alone (Fig. 3C) or Cl-IB-MECA alone (Fig. 4B). The coapplication of the two drugs, MRS1523 and Cl-IB-MECA, did not change the fEPSP amplitude under normoxic conditions (from 0.92±0.03 mV in the absence to 0.94±0.04 mV in the presence of the two drugs, n=13).
3.5. Selective stimulation of adenosine A3 receptors by a short application of Cl-IB-MECA did not affect fEPSP depression evoked by 2-min OGD and delayed the recovery of fEPSP amplitude after 5-min OGD
Because it has been demonstrated that A3 adenosine receptors undergo a rapid desensitization that is more evident and persistent when the agonist is applied for long periods [20], we chose to examine a different protocol, shortening the time of Cl-IB-MECA application. As shown in Fig. 5A, Cl-IB-MECA (10 nM), applied only during the 2 min of OGD application, did not modify the outcome of fEPSP depression induced by 2-min OGD from that obtained in the absence of drug (51.6 ± 7.7% in the absence and 55.6 ± 11.8% in the presence of Cl-IB-MECA, n = 7,). The data in Fig. 5B show that when Cl-IB-MECA was applied for 2 min at the end of an OGD episode of 5-min duration, it significantly delayed the time of fEPSP recovery (AUC: 9.7 ± 0.4 min in untreated OGD slices, n = 20, and 12.1 ± 0.7 min in the presence of the agonist, n = 10, P < 0.05, one-way ANOVA, Newman-Keuls multiple-comparison post hoc test). Similar results were obtained with different A3 agonists: AR132 (10 nM, n = 3), VT158 (5 nM, n = 2), and VT160 (5 nM, n = 2, data not shown). A summary of short and long application of Cl-IB-MECA during 5-min OGD is illustrated in Fig. 5C.
Fig. 5.
Short application Cl-IB-MECA did not modify fEPSP depression induced by 2-min OGD, but it significantly delays fEPSP recovery after 5-min OGD.
(A) Time course of fEPSP amplitude changes elicited by 2-min OGD in the absence and in the presence of Cl-IB-MECA (10 nM, n = 7). Data (mean ± S.E.M.) are expressed as a percentage of respective baseline values recorded before the respective OGD periods. As indicated by the open bar, the A3 agonist was applied for only 2 min, during OGD. Note that short application of Cl-IB-MECA did not induce significant change (P > 0.05, paired two-tailed Student's t test) in the fEPSP depression induced by 2-min OGD. (B) Time course of fEPSP amplitude changes elicited by 5-min OGD in the absence (n = 20) and in the presence of Cl-IB-MECA (10 nM, n = 10). Data (mean ± S.E.M.) are expressed as a percentage of respective baseline values recorded before the respective OGD periods. As indicated by the open bar, the A3 agonist was applied only for 2 min, at the end of 5-min OGD episodes. Note that short application of Cl-IB-MECA delayed fEPSP recovery after 5 min OGD. (C) Columns in the graph summarize the average time of fEPSP amplitude recovery (mean ± S.E.M.) after 5-min OGD in untreated OGD slices and during long or short application of Cl-IB-MECA. *P < 0.05, one-way ANOVA followed by Newman-Keuls multiple-comparison post hoc test versus untreated OGD slices; § P < 0.05, one-way ANOVA followed by Newman-Keuls multiple-comparison post hoc test versus all experimental groups. Solid bars indicate the duration of OGD.
4. Discussion
Our results indicate that A3 adenosine receptors play a role in neurotransmission of the CA1 region of the rat hippocampus during ischemia. A3 adenosine receptor antagonists facilitate the recovery of otherwise disrupted neurotransmission after a period of prolonged OGD, and during short periods of OGD they decrease the depression of neurotransmission brought about by OGD. Selective stimulation of A3 receptors, before and during an ischemic episode, produces changes in synaptic activity similar to those observed following their selective antagonism, indicating that A3 receptors undergo rapid desensitization following exposure to exogenous agonist.
