Abstract
To achieve an adequate response, cells of the immune system must be tightly regulated to avoid hypo- or hyper-responsiveness. One of the mechanisms used by the immune system to avoid excessive inflammation is the modulation of the response through inhibitory receptors containing immunoreceptor tyrosine based inhibitory motifs (ITIM). Here, we show that human neutrophils from peripheral blood express the ITIM containing CD300a (also known as IRp60 and CMRF-35H) receptor. By using the HL-60 differentiation model, we show that the expression of CD300a receptor is developmentally regulated. Stimulation of human neutrophils with LPS and GM-CSF increased the cell surface expression of CD300a as a result of the rapid translocation of an intracellular pool of the receptor to the cell surface. Co-ligation of CD300a with the immunoreceptor tyrosine based activating motif (ITAM) containing CD32a (FcγRIIa) activation receptor inhibited CD32a mediated signaling, whereas it did not inhibit Toll like receptor (TLR)-4 mediated reactive oxygen species (ROS) production. Therefore, at least for human neutrophils, the inhibitory signals mediated by the CD300a receptor may be selective in their action.
Keywords: neutrophils, CD300a, inhibitory receptor, reactive oxygen species, ITAM, ITIM
1. Introduction
Neutrophils play a major role in inflammation by generating pro-inflammatory agents during phagocytosis. In addition they also produce cytokines that modulate the response of other leukocytes and they themselves become activated by numerous cytokines, such as GM-CSF, and other pro-inflammatory agents, such as LPS (Nathan, 2006). Human neutrophils express two distinct Fc gamma receptors (FcγR) (FcγRIIa and FcγRIIIb), which are responsible for phagocytosis and antibody dependent cellular cytotoxicity (ADCC), and release of ROS. Cross-linking of FcγRIIa (CD32a) initiates a signaling cascade that begins with phosphorylation of tyrosine residues within the ITAM motif by src kinases, followed by Syk activation and a rapid intracellular Ca2+ increase as a consequence of phospholipase C (PLC)γ activation by Syk (Ghazizadeh et al., 1994; Shen et al., 1994). Excessive signaling through FcγRIIa appears to play a role in neutrophil initiated tissue damage (Nathan, 2006; Selvaraj et al., 2004). Therefore, FcγRIIa signaling within neutrophils needs to be regulated to avoid excessive inflammation.
CD300a is a receptor with three ITIM motifs within its cytoplasmic tail that belongs to a multigene family of activating/inhibitory receptors that are clustered in human chromosome 17 (17q25.1) (Speckman et al., 2003). CD300a is expressed on a variety of immune cells (Cantoni et al., 1999). The ligand for CD300a, as well as for the other members of the CD300 family, is unknown. It has been shown that the CD300a gene receptor ranks near the top of the human genes that show evidence of positive selection (Bustamante et al., 2005; Nielsen et al., 2005), suggesting an important role for this receptor. Genetic studies have found that a non-synonymous polymorphism within the immunoglobulin domain (R111Q) of the CD300a receptor is linked to psoriasis susceptibility (Speckman et al., 2003). Cross-linking of the CD300a receptor with a specific monoclonal antibody (mAb) has been shown to exert an inhibitory effect on natural killer (NK) cell activity through recruitment of src homology 2 domain (SH2)-containing tyrosine phosphatase (SHP)-1 and SHP-2 phosphatases (Cantoni et al., 1999). For human eosinophils, cross-linking of CD300a is able to suppress the effects of eotaxin, IL-5 and GM-CSF by also recruiting SHP-1 phosphatase (Munitz et al., 2006a) and, for mast cells it is able to inhibit Ig-E dependent, but not Ig-E independent, activities by recruiting SHP-1 and the SH2-containing inositide phosphatase (SHIP) phosphatases (Bachelet et al., 2005). Because of the effect of CD300a on eosinophils and mast cells, the possibility of targeting this receptor for treating allergic reactions is being explored. In a mouse model of experimental asthma, treatment of animals with a bispecific antibody fragment linking CCR3 to CD300a has been described to reverse airway inflammation and remodeling (Munitz et al., 2006b). In another in vivo model, a bispecific antibody fragment linking IgE to CD300a was able to abrogate allergic reactions (Bachelet et al., 2006).
