Abstract
Leptin, an adipose tissue-derived hormone, has been linked to cardiovascular outcomes; however data are limited in the US population, especially in women. To assess the association between leptin concentrations and history of myocardial infarction (MI) and stroke independently of traditional cardiovascular risk factors. We analyzed data from 6,239 subjects (mean age 47 years; 3,336 women) with measurements of serum leptin and full assessment of cardiovascular risk factors from the National Health and Nutrition Examination Survey (NHANES) III. Logistic regression was used to estimate the cross-sectional association of leptin concentrations (highest quartile versus lowest quartile) and history of MI, stroke and the composite endpoint of MI or stroke (MI/stroke). Sex-specific models of leptin were adjusted for age, race, dyslipidemia, hypertension, diabetes, smoking and obese status. There were 212 men with MI/stroke (5.4%), 154 with MI (4.1%), and 82 with stroke (1.7%). There were 135 women with MI/stroke (2.6%), 74 with MI (1.5%), and 78 with stroke (1.4%). In multivariate analysis, high leptin was significantly and independently associated with MI/stroke in both men (OR, 2.41; 95% CI, 1.20 to 4.93) and women (OR, 4.26; 95% CI, 1.75-10.73); with MI in men (OR, 3.16; 95% CI, 1.40 to 7.37) and women (OR, 3.96; 95% CI, 1.29 to 12.72); and with stroke in women (OR, 3.20; 95% CI, 1.04-10.54) but not in men (OR, 1.37; 95% CI.0.38 to 3.88). In conclusion, in the US population, increased leptin concentrations are significantly associated with MI/stroke in men and women, independently of traditional cardiovascular risk factors and obese status.
Keywords: leptin, cardiovascular risk factors, myocardial infarction, stroke, NHANES
Several known cardiovascular actions of leptin on blood pressure, sympathetic activation, insulin resistance, platelet aggregation, arterial thrombosis, angiogenesis, and inflammatory vascular responses (1-10) suggest that leptin may play an important role in the development of cardiovascular disease. Leptin is an adipose tissue-derived hormone that may represent an important pathophysiologic link between obesity and cardiovascular disease. Consistent with this notion are the results of previous studies showing a possible independent association between leptin and cardiovascular events in patients with and without established atherosclerosis. (11, 12) However, there is few data assessing the effects of leptin on cardiovascular risk and if this risk is mediated by or independently of traditional cardiovascular risk factors in the US population. This study was undertaken to assess the relationship between serum leptin levels and self-reported myocardial infarction and stroke, in the US population.
Methods
The National Health and Nutrition Examination Survey (NHANES) is a representative sample of the US non-institutionalized civilian population from 1988 to 1994. It consists of a periodic survey conducted by the United States National Center for Health Statistics designed to provide an estimate of the health of the nation. Detailed methods used in NHANES III are available for public access on the internet: http://www.cdc.gov/nchs/nhanes.htm
Out of a sample of 39 695 people selected for the NHANES III, 33 994 were interviewed and 30 818 submitted to an examination by a physician at a mobile examination center, including extensive anthropometric, physiological, and laboratory testing. NHANES information was obtained from an in-home interview followed by a medical evaluation and blood sample collection at a mobile examination center. For this study, the sample was restricted to adult subjects aged ≥17 years, yielding an initial sample size of 18,162; however we limited the present analysis of both surveys to men and non-pregnant women aged 20 to 89 years old. We excluded data for those judged by their interviewer to have provided unreliable data, and those missing data for serum leptin or the self reported myocardial infarction/stroke (n=11,923). This resulted in a final analytic sample of 6,239 subjects (2,903 men and 3,336 women).
Height and weight were obtained using standardized techniques and equipment. Blood pressure was measured with the participant in a seated position following 5 minutes of quiet rest by a board-eligible physician at the NHANES Mobile Examination Center.