The application of prolonged (7-min) OGD elicited a complete and irreversible depression of neurotransmission, which persists upon slice reperfusion with oxygenated and glucose-containing aCSF and is accompanied by the appearance of AD, an unequivocal sign of neuronal injury during ischemia [26]. Here we report that two selective A3 receptor antagonists, the newly synthesized LJ1251 [33] and MRS1523 share an equal effect. Both drugs in fact block the occurrence of AD and significantly protect from the irreversible disruption of excitatory neurotransmission caused by prolonged, severe (7-min) OGD episodes. These results are consistent with those obtained in the same brain region when different A3 antagonists were used [14]. LJ1251 and MRS 1523 possess high affinity for the rat A3 receptors (Ki= 3.89 nM [33] and 113 nM [31], respectively). The apparent discrepancy between the EC50 value of LJ1251 (0.037 nM) found in our experiments and the binding Ki value of the compound, might depend on the specific environment and coupling of A3 receptors in the cell membrane of native tissue and may differ from that assessed in receptor binding experiments. Alternatively, the paucity of A3 receptors in native tissue [6] allows one to speculate that occupancy of a substantial fraction of A3 receptors is required for evoking cell response(s). Thus, blocking a relatively small fraction of A3 receptors may be sufficient to antagonize the effect of endogenous adenosine released during OGD. Alterations in AD characteristics caused by A3 antagonists may be attributable to their actions on glutamate-mediated cellular responses. The time window of A3 receptor-mediated effects found in the present work and reported by Pugliese et al [14] overlaps with the delay that can be obtained by treating the slices with glutamate receptor antagonists [25,40,41]. NMDA receptors are essential to AD initiation and propagation [26]. Block of A3 receptors, in removing A3 receptor-mediated impairment of the feedback inhibition of glutamate release exerted by specific metabotropic glutamate receptor subtypes [42], may reduce the participation of glutamate in triggering the AD.
Unlike the A3 antagonists, the selective A1 receptor antagonist DPCPX impairs the recovery of synaptic potentials after a hypoxic insult [43]. In rats in vivo the nonselective adenosine receptor antagonist theophylline increases the incidence of spreading depression, the analogue of AD in normoxic conditions, and decreases the latency of spreading-depression occurrence elicited by microdialysis of high K+ perfusate through the hippocampal CA1 area [44]. Moreover, it has been demonstrated that the block of A1 receptors by theophylline or CPT (8-cyclopentyl-1,3-dimethylxanthine) shortens the onset of AD evoked by hypoxia in the CA1 region of gerbil hippocampal slices [45]. Therefore, during a prolonged severe OGD insult, the roles of A1 and A3 receptors drastically diverge. On the whole, the data support the well-established neuroprotective role of A1 receptors [9,12,41] and confirm an opposite, deleterious, role of A3 receptors during prolonged ischemia [14].
We then demonstrated that the block of A3 receptors by the selective A3 receptor antagonist MRS1523 reduces fEPSP inhibition caused by 2-min OGD and induces a faster recovery of fEPSP amplitude after 5-min OGD application. Unlike prolonged (7-min) OGD, depression of synaptic responses caused by OGD episodes of short duration (2 or 5 min) is fully reversible [13,27,28] and is not sufficient to trigger AD. The decrease in fEPSP depression or the faster recovery of fEPSP amplitude during the selective block of A3 receptors indicates an inhibitory role of A3 receptors on synaptic transmission during brief OGD. We previously demonstrated that depression of synaptic potentials during 2- or 5-min OGD is almost completely antagonized in the presence of the selective A1 adenosine receptor antagonist DPCPX [10,27,28]. Most of the known neuroprotective effects of adenosine during ischemia both in vivo and in vitro are attributed to the depression of synaptic activity induced by the activation of A1 receptors. Therefore, the decrease in synaptic depression brought about by A3 adenosine receptor antagonists suggests that A1 and A3 receptors cooperate in inhibiting fEPSP amplitude during the first minutes of OGD and share a neuroprotective role in these pathological conditions. This interpretation is in line with the observation that selective activation of A3 adenosine receptors under hypoxic conditions brings about inhibition of excitatory neurotransmission in rat cortical neurons [22] and that mice lacking A3 adenosine receptors have increased neurodegeneration in response to repeated episodes of moderate hypoxia [23].