Because activated neutrophils generated in response to “foreign” agents are a primary source of inflammatory mediators, knowledge of the mechanisms that regulate neutrophil activation has potential usefulness for designing therapies. Here, we report that CD300a is expressed on human neutrophils and that its rapid up-regulation by activated neutrophils does not require new protein synthesis, and that it is capable of modulating neutrophil responses.
2. Materials and methods
2.1. Cell Isolation, culture and FACS analysis
Neutrophils were freshly isolated by density gradient centrifugation with lymphocyte separation medium (MP Biomedical) from granulocytes preparations obtained from the NIH blood bank, After isolation, they were cultured at a concentration of 3 × 106/ml in RPMI (Biosource), 10% fetal calf serum (Hyclone), Glutamax (Invitrogen) and sodium pyruvate (Biosource), either in the absence or presence of LPS (Sigma), GM-CSF, IFN-γ, IL-10 and TGF-β (R&D Systems). To study CD300a expression during neutrophil differentiation, HL-60 cells (ATCC) were treated with all trans-retinoic acid (RA) (Sigma). The cell surface expression of CD11b, CD14, CD69 and CD300a was analyzed with a FACSort (BD Biosciences) using specific antibodies (Beckman Coulter).
2.2. ROS production assay
Freshly isolated neutrophils were washed and resuspended at 2 × 106/ml in Hank’s Balanced Salt Solution (HBSS) (Biosource) with 5% of fetal calf serum. Cells were incubated on ice with anti-CD32a (clone IV.3) mAb (StemCell Technologies) or LPS, anti-CD300a (clone E59.126) mAb and/or isotype controls for 15-30 min. Then, cells were transferred to 96 well plates, followed by the addition of pre-warmed Diogenes (National Diagnostics) and goat anti-mouse Fab’ (Jackson ImmunoResearch) to cross-link the antibodies. Luminescence was immediately measured with a Luminoskan Ascent (Thermo Labsystems).
2.3. Calcium mobilization assay
This assay was performed as previously described (Maasho et al., 2005). Neutrophils were labeled with Fluo-4 and Fura Red (Invitrogen) for 30 min in a 30 °C water bath. To establish a baseline, cells were first acquired with a FACSort for 30 sec at which point the primary antibodies were added: anti-CD32a, anti-CD300a and/or isotype control mAb. Then, at 60 sec, the primary antibodies were cross-linked with goat anti-mouse Fab’ and fluorescence was measured in real time. Data were analyzed using the FlowJo software package (Treestar).
3. Results
3.1. CD300a expression during human neutrophil development
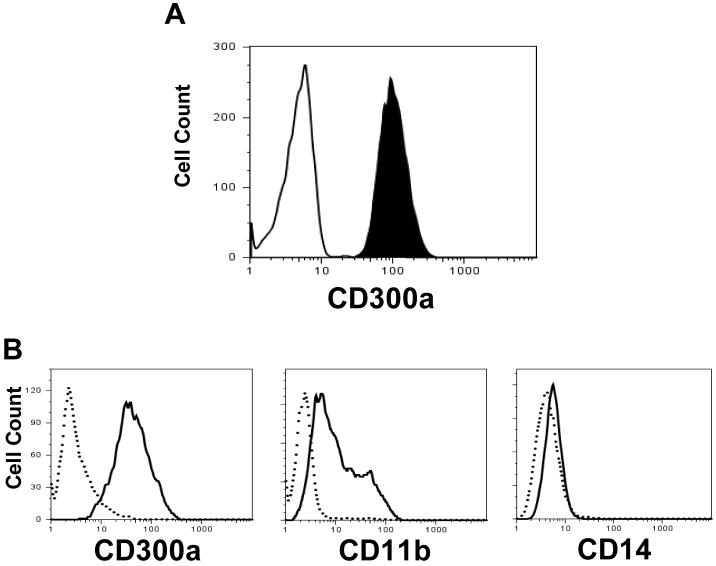
