Abstract
In this letter, we report the first observation of surface plasmon-coupled chemiluminescence (SPCC), where the luminescence from chemically induced electronic excited states couples to surface plasmons in a thin continuous silver film. The SPCC is highly directional and predominantly p-polarized, strongly suggesting that the emission is from surface plasmons instead of the luminophores directly themselves. This indicates that surface plasmons can be directly excited from chemically induced excited states. With a wealth of assays that employ chemiluminescence based detection currently in use, then our findings suggest new chemiluminescence sensing strategies based on localized, directional and polarized chemiluminescence detection.
Keywords: Surface plasmon-coupled emission (SPCE), surface plasmons, radiative decay engineering, plasmon controlled fluorescence (PCF), metal enhanced fluorescence (MEF), surface enhanced fluorescence (SEF), chemiluminescence, luminescence, fluorescence
1.0 Introduction
The utility of chemiluminescence reactions and materials as an analytical tool is widely established [1–4]. Compared to fluorescence-based detection, chemiluminescence offers practical simplicity, significantly reduced background interference as the entire sample is not externally excited and the fact that no optical filters are required as there is no external excitation. Chemiluminescent detection however, is currently limited by the choice of probes available, and in some cases the toxicity of reagents and the need for particular reagents to create chemically induced electronic excited states [1–5]. In order to enhance the utility of chemiluminescence based detection technologies, there is an urgent unequivocal need for increased luminescence yields as well as signal collection efficiency, since this would benefit overall detectability and therefore in the context of bioassays, the sensitivity towards a particular analyte [1–5].
In the last few years, we have published numerous reports where we describe the use of metallic surfaces and nanoparticles to modify the emissive properties of fluorophores [5–10]. We reported that under appropriate conditions, close proximity of fluorophores to silver nanoparticles can lead to increased quantum yields and radiative decay rates, increased photostability and decreased lifetimes [10]. We explained the effects of metals on fluorescence using a simple concept based on radiating plasmons called the radiating plasmon model (RPM) [11–12]. In addition, we have also reported the first observation of metal-enhanced chemiluminescence (MEC) where Silver Island films (a non-continuous surface) in close proximity to chemiluminescing species, significantly enhanced the luminescence intensity [13–14]. This suggested that surface plasmons can be directly excited by chemically induced electronically excited luminophores [13–14].
Our studies on fluorophoremetal interactions have led us to demonstrate that resonance interactions can occur between excited fluorophores in close proximity to thin continuous films of metal attached to glass prisms, resulting in the generation of surface plasmons and the directional emission of the photophysical properties of the excited fluorophores on the glass side. We subsequently termed this phenomenon as surface plasmon-coupled emission (SPCE) [8–9, 15–20]. We have also shown that SPCE can be generated on gold films by electrochemical excitation of [Ru(bpy)3]2+ near a gold electrode [21]. This was a significant finding because it clearly demonstrated that surface plasmons can be excited by electrochemically induced electronic excited states. It also showed that electrochemiluminescence can be coupled to plasmons on thin metal films, resulting in directional emission, as compared to the more classical fluorophore isotropic emission.
In this letter, we subsequently report for the first time, the observation of surface plasmon-coupled chemiluminescence (SPCC), where the luminescence from chemically induced electronic excited states couple to surface plasmons in a thin continuous silver film. This results in highly directional and polarized emission of the luminescence from the prism side, Figure 1. This strongly indicates that surface plasmons can also be directly excited from chemically induced excited luminophores, which in turn radiate the photophysical properties of the excited luminophore.
Figure 1.