Prolonged ischemic conditions could play a pivotal role in switching the effects of A3 receptor stimulation from A1-like inhibition to potentiation of an excitotoxic glutamate effect. The activation of phospholipase C by adenosine A3 receptors has been reported in striatal and hippocampal slices [46]. Rat cortical neurons exposed to hypoxia in vitro show an increase in activation of protein kinase C (PKC) after selective A3 receptor stimulation [47]. Similarly to what is described in the heart, PKC-dependent activation of KATP channels may enhance adenosine protection [48], but if OGD is applied long enough to be considered severe, protracted PKC activation induced by A3 receptors could account for an increase in intracellular calcium, which may participate in increasing tissue excitability and thus lead to irreversible synaptic failure.
Unexpectedly, our results show that the effects induced by Cl-IB-MECA, during OGD of different durations, are similar to those elicited by adenosine A3 antagonists. Cl-IB-MECA reduces fEPSP depression induced by 2-min OGD, induces a faster recovery of fEPSPs after 5-min OGD, and permits a full recovery of neurotransmission after prolonged (7-min) OGD. A decrease in fEPSP depression, brought about by A3 agonists, hypothetically might be attributed to A2A receptor stimulation. It was, in fact, previously demonstrated that selective stimulation of A2A receptors by CGS 21680 decreases fEPSP depression induced by 2-min OGD and that the effect is antagonized by the selective A2A antagonist ZM 241385 [28]. However, the concentration of Cl-IB-MECA used in the present study (10 nM) is highly selective for A3 versus A2A receptors [32], and the effect of Cl-IB-MECA is not antagonized by ZM 241385, indicating that Cl-IB-MECA-induced depression of fEPSP inhibition during 2-min OGD is not mediated by A2A adenosine receptors. Specific involvement of A3 receptors in the observed effects is also indicated by the observation that five chemically related compounds demonstrated similar effects to those elicited by Cl-IB-MECA. Moreover, the concentrations of Cl-IB-MECA and of MRS1523 used in the present study induce maximal effects. Therefore, the observation that the effects of Cl-IB-MECA and MRS1523 during 2- or 5- min OGD are not additive indicates that the effect of both drugs on fEPSP depression evoked by OGD is mediated by the same system (i.e., A3 receptors).
A reducing effect of Cl-IB-MECA on fEPSPs inhibition induced by adenosine has been attributed to a desensitization of A1 receptors [49]. Our results do not support such a mechanism because (i) the selective A3 receptor antagonist MRS1523 not only does not potentiate fEPSP inhibition induced by 2- and 5-min OGD but attenuates this effect and (ii) Cl-IB-MECA protects against AD appearance and irreversible disruption of excitatory neurotransmission caused by prolonged (7-min) OGD episodes, whereas A1 antagonism shortens the onset of AD evoked by hypoxia [43].
Therefore, the effects of Cl-IB-MECA are likely attributable to a rapid desensitization of A3 receptors. Further evidence in support of A3 desensitization is provided by the observation that the perfusion protocol of 15 min Cl-IB-MECA application still allows a significant recovery of neurotransmission when it was applied 20 min before 7-min OGD. It is worth noting that when Cl-IB-MECA was applied for a short time, it induced a slower recovery of fEPSPs after 5-min OGD instead of shortening the recovery as after long application. This indicates that a short application of Cl-IB-MECA is not sufficient to trigger receptor desensitization. The adenosine A3 receptor is particularly susceptible to phosphorylation by G protein-coupled receptor kinases [17,50]. Studies using CHO cells stably expressing the A3 receptors have demonstrated that, following a few minutes of exposure to the agonist, there is rapid phosphorylation of the A3 receptor, a reduction in number of A3 high affinity agonist binding sites [16], and receptor internalization [19]. In human astrocytoma cells, brief exposure (1-60 min) to nanomolar concentrations of Cl-IB-MECA results in a rapid uncoupling of A3 receptors and receptor internalization while a long-lasting (1-24 h) Cl-IB-MECA treatment leads to receptor down-regulation which recovers after several hours [20]. On this basis, in our experiments, 9 to 15 min of treatment with Cl-IB-MECA likely produces uncoupling and internalization of the A3 receptor. However, we cannot exclude subsequent down-regulation of receptor.