A morning, fasting venous blood sample was collected. Serum specimens were stored at −70°C and went through at least one freeze-thaw cycle during an average of 8 years of storage before measurement of leptin concentrations. Leptin has been shown to remain stable through five freeze-thaw cycles and after storage for as long as 29 years. (13) Serum leptin concentrations were measured by radioimmunoassay at Linco Research, Inc. (St. Charles, MO, USA). The minimum detectable concentration of the assay is 0.5 ng/ml. Intra- and interassay coefficients of variation are both <5%. (14) Cholesterol was measured quantitatively by a peroxidase-catalyzed reaction and high-density lipoprotein (HDL) was measured following the precipitation of the other lipoproteins using a Hitachi 704 Analyzer (Boehringer Mannheim Diagnostics). Low density lipoprotein (LDL) was calculated by using the formula: LDL = total cholesterol - (tryglycerides/5) - HDL. LDL-cholesterol was not calculated and reported as a missing value if the triglyceride level was >400 mg/dl. Fasting plasma glucose was measured using a modified hexokinase enzymatic method (Roche Cobas Mira). For the analysis of glucose, morning and ≥8 hours fasting samples were used. More detailed methodology and laboratory procedures of NHANES III are published elsewhere. (15)
Subjects were considered to have dyslipidemia if they reported current usage of medications to lower blood cholesterol, self reported diagnostic of hypercholesterolemia, HDL-cholesterol <40 mg/dl in men and <50 mg/dl in women, and/or LDL-cholesterol ≥160 mg/dl. (16) Subjects were considered to have diabetes if they reported current usage of anti-diabetic medications (insulin and oral medications), self reported diagnosis of diabetes and/or if their fasting plasma glucose was ≥126 mg/dl. (17) Subjects were considered to be hypertensive if they were taking antihypertensive medications, self reported diagnosis of hypertension and/or if their systolic pressure was ≥140 mm Hg or diastolic pressure was ≥90 mm Hg. (18) Subjects were considered to be in the smoking group if they were current, former or ever smokers (more than 100 cigarettes in their life). Obese status was defined as BMI≥30 and/or waist circumference ≥102 cm in men and ≥88 cm in women. We decided to combine measures of total and central obesity since BMI alone might not be the best measure of obesity and/or metabolic risk. (19, 20)
Presence of myocardial infarction (MI) was defined as self-reported MI based on the interview question: “Has a doctor ever told you that you had a heart attack?”. Presence of stroke was defined as self-reported stroke based on the interview question: “Has a doctor ever told you that you had a stroke?”. Our definition of MI and stroke was based upon information available in the NHANES III database. A composite endpoint (MI/stroke) was created (21) based on the presence of self-reported MI and/or stroke.
The analysis of the NHANES III data was conducted following the guidelines in ‘Analytic and Reporting Guidelines: The Third National Health and Nutrition Examination Survey, NHANES III (1988 to 1994)’. Data were summarized by calculating means and standard deviations for quantitative variables and percentages for qualitative variables. Mean leptin levels were significantly different between sexes (in men 6.1 ±5; in women 18.0 ±13, P<0.0001) so we stratified analyses by sex.
We defined “high leptin” as the highest sex-specific quartile of serum leptin (≥7.6 in men; ≥23.6 in women) when compared to the lowest quartile (used as reference). Also, we defined “high leptin/BMI ratio” as the highest sex-specific quartile of the serum leptin/BMI ratio (≥0.269 in men; ≥0.783 in women) when compared to the lowest quartile (used as reference). The calculated prevalences were adjusted (weighted) to the general United States population using weights calculated for that purpose by the National Center for Health Statistics.
We assessed the association between leptin levels and individual cardiovascular risk factors with Spearman correlation coefficients (due to the use of log leptin due to the skewness of the leptin distribution) which related the individual risk scores and leptin levels. We created logistic regression models to determine the association of high leptin, high leptin/BMI ratio, and cardiovascular risk factors with self-reported MI, stroke and MI/stroke. Leptin and each of the cardiovascular risk factors were included as dichotomous variables. We stratified the analyses by sex and controlled for age and race as possible confounders. Additional adjustments were used for high leptin and high leptin/BMI ratio that included age, race, dyslipidemia, high blood pressure, diabetes, smoking and obese status. All analyses were performed using the SAS windows version and SUDAAN (22).
Results
There were 6,239 subjects with serum leptin measurements who reported a history of MI or stroke. There were 212 men with MI/stroke (unadjusted prevalence 7.3%; adjusted prevalence 5.4%), 154 with MI (unadjusted prevalence 5.3%; adjusted prevalence 4.1%), and 82 with stroke (unadjusted prevalence 2.8%; adjusted prevalence 1.7%). There were 135 women with MI/stroke (unadjusted prevalence 4.0%; adjusted prevalence 2.6%), 74 with MI (unadjusted prevalence 2.2%; adjusted prevalence 1.5%), and 78 with stroke (unadjusted prevalence 2.3%; adjusted prevalence 1.4%).