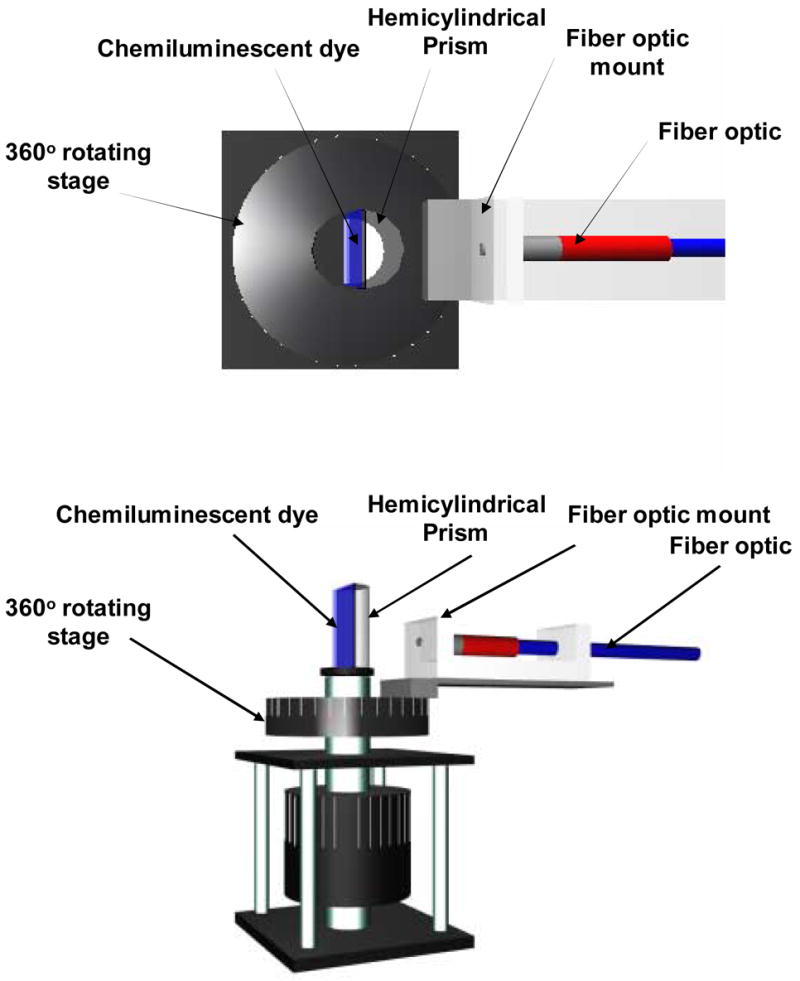
Experimental geometry used for surface plasmon coupled chemiluminescence (SPCC). Top – View from the top, Bottom – side view.
2.0 Materials and Methods
Premium quality APS-coated glass slides (75×25 mm), silver wire (99.99+% purity) and silicon monoxide pieces (99.99% purity) were obtained from Sigma-Aldrich (St. Loius, MO). CoverWell imaging chamber gaskets with adhesive (20 mm diameter, 1 mm deep) were obtained from Molecular Probes (Eugene, OR). The smaller imaging chambers were built in-house using electrical black tape, double sticky tape and microscope coverslips. Standard chemiluminescence kits from Omnioglow (West Springfield, MA) were used as the source of chemiluminescence. These kits contain the reacting chemicals encapsulated inside a plastic tube. Inside the plastic tube lies a glass capsule containing the activating agent (in this case hydrogen peroxide). Activation of the chemicals is accomplished with a bend, snap, and a vigorous shake of the plastic tube which breaks the glass capsule containing the peroxide and mixes the chemicals to begin the chemiluminescence reaction.
45 nm of silver was deposited on APS-coated glass slides using an Edwards Auto 306 Vacuum Evaporation chamber (West Sussex, UK) under ultra high vacuum (< 3 × 10−6 Torr). In each case, the metal deposition step was followed by the deposition of 5 nm of silica via evaporation without breaking vacuum. This step served to protect the metal surface from attack by the various chemical species present in the chemiluminescence assay.
The Surface Plasmon Coupled Chemiluminescence (SPCC) experiments were performed by first bending the plastic tube of the chemiluminescence kit and shaking it vigorously. This allowed the reaction mixtures to mix and begin to luminesce. The tube was then cut, and 150 μL of the reacting fluid was then placed in an imaging chamber gasket with adhesive (20 mm diameter, 1 mm deep). This gasket was then pressed against a silver coated and silica capped microscope glass slide until they were stuck together creating a chamber containing the chemiluminescent dye on the surface of the metal coated glass slide. For smaller samples, approximately 50 μl of the reacting fluid was placed in an imaging chamber built in-house attached to an (APS-coated) continuous metal coated and silica capped microscope glass slide.