When considering that A3 receptors encounter desensitization after prolonged exposure to A3 agonists, we also have to consider that, under the OGD conditions used in the present study, endogenous adenosine released by the ischemic stimulus is not sufficient to cause A3 receptor desensitization. During hypoxic/ischemic conditions, adenosine is released from hippocampal slices, reaching concentrations up to 30 μM after 5-min OGD [10,41]. However, the efficacy of A3 antagonists in blocking the depression induced by OGD of different durations indicates that endogenous adenosine concentrations reached in the extracellular milieu alone are not sufficient to desensitize A3 receptors. Therefore, a stimulation as massive as that reached in the presence of endogenous adenosine plus exogenous A3 agonists is necessary to induce substantial A3 receptor plastic adjustments.
Conclusions
Our data indicate that the block of A3 receptors, stimulated by adenosine released during ischemia, counteracts fEPSP depression induced by OGD of different durations. A novel A3 antagonist, LJ1251, is protective at very low concentrations. Adenosine A3 receptors stimulated by adenosine released during brief periods of ischemia might exert A1-like protective effects on neurotransmission. Prolonged periods of ischemia would transform the A3 receptor-mediated effects from protective to injurious. Moreover, our data support the notion that A3 receptors undergo desensitization during exposure to an exogenous A3 agonist.
Increased knowledge of molecular mechanisms of A3 receptors during cerebral ischemia may increase our understanding of the utility of selective A3 agonists/antagonists for treatment of ischemic and other neurodegenerative disorders.
Acknowledgments
This work was supported by grants from the University of Florence, the “Fondazione Monte del Paschi di Siena”, Siena, Italy and the Italian Ministry of Health (MIUR) and by the NIDDK Intramural Research Program, National Institutes of Health (Bethesda, MD, USA).
Abbreviations
- AD
anoxic depolarization
- aCSF
artificial cerebral spinal fluid
- AR132
N6-methyl-2-phenylethynyladenosine
- AUC
area under curve
- CHO
Chinese hamster ovary
- Cl-IB-MECA
1-[2-chloro-6[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-β-D-ribofuranuronamide
- DMSO
dimethylsulfoxide
- fEPSP
field excitatory post synaptic potential
- LJ1251
(2R,3R,4S)-2-(2-chloro-6-(3-iodobenzylamino)-9H-purin-9-yl)tetrahydrothiophene-3,4-diol
- MRS1523
3-propyl-6-ethyl-5-[(ethylthio)carbonyl]-2-phenyl-4-propyl-3-pyridine carboxylate
- OGD
oxygen glucose deprivation
- PKC
protein kinase C
- SD
spreading depression
- VT72
N6-methoxy-2-phenylethynyladenosine
- VT158
N6-methoxy-2-phenylethynyl-5′-N-methylcarboxamidoadenosine
- VT160
N6-methoxy-2-(2-pyridinyl)-ethynyl-5′-N-methylcarboxamidoadenosine
- VT163
N6-methoxy-2-p-acetylphenylethynyl-5′-N-methylcarboxamidoadenosine
- ZM241385
4-(2-[7-amino-2-(2-furyl)(1,2,4)triazolo(2,3-a)(1,3,5,)triazin-5-yl amino]ethyl)phenol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou QY, Li C, Olah ME, Johnson RA, Stiles GL, Civelli O. Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor. Proc Natl Acad Sci USA. 1992;89:7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC. Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz-Hernandez M, Pereira MF, Pintor J, Cunha RA, Ribeiro JA, Miras-Portugal MT. Modulation of the rat hippocampal dinucleotide receptor by adenosine receptor activation. J Pharmacol Exp Ther. 2002;301:441–450. doi: 10.1124/jpet.301.2.441. [DOI] [PubMed] [Google Scholar]
- 5.Lopes LV, Rebola N, Pinheiro PC, Richardson PJ, Oliveira CR, Cunha RA. Adenosine A3 receptors are located in neurons of the rat hippocampus. Neuroreport. 2003;14:1645–1648. doi: 10.1097/00001756-200308260-00021. [DOI] [PubMed] [Google Scholar]