Table 1 shows sex-specific descriptive characteristics. Men with MI/stroke were significantly older, more likely to be smokers and have high leptin, dyslipidemia, hypertension, and diabetes than men with no events. Women with MI/stroke were significantly older, heavier, and more likely to have high leptin, dyslipidemia, hypertension, and diabetes than women with no events. Nonetheless, we did not find a significant sex interaction in the relationship between leptin and MI/stroke.
Table 1.
Sex-Specific Descriptive Characteristics of Subjects with High Leptin and Traditional Cardiovascular Risk Factors by Self-Reported Events
| No Event | MI/ Stroke | MI | Stroke | |||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | Men | Women | |
| (n=2691) | (n=3201) | (n=212) | (n=135) | (n=154) | (n=74) | (n=82) | (n=78) | |
| Age (yrs) (M ±SD) | 45.9 ±18 | 45.8 ±18 | 68.5 ±13* | 69.8 ±13* | 68.7 ±12* | 69.3 ±13* | 69.5 ±13* | 70.7 ±13* |
| BMI (kg/m2) (M ±SD) | 26.7 ±5 | 27.5 ±6 | 27.2 ±5 | 29.1 ±7‡ | 27.4 ±5§ | 29.5 ±8§ | 26.5 ±4 | 28.9 ±6§ |
| Leptin (ng/ml) (M ±SD) | 5.9 ±4 | 17.8 ±12 | 8.4 ±6* | 23.4 ±15* | 8.6 ±7* | 24.5 ±17* | 7.5 ±5‡ | 22.5 ±12† |
| High leptin | 642 (24%) | 775 (24%) | 89 (42%)* | 64 (47%)* | 67 (43%)* | 34 (46%)* | 31 (38%)‡ | 38 (49%)* |
| Dyslipidemia | 1256 (47%) | 1812 (57%) | 140 (66%)* | 99 (73%)* | 107 (69%)* | 53 (72%)§ | 51 (62%)‡ | 59 (76%)† |
| Hypertension | 961 (37%) | 1129 (36%) | 150 (73%)* | 102 (77%)* | 107 (71%)* | 52 (72%)* | 60 (76%)* | 61 (79%)* |
| Diabetes mellitus | 242 (9) | 264 (8%) | 52 (25%)* | 42 (31%)* | 37 (24%)* | 20 (27%)* | 20 (24%)* | 26 (33%)* |
| Smokers | 1681 (62) | 1216 (38%) | 162 (76%)* | 60 (44%) | 121 (78%)* | 35 (47%) | 61 (74%)§ | 33 (42%) |
P<0.0001 vs. no event
P<0.001 vs. no event
P<0.01 vs. no event
P<0.05 vs. no event
Based on 6,239 subjects with leptin measurements reporting MI and stroke history
As shown in Table 2, we found a significant correlation between leptin levels and all cardiovascular risk factors, except for smoking. Obesity had the strongest correlation with leptin levels in both men and women.
Table 2.
Spearman Correlation Coefficients Relating Baseline Serum Leptin to Individual Traditional Cardiovascular Risk Factors
| Variable | Men
(n=2903) |
Women
(n=3336) |
|---|---|---|
| Age | 0.18* | 0.09* |
| Dyslipidemia | 0.17* | 0.14* |
| Hypertension | 0.23* | 0.20* |
| Diabetes mellitus | 0.12* | 0.06* |
| Smokers | 0.02 | -0.02 |
| Obesity | 0.60* | 0.59* |
P<0.01
Table 3 shows the age- and race-adjusted association of MI and stroke with high leptin and cardiovascular risk factors (dyslipidemia, hypertension, diabetes, and smoking). The association of leptin with MI and stroke was further adjusted for obese status.
Table 3.