The metal coated slides containing the chemiluminescent dyes were attached to a hemicylindrical prism made with BK7 glass (n = 1.52) and the refractive index were matched using spectrophotometric grade glycerol (n = 1.475) between the back of the glass slide (uncoated side) and the prism. This unit was then placed on a precise 360° rotatory stage which was built in-house. The rotatory stage allowed the collection of light at all angles around the sample chamber, Figure 1. An Ocean Optics low OH 600 μm diamerter optical fiber with NA of 0.22 (Dunedin, FL) used for collecting the chemiluminecence signals was mounted on a holder that was screwed onto the base of the rotatory stage. A pictorial representation of the setup is presented in Figure 1. Surface Plasmon Coupled Chemiluminescence (SPCC) spectra were collected using a model SD 2000 Ocean Optics spectrometer (Dunedin, FL) connected to the optical fiber. The spectra were collected with an integration time of 0.5 seconds. Unpolarized, p and s-polarized signal information were collected for the SPCC signal (from 0 – 180° with respect to the front of the prism) and the free-space signal (from 180 – 360° with respect to the front of the prism). A time dependent decay study was performed on the chemiluminescent dye to study the comparative time dependent decay profile of the SPCC signal and the free-space signal.
3.0 Results and Discussion
Figure 2 (Middle) shows the surface plasmon-coupled chemiluminescence (SPCC) and the free-space emission from the sample chamber. It is important to note that the free-space emission is of much higher magnitude than the SPCC signal. This is because the sample chamber was 1 mm thick and hence only the luminophores within approximately 250 nm of the surface of silver are known to excite surface plasmons [8–9]. Hence the majority of the luminophores in the chamber did not couple to plasmons and so radiated their energy out in the form of free-space emission. We subsequently attempted to use very thin films of liquid to alleviate this effect. However, the hydrophobic nature of the surface globulated the chemiluminescence liquid, preventing films less < 250 nm thick to be produced. Given that clinical/biochemical assays are typically substantially smaller than 250 nm, indeed as we have shown in numerous SPCE publications [15–20], then similarly we expect surface-bound chemiluminescence assays to be also highly useful.
Figure 2.
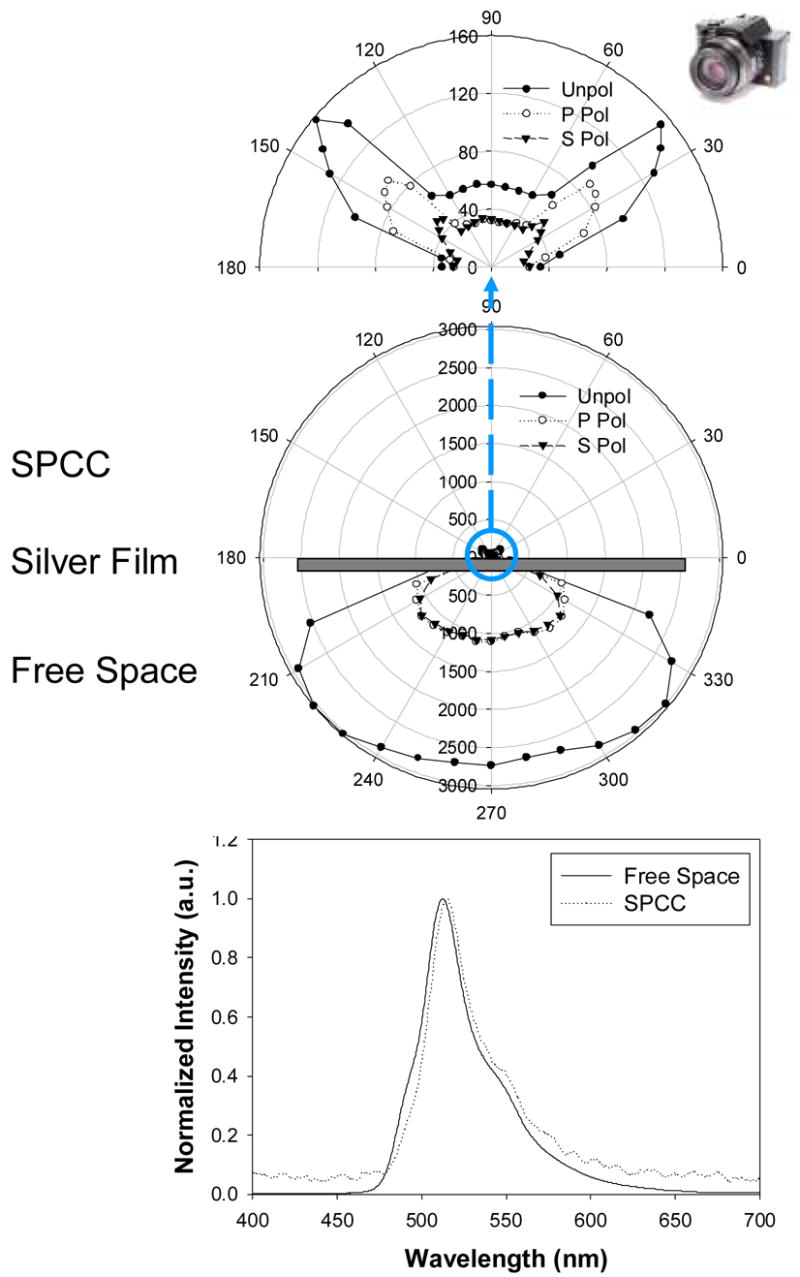
Surface Plasmon coupled chemiluminescence from 45 nm silver films. Top – enlarged directional SPCC, Middle – free-space chemiluminescence and SPCC, Bottom – Emission spectra of both the free-space chemiluminescence and SPCC.