- 6.Ji XD, Gallo-Rodriguez C, Jacobson KA. A selective agonist affinity label for A3 adenosine receptors. Biochem Biophys Res Commun. 1994;203:570–576. doi: 10.1006/bbrc.1994.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunha RA, Constantino MC, Sebastiao AM, Ribeiro JA. Modification of A1 and A2A adenosine receptor binding in aged striatum, hippocampus and cortex of the rat. Neuroreport. 1995;6:1583–1588. doi: 10.1097/00001756-199507310-00029. [DOI] [PubMed] [Google Scholar]
- 8.Cunha RA, Johansson B, Constantino MD, Sebastiao AM, Fredholm BB. Evidence for high-affinity binding sites for the adenosine A2A receptor agonist [3H]CGS 21680 in the rat hippocampus and cerebral cortex that are different from striatal A2A receptors. Naunyn Schmiedebergs Arch Pharmacol. 1996;353:261–271. doi: 10.1007/BF00168627. [DOI] [PubMed] [Google Scholar]
- 9.Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- 10.Latini S, Bordoni F, Corradetti R, Pepeu G, Pedata F. Temporal correlation between adenosine outflow and synaptic potential inhibition in rat hippocampal slices during ischemia-like conditions. Brain Res. 1998;794:325–328. doi: 10.1016/s0006-8993(98)00304-7. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson KA. Adenosine A3 receptors: novel ligands and paradoxical effects. Trends Pharmacol Sci. 1998;19:184–191. doi: 10.1016/s0165-6147(98)01203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pugliese AM, Latini S, Corradetti R, Pedata F. Brief, repeated, oxygen-glucose deprivation episodes protect neurotransmission from a longer ischemic episode in the in vitro hippocampus: role of adenosine receptors. Br J Pharmacol. 2003;140:305–314. doi: 10.1038/sj.bjp.0705442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pugliese AM, Coppi E, Spalluto G, Corradetti R, Pedata F. A3 adenosine receptor antagonists delay irreversible synaptic failure caused by oxygen and glucose deprivation in the rat CA1 hippocampus in vitro. Br J Pharmacol. 2006;147:524–532. doi: 10.1038/sj.bjp.0706646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Lubitz DK, Lin RC, Popik P, Carter MF, Jacobson KA. Adenosine A3 receptor stimulation and cerebral ischemia. Eur J Pharmacol. 1994;263:59–67. doi: 10.1016/0014-2999(94)90523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer TM, Benovic JL, Stiles GL. Agonist-dependent phosphorylation and desensitization of the rat A3 adenosine receptor. Evidence for a G-protein-coupled receptor kinase-mediated mechanism. J Biol Chem. 1995;270:29607–29613. doi: 10.1074/jbc.270.49.29607. [DOI] [PubMed] [Google Scholar]
- 17.Palmer TM, Stiles GL. Identification of threonine residues controlling the agonist-dependent phosphorylation and desensitization of the rat A3 adenosine receptor. Mol Pharmacol. 2000;57:539–545. [PubMed] [Google Scholar]
- 18.Ferguson G, Watterson KR, Palmer TM. Subtype-specific kinetics of inhibitory adenosine receptor internalization are determined by sensitivity to phosphorylation by G protein-coupled receptor kinases. Mol Pharmacol. 2000;57:546–552. [PubMed] [Google Scholar]
- 19.Trincavelli ML, Tuscano D, Cecchetti P, Falleni A, Benzi L, Klotz KN, Gremigni V, Cattabeni F, Lucacchini A, Martini C. Agonist-induced internalization and recycling of the human A3 adenosine receptors: role in receptor desensitization and resensitization. J Neurochem. 2000;75:1493–1501. doi: 10.1046/j.1471-4159.2000.0751493.x. [DOI] [PubMed] [Google Scholar]
- 20.Trincavelli ML, Tuscano D, Marroni M, Falleni A, Gremigni V, Ceruti S, Abbracchio MP, Jacobson KA, Cattabeni F, Martini C. A3 adenosine receptors in human astrocytoma cells: agonist-mediated desensitization, internalization, and down-regulation. Mol Pharmacol. 2002;62:1373–1384. doi: 10.1124/mol.62.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trincavelli ML, Tuscano D, Marroni M, Klotz KN, Lucacchini A, Martini C. Involvement of mitogen protein kinase cascade in agonist-mediated human A3 adenosine receptor regulation. Biochim Biophys Acta. 2002;1591:55–62. doi: 10.1016/s0167-4889(02)00248-3. [DOI] [PubMed] [Google Scholar]