Age and Race-Adjusted Cross-Sectional Association of MI and Stroke with High Leptin and the Traditional Cardiovascular Risk Factors in Men
| MI/ Stroke | MI | Stroke | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Dyslipidemia | 1.93 | 1.41-2.65 | <0.0001 | 2.21 | 1.54-3.23 | <0.0001 | 1.55 | 0.97-2.49 | 0.0656 |
| Hypertension | 2.06 | 1.47-2.92 | <0.0001 | 1.79 | 1.22-2.65 | 0.0030 | 2.17 | 1.27-3.85 | 0.0055 |
| Diabetes | 1.80 | 1.24-2.58 | 0.0015 | 1.71 | 1.12-2.57 | 0.0105 | 1.55 | 0.88-2.62 | 0.1067 |
| Smoking | 1.60 | 1.14-2.29 | 0.0076 | 1.83 | 1.22-2.80 | 0.0039 | 1.35 | 0.81-2.31 | 0.2539 |
| High leptin | 2.49 | 1.52-4.22 | 0.0004 | 2.62 | 1.48-4.91 | 0.0015 | 1.76 | 0.86-3.92 | 0.1368 |
| High leptin/BMI ratio | 2.04 | 1.27-3.37 | 0.0039 | 1.99 | 1.16-3.58 | 0.0153 | 1.98 | 0.95-4.54 | 0.0821 |
| Adjusted-High leptin* | 2.41 | 1.20-4.93 | 0.0139 | 3.16 | 1.40-7.37 | 0.0061 | 1.37 | 0.48-3.88 | 0.5462 |
Adjusted for Age, Race plus Dyslipidemia, Hypertension, Diabetes, Smoking and Obese status
High leptin was defined as the highest quartile of serum leptin (> 7.5 in men) when compared to the lowest quartile.
In multivariate analysis, high leptin was significantly and independently associated with MI/stroke in both men (OR, 2.41; 95% CI, 1.20 to 4.93) and women (OR, 4.26; 95% CI, 1.75-10.73), after adjusting for age, race, dyslipidemia, hypertension, smoking and obese status. Also, after these adjustments, high leptin was significantly and independently associated with MI in men (OR, 3.16; 95% CI, 1.40 to 7.37) and women (OR, 3.96; 95% CI, 1.29 to 12.72); and with stroke in women (OR, 3.20; 95% CI, 1.04-10.54).
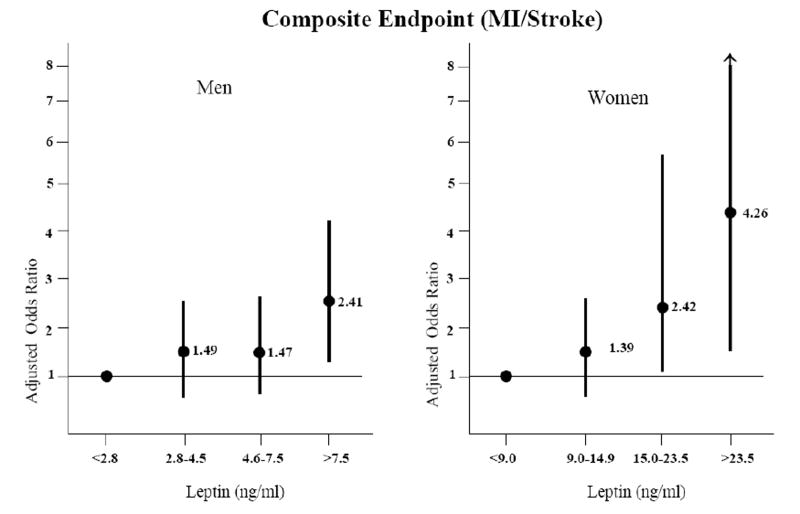
When leptin was corrected for BMI and expressed as leptin/BMI ratio, a similar relationship was observed for MI/stroke (in men OR, 2.04; 95% CI, 1.27-3.37; in women OR, 2.89; 95% CI, 1.72-5.00). Figure 1 shows the sex-specific adjusted OR between high leptin and MI/stroke by quartiles of leptin concentrations.
Figure 1.
Sex-specific association between leptin (determined by the sample quartiles) and odds ratios for the composite endpoint (MI/stroke) after adjustment for age, race, dyslipidemia, hypertension, diabetes, smoking and obese status. Each quartile of leptin is compared to the lowest one (used as reference).
When we compared the 6,239 subjects with complete data with the 10,195 subjects with missing leptin data but with self-reported MI and/or stroke, subjects with missing data were on average slightly older for both men (49.4 versus 47.5 years; P<0.0001) and women (49.3 versus 46.2 years; P<0.0001); however, they did not differ significantly with respect to sex and obese status (where reported). The percentage of subjects having a history of MI or stroke was much higher among the subjects with missing data for both men (9.9% versus 7.3%, P<0.0001) and women (7.1% versus 4.1%, P<0.0001).