Figure 2 (Top) is an enlarged figure showing the highly directional and p-polarized SPCC emission, suggesting that the observed signal is due to surface plasmons. This is in stark contrast to that of the free-space emission which does not show any polarization or directional preference. It is however worth noting that the signal at the SPCC peak angle is not entirely p-polarized. This is in contrast with our past experiences with optically pumped SPCE experiments where the SPCE signal was almost entirely p-polarized [8–9, 15–20]. Figure 2 (Bottom) is the normalized SPCC and free-space emission spectra showing a high degree of overlap between the spectra. This suggests the plasmon-coupled chemiluminescence has not undergone any changes in its photophysical properties, but is simply directional instead of isotropic.
Figure 3 shows photographs taken from the prism side at the SPCC angle (40 deg) without polarizers and also with p and s-polarization. The figure clearly shows that the emission at the SPCC peak angle is predominantly p-polarized thus suggesting that surface plasmons are responsible for the SPCC signal, as has been demonstrated for fluorophores [8, 9]. The reason the p-polarized signal intensity at the SPCC peak angle (in both Figure 2 and 3) is lower in magnitude than the unpolarized signal is because: (i) the entire SPCC signal consists of both p and to a lesser degree s-polarized light and (ii) the sheet polarizers used in the experiment have only 30 – 40 % peak transmission efficiency for both polarizations.
Figure 3.
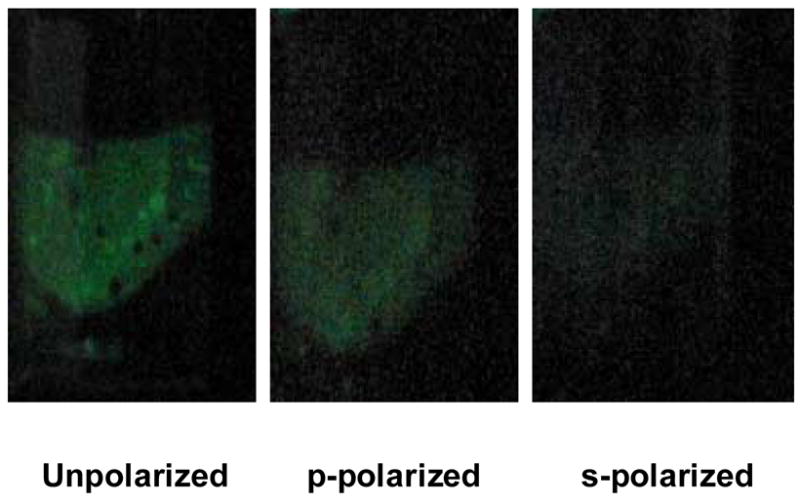
Photographs of the coupled emission at various polarizations taken at 40 degrees. See location of camera in Figure 2 (Top).