- 22.Hentschel S, Lewerenz A, Nieber K. Activation of A3 receptors by endogenous adenosine inhibits synaptic transmission during hypoxia in rat cortical neurons. Restor Neurol Neurosci. 2003;21:55–63. [PubMed] [Google Scholar]
- 23.Fedorova IM, Jacobson MA, Basile A, Jacobson KA. Behavioral characterization of mice lacking the A3 adenosine receptor: sensitivity to hypoxic neurodegeneration. Cell Mol Neurobiol. 2003;23:431–447. doi: 10.1023/A:1023601007518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen GJ, Harvey BK, Shen H, Chou J, Victor A, Wang Y. Activation of adenosine A3 receptors reduces ischemic brain injury in rodents. J Neurosci Res. 2006;84:1848–1855. doi: 10.1002/jnr.21071. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka E, Yamamoto S, Kudo Y, Mihara S, Higashi H. Mechanisms underlying the rapid depolarization produced by deprivation of oxygen and glucose in rat hippocampal CA1 neurons in vitro. J Neurophysiol. 1997;78:891–902. doi: 10.1152/jn.1997.78.2.891. [DOI] [PubMed] [Google Scholar]
- 26.Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev. 2001;81:1065–1096. doi: 10.1152/physrev.2001.81.3.1065. [DOI] [PubMed] [Google Scholar]
- 27.Latini S, Bordoni F, Pedata F, Corradetti R. Extracellular adenosine concentrations during in vitro ischaemia in rat hippocampal slices. Br J Pharmacol. 1999;127:729–739. doi: 10.1038/sj.bjp.0702591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Latini S, Bordoni F, Corradetti R, Pepeu G, Pedata F. Effect of A2A adenosine receptor stimulation and antagonism on synaptic depression induced by in vitro ischaemia in rat hippocampal slices. Br J Pharmacol. 1999;128:1035–1044. doi: 10.1038/sj.bjp.0702888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson WW, Collingridge GL. The LTP Program: a data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J Neurosci Methods. 2001;108:71–83. doi: 10.1016/s0165-0270(01)00374-0. [DOI] [PubMed] [Google Scholar]
- 30.Pedata F, Latini S, Pugliese AM, Pepeu G. Investigations into the adenosine outflow from hippocampal slices evoked by ischemia-like conditions. J Neurochem. 1993;61:284–289. doi: 10.1111/j.1471-4159.1993.tb03566.x. [DOI] [PubMed] [Google Scholar]
- 31.Li AH, Moro S, Melman N, Ji XD, Jacobson KA. Structure-activity relationships and molecular modeling of 3, 5-diacyl-2,4-dialkylpyridine derivatives as selective A3 adenosine receptor antagonists. J Med Chem. 1998;41:3186–3201. doi: 10.1021/jm980093j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Müller CE. Medicinal chemistry of adenosine A3 receptor ligands. Curr Top Med Chem. 2003;3:445–462. doi: 10.2174/1568026033392174. [DOI] [PubMed] [Google Scholar]
- 33.Jeong LS, Choe SA, Gunaga P, Kim HO, Lee HW, Lee SK, Tosh D, Patel A, Palaniappan KK, Gao ZG, Jacobson KA, Moon HR. Discovery of a new nucleoside template for human A3 adenosine receptor ligands: Truncated 4′-tihoadenosine derivatives as highly potent and selective antagonists. J Med Chem. 2007 doi: 10.1021/jm070259t. in press. [DOI] [PubMed] [Google Scholar]
- 34.Jacobson KA, Siddiqi SM, Olah ME, Ji XD, Melman N, Bellamkonda K, et al. Structure-activity relationships of 9-alkyladenine and ribose-modified adenosine derivatives at rat A3 adenosine receptors. J Med Chem. 1995;38:1720–1735. doi: 10.1021/jm00010a017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HO, Ji XD, Melman N, Olah ME, Stiles GL, Jacobson KA. Selective ligands for rat A3 adenosine receptors: structure-activity relationships of 1,3-dialkylxanthine 7-riboside derivatives. J Med Chem. 1994;37:4020–4030. doi: 10.1021/jm00049a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volpini R, Costanzi S, Lambertucci C, Taffi S, Vittori S, Klotz KN, Cristalli G. N6-Alkyl-2-alkynyl derivatives of adenosine as potent and selective agonists at the human adenosine A3 receptor and a starting point for searching A2B ligands. J Med Chem. 2002;45:3271–3279. doi: 10.1021/jm0109762. [DOI] [PubMed] [Google Scholar]