Discussion
The novel finding of this cross-sectional analysis of a representative sample of the US civilian population is that high leptin is significantly associated with MI/stroke in men and women, independently of several cardiovascular risk factors and obese status. These findings suggest that leptin may have clinical utility in identifying men at women at increased risk for MI and stroke.
In men, leptin was found to be a modest but independent predictor of coronary events during a five-year follow-up period in the West of Scotland Coronary Prevention Study (WOSCOPS). (23) Conversely, a smaller study of men in the Quebec Cardiovascular Study population found no association between leptin levels and coronary events. (24)
Recently, Wolk et al reported that in 361 subjects from a prospective cohort of patients undergoing clinically indicated coronary angiography, leptin was an independent predictor of future cardiovascular events.(12) In a nested case-referent study of 276 cases for a first-ever stroke, Södeberg et al reported that high leptin predicted stroke in men independently of traditional risk factors. (11)
Obesity is known to be associated with high leptin; therefore, obesity represents a major confounder in the association between leptin and cardiovascular events. Based on this, to have a more accurate estimate, we decided to control for obese status using measures of both, total and central body fat. In our analyses, leptin remained significantly associated with MI/stroke even after adjusting for obese status, supporting the independent effect of leptin in the association with MI/stroke.
In addition, leptin levels are higher in women than men, suggesting an important biological sex-specific difference. It is possible that women may have lower responses or are less sensitive to the actions of leptin in the central sympathetic nervous system. (25) Our stratified analyses showed an increased risk of nearly two-fold for MI/stroke in women with high leptin when compared to men.
NHANES was designed with the aim that the results of this analysis are generalizable to all persons in the US. NHANES uses a stratified multistage probability sampling design to produce estimates generalizable to the US population. Comparison of weighted-prevalence rates between the high leptin group and excluded subjects showed a higher prevalence for MI/stroke (for high leptin 7.9%; for excluded patients 5.7%, p<0.0001), for MI (for high leptin 5.8%; for excluded patients 4.1%, p<0.0001), and for stroke (for high leptin 2.7%; for excluded patients 2.1%, p<0.0001) in subjects with high leptin than in excluded subjects. Due to the fact that the percentage of subjects having a history of MI or stroke was much higher among the subjects with missing leptin data, we believe that the strength and significance of the association of leptin with cardiovascular events could possibly be further enhanced in a cohort with a greater number of cardiovascular events.
Due to its cross-sectional design, fatal events were not considered, limiting this study’s ability to determine causal relation between risk factors and cardiovascular outcomes; hence, we do not know if high leptin causes adverse cardiovascular events or if the presence of cardiovascular events raises the levels of leptin. Unfortunately NHANES does not account for the severity of cardiovascular event, such that, individuals with MI and stroke might incur disability that would tend to make them more sedentary and gain weight, maybe increasing leptin concentrations from its pre-event levels. The opposite bias could instead be present if the events are predominantly of modest severity and act to motivate healthier behaviors. However, without any measure of event severity it is impossible to determine just how strong the reverse-causality bias might be in this particular instance, so the results should be carefully interpreted.
Furthermore, MI and stroke history in the present analysis are based on self-report of physician – diagnosed disease and thus subject to recall bias. Using self-reported definitions of cardiovascular disease (without including information from the Rose Questionnaire, for example) reduces the prevalence of cardiovascular disease by 2–3%. (26) However, we would not expect this to significantly alter our results.
Other known confounders for cardiovascular outcomes such as physical activity and diet, were not included in these analyses. We decided not to include these confounders due to the nature of the NHANES cross-sectional design which limits their use due to the possible reverse causality of these variables. (27)
Although available information indicates that leptin remains stable through 5 freeze-thaw cycles (15), NHANES methodology states that there is the possibility that serum could have undergone > 5 freeze-thaw cycles during the average 8 years of storage. Based on this, leptin measurements could be compromised; however, we believe that this occurrence would direct the results towards the null hypothesis or no association.
Table 4.