At first we were surprised by the broadness of the SPCC peak angle, which varied between 20–25 degrees, Figure 2 (Top). This is unlike our previous experiences with optically pumped SPCE studies for a thin layer of fluorophores, where the SPCE peak angle was approximately 1–2 degrees [8–9, 15–20]. In order to investigate if the broadness of the SPCC peak is dependent on the surface area of the sample, we repeated the experiment with a sample chamber built in-house that had approximately half the surface area when compared to the samples made with commercially available imaging chambers that had been used thus far. Figure 4 (Top) shows the unpolarized, p and s-polarized SPCC signal from the small sample chamber (built in-house) and Figure 4 (Bottom) shows the parallel SPCC signals from the commercially available sample chamber. It is clear from this figure that the broadness of the SPCC peak angle is not significantly affected by the surface area of the sample. An interesting observation in Figure 4 (Top) is the decay in the SPCC signal in the region between 90 – 180 degrees when compared to that in the 0 – 90 degrees. This is because we collected data sequentially from 0 through 360 degrees. As a result, for the small chamber where there was a lower volume of reactants, by the time we got to the region between 90 – 180 degrees we see a signal reduction due to the depletion of reactants (depletion of excited states) over time. Recently our laboratory has reported the effects of fluorophores in thick plastic films (> 250 nm) above 50 nm thick metallic continuous surfaces [22]. Similar to our findings here, these reports show a broader angle dependence of SPCE, due to waveguide modes [22]. Subsequently we attribute the broad angle distribution shown in figure 2 and 4 to this waveguide effect, given that our solution of chemiluminescence was ⋙ 250 nm, some estimated 1 mm.
Figure 4.
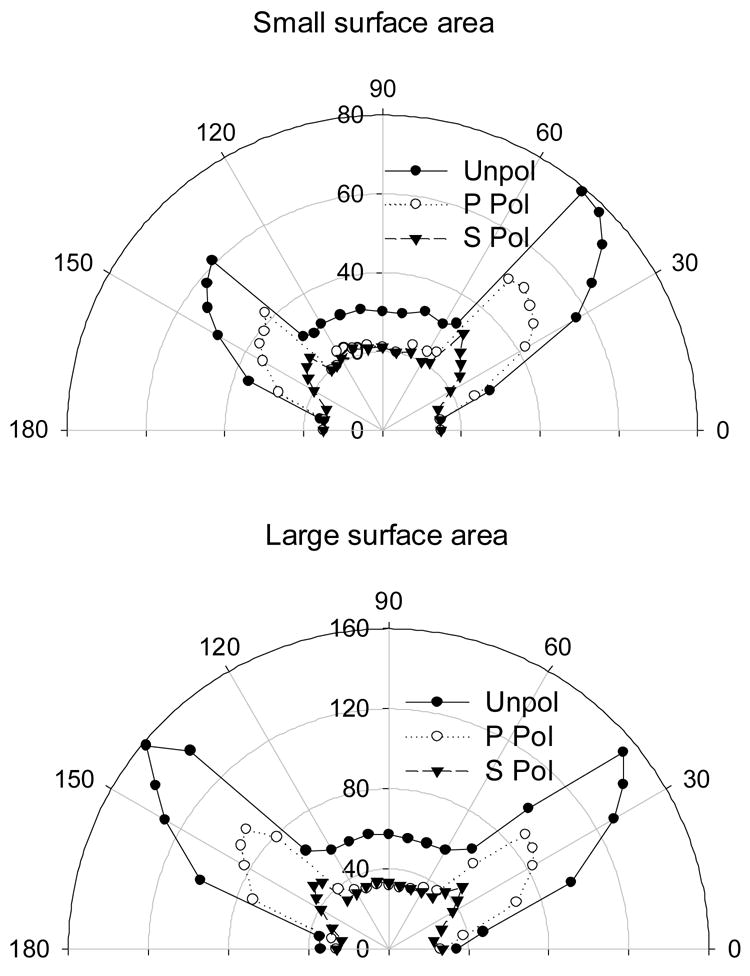
Surface Plasmon Coupled Chemiluminescence (SPCC) from both small and large sample chambers, Top and Bottom respectively.
Finally we undertook several experiments to determine the rate of decay of luminescence as a function of time for both the free-space emission as well as the SPCC emission (with p-polarizers so that only plasmon coupled emission was measured). The results of these experiments is shown in Figure 5 where the top image shows the decay of free-space and SPCC emission as a function of time, the bottom image showing both the decay intensities normalized to their respective values at t = 0. The rate of loss of luminescence, which is a due to the depletion of solution reactants and therefore depletion over time of excited states, was found to follow first-order decay kinetics and can be modeled to an exponential function of the form:
Figure 5.