- 37.Volpini R, Lambertucci C, Taffi S, Vittori S, Klotz KN, Cristalli G. A3 adenosine receptors: synthesis and biological evaluation of new potent and selective ligands. Collection Symposium Series. 2005;7:297–300. [Google Scholar]
- 38.Dal Ben D, Lambertucci C, Taffi S, Vittori S, Volpini R, Klotz KN, Cristalli G. Molecular modeling study of 2-phenylethynyladenosine (PEAdo) derivatives as highly selective A3 adenosine receptor ligands. Purinergic Signalling. 2006;2:589–594. doi: 10.1007/s11302-006-9010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volpini R, Dal Ben D, Lambertucci C, Taffi S, Vittori S, Klotz KN, Cristalli G. N6-Methoxy-2-alkynyladenosine derivatives as highly potent and selective ligands at the human A3 adenosine receptor. J Med Chem. 2007;50:1222–1230. doi: 10.1021/jm060963u. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto S, Tanaka E, Shoji Y, Kudo Y, Inokuchi H, Higashi H. Factors that reverse the persistent depolarization produced by deprivation of oxygen and glucose in rat hippocampal CA1 neurons in vitro. J Neurophysiol. 1997;78:903–911. doi: 10.1152/jn.1997.78.2.903. [DOI] [PubMed] [Google Scholar]
- 41.Pearson T, Damian K, Lynas RE, Frenguelli BG. Sustained elevation of extracellular adenosine and activation of A1 receptors underlie the post-ischaemic inhibition of neuronal function in rat hippocampus in vitro. J Neurochem. 2006;97:1357–1368. doi: 10.1111/j.1471-4159.2006.03823.x. [DOI] [PubMed] [Google Scholar]
- 42.Macek TA, Schaffhauser H, Conn PJ. Protein kinase C and A3 adenosine receptor activation inhibit presynaptic metabotropic glutamate receptor (mGluR) function and uncouple mGluRs from GTP-binding proteins. J Neurosci. 1998;18:6138–6146. doi: 10.1523/JNEUROSCI.18-16-06138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sebastiao AM, de Mendonca A, Moreira T, Ribeiro JA. Activation of synaptic NMDA receptors by action potential-dependent release of transmitter during hypoxia impairs recovery of synaptic transmission on reoxygenation. J Neurosci. 2001;21:8564–8571. doi: 10.1523/JNEUROSCI.21-21-08564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaku T, Hada J, Hayashi Y. Endogenous adenosine exerts inhibitory effects upon the development of spreading depression and glutamate release induced by microdialysis with high K+ in rat hippocampus. Brain Res. 1994;658:39–48. doi: 10.1016/s0006-8993(09)90008-7. [DOI] [PubMed] [Google Scholar]
- 45.Lee KS, Lowenkopf T. Endogenous adenosine delays the onset of hypoxic depolarization in the rat hippocampus in vitro via an action at A1 receptors. Brain Res. 1993;609:313–315. doi: 10.1016/0006-8993(93)90888-t. [DOI] [PubMed] [Google Scholar]
- 46.Abbracchio MP, Brambilla R, Ceruti S, Kim HO, von Lubitz DK, Jacobson KA, Cattabeni F. G protein-dependent activation of phospholipase C by adenosine A3 receptors in rat brain. Mol Pharmacol. 1995;48:1038–1045. [PubMed] [Google Scholar]
- 47.Nieber K, Hentschel S. Signalling pathways of the adenosine A3 receptors in rat cortical neurons. Proceedings of the 8th International Symposium on adenosine and adenine nucleotides; Ferrara, Italy. May 24-28, 2006. [Google Scholar]
- 48.Liang BT. Protein kinase C-dependent activation of KATP channel enhances adenosine-induced cardioprotection. Biochem J. 1998;336:337–343. doi: 10.1042/bj3360337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunwiddie TV, Diao L, Kim HO, Jiang JL, Jacobson KA. Activation of hippocampal adenosine A3 receptors produces a desensitization of A1 receptor-mediated responses in rat hippocampus. J Neurosci. 1997;17:607–614. doi: 10.1523/JNEUROSCI.17-02-00607.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Ann Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]