Age and Race-Adjusted Cross-Sectional Association of MI and Stroke with High Leptin and the Traditional Cardiovascular Risk Factors in Women
| MI/ Stroke | MI | Stroke | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Dyslipidemia | 1.76 | 1.18-2.67 | 0.0060 | 1.56 | 0.94-2.69 | 0.0903 | 1.97 | 1.17-3.45 | 0.0128 |
| Hypertension | 1.88 | 1.21-3.00 | 0.0057 | 1.36 | 0.78-2.46 | 0.2784 | 2.07 | 1.16-3.88 | 0.0165 |
| Diabetes | 2.90 | 1.89-4.38 | <0.0001 | 2.28 | 1.28-3.92 | 0.0038 | 2.87 | 1.64-4.77 | <0.0001 |
| Smoking | 1.84 | 1.26-2.67 | 0.0014 | 1.97 | 1.21-3.22 | 0.0061 | 1.66 | 1.02-2.69 | 0.0364 |
| High leptin | 3.42 | 2.01-6.03 | <0.0001 | 3.29 | 1.65-7.08 | 0.0012 | 3.22 | 1.65-6.69 | 0.0009 |
| High leptin/BMI ratio | 2.89 | 1.72-5.00 | <0.0001 | 3.11 | 1.58-6.53 | 0.0016 | 2.56 | 1.33-5.16 | 0.0061 |
| Adjusted-High leptin* | 4.26 | 1.75-10.73 | 0.0016 | 3.96 | 1.29-12.72 | 0.0178 | 3.20 | 1.04-10.54 | 0.0474 |
Adjusted for Age, Race plus Dyslipidemia, Hypertension, Diabetes, Smoking and Obese status
High leptin was defined as the highest quartile of serum leptin (>23.5 in women) when compared to the lowest quartile.
Acknowledgments
The data reported here have been analyzed using NHANES files available for public use. All the analysis, interpretation and/or conclusion reached in this paper are work of the authors and not of the National Center for Health Statistics.
Dr. Virend K. Somers is supported in part by NIH grants HL-65176, HL-70302, HL-73211 and M01-RR00585.
Dr. Lopez-Jimenez is a recipient of a Clinical Scientist Development Award from the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Considine RV. Human leptin: an adipocyte hormone with weight-regulatory and endocrine functions. Semin Vasc Med. 2005;5(1):15–24. doi: 10.1055/s-2005-871738. [DOI] [PubMed] [Google Scholar]
- 2.Cooke JP, Oka RK. Does leptin cause vascular disease? Circulation. 2002;106:1904–1905. doi: 10.1161/01.cir.0000036864.14101.1b. [DOI] [PubMed] [Google Scholar]
- 3.Montani JP, Antic V, Yang Z, Dulloo A. Pathways from obesity to hypertension: from the perspective of a vicious triangle. Int J Obes Relat Metab Disord. 2002;26(Suppl 2):S28–38. doi: 10.1038/sj.ijo.0802125. [DOI] [PubMed] [Google Scholar]
- 4.Beltowski J, Wojcicka G, Gorny D, Marciniak A. Human leptin administered intraperitoneally stimulates natriuresis an decreases renal medullary Na+, K+-ATPase activity in the rat– impaired effect in dietary-induced obesity. Med Sci Monit. 2002;8:BR221–229. [PubMed] [Google Scholar]
- 5.Konstantinides S, Schafer K, Koschnick S, Loskutoff DJ. Leptin-dependent platelet aggregation and arterial thrombosis suggests a mechanism for atherothrombotic disease in obesity. J Clin Invest. 2001;108:1533–1540. doi: 10.1172/JCI13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodary PF, Westrick RJ, Wickenheiser KJ, Shen Y, Eitzman DT. Effect of leptin on arterial thrombosis following vascular injury in mice. JAMA. 2002;287:1706–1709. doi: 10.1001/jama.287.13.1706. [DOI] [PubMed] [Google Scholar]
- 7.Chu NF, Spiegelman D, Rifai N, Hotamisligil GS, Rimm EB. Glycemic status and soluble tumor necrosis factor receptor levels in relation to plasma leptin concentrations among normal weight and overweight US men. Int J Obes Relat Metab Disord. 2000;24:1085–1092. doi: 10.1038/sj.ijo.0801361. [DOI] [PubMed] [Google Scholar]
- 8.Chaldakov GN, Fiore M, Stankulov IS, Hristova M, Antonelli A, Manni L, Ghenev PI, Angelucci F, Aloe L. NGF, BDNF, leptin, and mast cells in human coronary atherosclerosis and metabolic syndrome. Arch Physiol Biochem. 2001;109:357–360. doi: 10.1076/apab.109.4.357.4249. [DOI] [PubMed] [Google Scholar]
- 9.Soderberg S, Ahren B, Stegmayr B, Johnson O, Wiklund PG, Weinehall L, Hallmans G, Olsson T. Leptin is a risk marker for first-ever hemorrhagic stroke in a population-based cohort. Stroke. 1999;30:328–337. doi: 10.1161/01.str.30.2.328. [DOI] [PubMed] [Google Scholar]