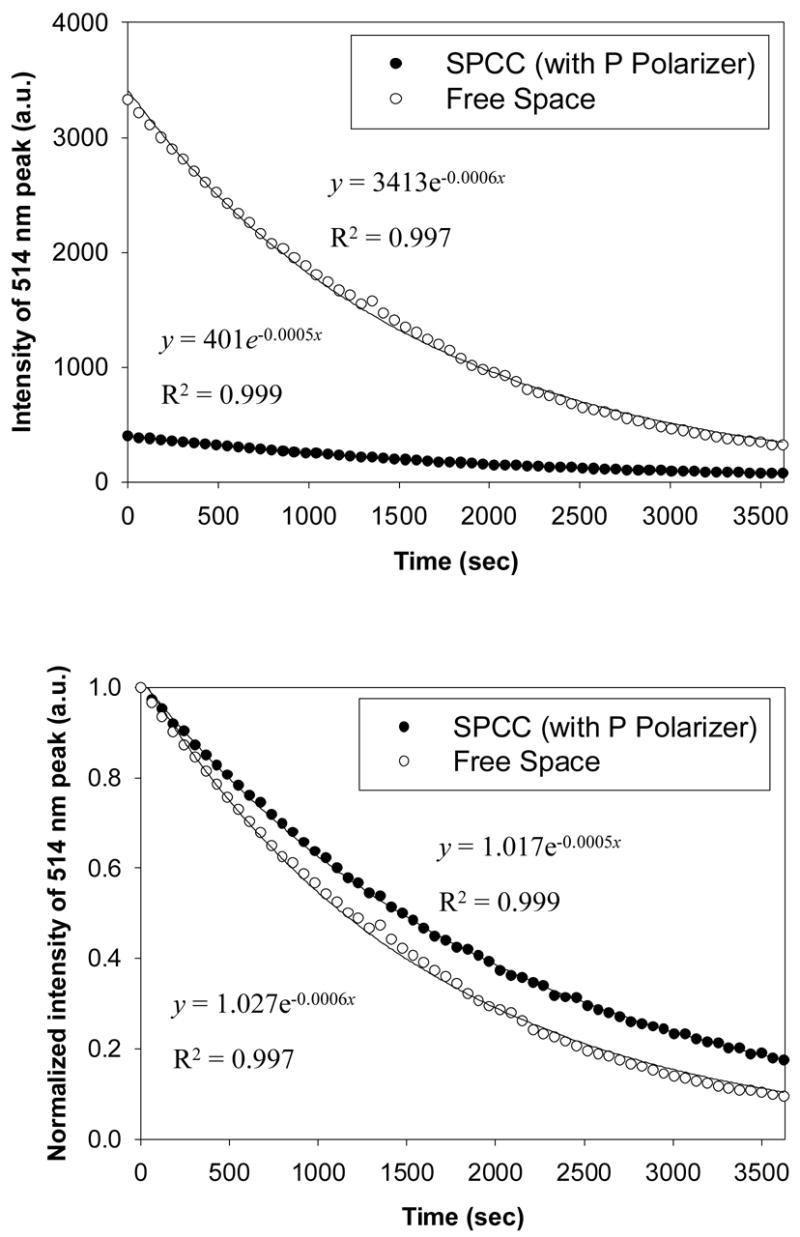
Chemiluminescence intensity decays both free-space and coupled (Top), and normalized to the same initial intensity (Bottom).
| (1) |
where C is the intensity at time t = α, B is a pre-exponential factor, and k is the rate of luminescence depletion, units s−1. The rate of depletion of the SPCC signal was found to be only minimally greater than the free-space emission, 0.0006 versus 0.0005 s−1, respectively. Since both the SPCC signal and the free-space emission signal decay is highly dependent on the rate of depletion of the same reactants (depletion of excited states) in the sample chamber over time, it is not surprising that the measured decay rate for both the signals as shown in Figure 5 are almost identical. However, this finding does clearly show that there are no localized catalytic effects of the silver on the chemiluminescence reaction. Since the SPCC only originates from the first 250 nm of solution close to the film, then any catalytic effect would be manifested by a different chemiluminescence decay rate, which is clearly not observed.
4.0 Conclusions
In this letter we conclude that chemically induced electronic excited states of luminophores can excite surface plasmons on thin films of continuous silver, producing highly polarized and directional emission. We believe this phenomenon is not restricted to the commercially available kits that were used in this study, but moreover, can be extended to the myriad of chemiluminescent reactions employed in biotechnology today to increase signal collection efficiency and hence the sensitivity of such assays. Interestingly, the physical dimensions of these assays are compatible with an approximately 250 nm coupling region [15–20], potentially alleviating unwanted background distal fluorescence and therefore increasing assay sensitivity. In this regard, work is underway in our laboratories and will be reported in due course.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garcia-Campana AM, Baeyens WR. Analytical Chemistry. Marcel Dekker; New York: 2001. [Google Scholar]
- 2.Wampler JE. In: Chemi- and Bioluminescence. Burr JG, editor. Marcel Dekker; New York: 1985. p. 1. [Google Scholar]
- 3.Berthold F. In: Luminescence Immunoassays and Molecular Applications. Van Dyke K, Van Dyke R, editors. CRC Press; Boca Raton: 1990. p. 11. [Google Scholar]
- 4.Nieman T. Chemiluminescence: Theory and Instrumentation, Overview, in Encyclopedia of Analytical Science. Academic Press; Orlando: 1995. [Google Scholar]
- 5.Lakowicz JR. Principles of Fluorescence Spectroscopy. Kluwer/Academic Plenum; New York: 1997. [Google Scholar]
- 6.Lakowicz JR. Anal Biochem. 2001;298:1. doi: 10.1006/abio.2001.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakowicz JR. Anal Biochem. 2002;301:261. doi: 10.1006/abio.2001.5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakowicz JR. Anal Biochem. 2004;324:153. doi: 10.1016/j.ab.2003.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gryczynski I, Malicka J, Gryczynski Z, Lakowicz JR. Anal Biochem. 2004;324:170. doi: 10.1016/j.ab.2003.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geddes CD, Aslan K, Gryczynski I, Malicka J, Lakowicz JR. In: Annual Reviews in Fluorescence. Geddes CD, Lakowicz JR, editors. Kluwer Academic/Plenum; New York: 2004. p. 356. [Google Scholar]
- 11.Aslan K, Leonenko Z, Lakowicz JR, Geddes CD. J Fluoresc. 2005;15:643. doi: 10.1007/s10895-005-2970-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lakowicz JR. Anal Biochem. 2005;337:171. doi: 10.1016/j.ab.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowdhury MH, Aslan K, Malyn SN, Lakowicz JR, Geddes CD. Appl Phys Lett. 2006;88:173104. doi: 10.1063/1.2195776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chowdhury MH, Aslan K, Malyn SN, Lakowicz JR, Geddes CD. J Fluoresc. 2006:16. doi: 10.1007/s10895-006-0082-z. published online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gryczynski I, Malicka J, Nowaczyk K, Gryczynski Z, Lakowicz JR. J Phys Chem B. 2004;108:12073. doi: 10.1021/jp0312619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gryczynski I, Malicka J, Gryczynski Z, Lakowicz JR. J Phys Chem B. 2004;108:12568. doi: 10.1021/jp040221h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geddes CD, Gryczynski I, Malicka J, Gryczynski Z, Lakowicz JR. J Fluoresc. 2004;14:119. doi: 10.1023/b:jofl.0000014809.64610.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gryczynski I, Malicka J, Gryczynski Z, Nowaczyk K, Lakowicz JR. Anal Chem. 2004;76:4076. doi: 10.1021/ac040004c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matveeva E, Gryczynski Z, Gryczynski I, Malicka J, Lakowicz JR. Anal Chem. 2004;76:6287. doi: 10.1021/ac0491612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gryczynski I, Malicka J, Jiang W, Fischer H, Chan WCW, Gryczynski Z, Grudzinski W, Lakowicz JR. J Phys Chem B. 2005;109:1088. doi: 10.1021/jp046173i. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Gryczynski Z, Lakowicz JR. Chem Phys Lett. 2004;393:483. doi: 10.1016/j.cplett.2004.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gryczynski I, Malicka J, Nowaczyk K, Gryczynski Z, Lakowicz JR. Thin Solid Films. 2006;510:15. doi: 10.1016/j.tsf.2005.07.312. [DOI] [PMC free article] [PubMed] [Google Scholar]