- 10.Wolk R, Deb A, Caplice NM, Somers VK. Leptin receptor and functional effects of leptin in human endothelial progenitor cells. Atherosclerosis. 2005;183(1):131–139. doi: 10.1016/j.atherosclerosis.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 11.Soderberg S, Stegmayr B, Stenlund H, Sjostrom LG, Agren A, Johansson L, Weinehall L, Olsson T. Leptin, but not adiponectin, predicts stroke in males. J Intern Med. 2004;256(2):128–136. doi: 10.1111/j.1365-2796.2004.01351.x. [DOI] [PubMed] [Google Scholar]
- 12.Wolk R, Berger P, Lennon RJ, Brilakis ES, Johnson BD, Somers VK. Plasma leptin and prognosis in patients with established coronary atherosclerosis. J Am Coll Cardiol. 2004;44(9):1819–1824. doi: 10.1016/j.jacc.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 13.Ruhl CE, Everhart JE. Leptin concentrations in the United States: relations with demographic and anthropometric measures. Am J Clin Nutr. 2001;74(3):295–301. doi: 10.1093/ajcn/74.3.295. [DOI] [PubMed] [Google Scholar]
- 14.Ruhl CE, Everhart JE. Relationship of serum leptin concentration with bone mineral density in the United States population. J Bone Miner Res. 2002;17(10):1896–1903. doi: 10.1359/jbmr.2002.17.10.1896. [DOI] [PubMed] [Google Scholar]
- 15.http://www.cdc.gov/nchs/about/major/nhanes/NHANESIII_Reference_Manuals.htm
- 16.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3413–3421. [PubMed] [Google Scholar]
- 17.American Diabetes Association. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 18.Chobanian A, Bakris G, Black H, Cushman W. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 19.Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Mookadam F, Lopez-Jimenez F. Association between Body Weight with Mortality and with Cardiovascular Events in Patients with Coronary Disease: A Systematic Review of Cohort Studies. The Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 20.Sierra-Johnson J, Johnson BD, Bailey KR, Turner ST. Relationships between insulin sensitivity and measures of body fat in asymptomatic men and women. Obes Res. 2004;12(12):2070–2077. doi: 10.1038/oby.2004.258. [DOI] [PubMed] [Google Scholar]
- 21.Ninomiya JK, L’Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. Association of the Metabolic Syndrome with History of Myocardial Infarction and Stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004;109:42–46. doi: 10.1161/01.CIR.0000108926.04022.0C. [DOI] [PubMed] [Google Scholar]
- 22.SAS Institute Inc. JMP statistics and graphic guide 6.0 version 1995 [Google Scholar]
- 23.Wallace AM, McMahon AD, Packard CJ. Plasma leptin and the risk of cardiovascular disease in the West Of Scotland Coronary Prevention Study (WOSCOPS) Circulation. 2001;104:3052–3056. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 24.Couillard C, Lamarche B, Mauriege P. Leptinemia is not a risk factor for ischemic heart disease in men Prospective results from the Quebec Cardiovascular Study. Diabetes Care. 1998;21:782–786. doi: 10.2337/diacare.21.5.782. [DOI] [PubMed] [Google Scholar]
- 25.Wauters M, Van Gaal L. Gender differences in leptin levels and physiology: a role for leptin in human reproduction. J Gend Specif Med. 1999 Sep-Oct;2(5):46–51. [PubMed] [Google Scholar]
- 26.LaCroix AZ, Haynes SG, Savage DD, Havlik RJ. Rose Questionnaire angina among United States black, white, and Mexican-American women and men. Prevalence and correlates from the second national and Hispanic health and nutrition examination surveys. Am J Epidemiol. 1989;129:669–686. doi: 10.1093/oxfordjournals.aje.a115183. [DOI] [PubMed] [Google Scholar]
- 27.LaMonte MJ, Nichaman MZ, Blair SN. Physical activity and the metabolic syndrome association with myocardial infarction and stroke. Circulation. 2004;109(22):e314. doi: 10.1161/01.CIR.0000129350.05560.EB. [DOI] [PubMed] [Google Scholar]