We first investigated the expression of the CD300a inhibitory receptor on freshly isolated human peripheral blood cells. Neutrophils showed a quite uniform expression of CD300a (Fig. 1A). Next, we determined if the expression of this receptor is developmentally regulated on neutrophils. To do that, we chose the well defined model for neutrophil differentiation that utilizes HL-60 cells treated with RA (Breitman et al., 1980). In this model, treatment of undifferentiated HLA-60 cells with RA induces differentiation as detected by the expression of the CD11b receptor, but not the CD14 receptor. When undifferentiated HL-60 cells are induced to differentiate into monocytes both CD11b and CD14 expression is induced (Quesada et al., 1996). Untreated HL-60 cells do not express the CD300a receptor on the cell surface; however, when we induced neutrophil differentiation by adding RA a dramatic increase in CD300a cell surface expression was observed, indicating that the CD300a receptor expression is developmentally regulated (Fig. 1B). In fact CD300a expression is acquired earlier during differentiation than CD11b (data not shown). When HL-60 cells were induced to differentiate into monocytes by adding calcitriol instead of RA, the expression of CD300a was also induced, which is in agreement with the uniform expression of CD300a observed on peripheral blood monocytes (data not shown).
Fig. 1.
CD300a expression by neutrophils. (A) Expression of CD300a on freshly isolated human peripheral blood neutrophils (filled histogram). Empty histogram is background staining obtained with isotype control mAb. (B) Differentiated neutrophils obtained by treating HL-60 cells with 10-6 M RA for 4 days. The expression of CD300a, CD11b and CD14 in differentiated cells (continuous line) compared with non-differentiated cells (dotted line) is shown. These data are representative of 3 independent experiments.
3.2. Inflammatory agents up-regulate the expression of CD300a on human neutrophils
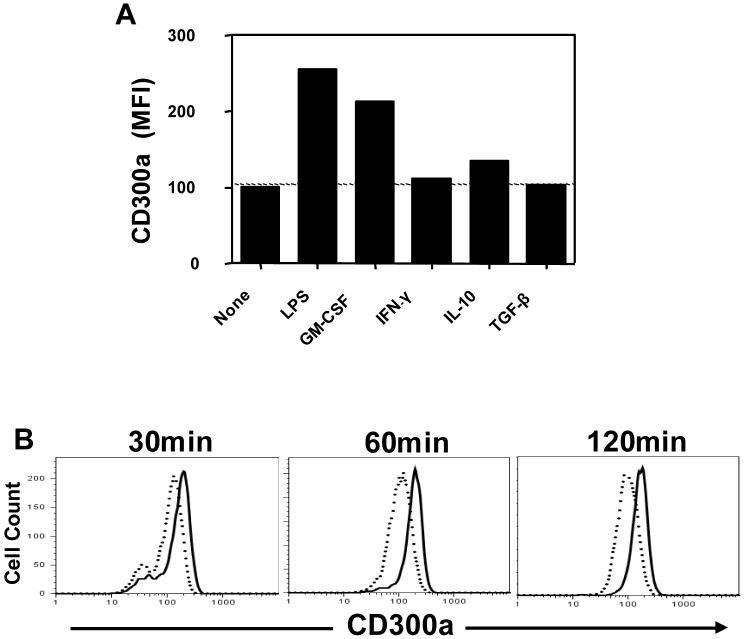
Neutrophils can be activated by a diverse array of agents (Nathan, 2006). In order to avoid hyper-responsiveness, one might expect that the expression of inhibitory receptors to be induced or up-regulated on such activated cells. Here we checked the expression of CD300a by neutrophils in response to exposure of the cells to pro-inflammatory agents. In Figure 2A, we show that GM-CSF or LPS induces a significant increase in the cell surface expression of CD300a. Neither IFN-γ nor anti-inflammatory agents TGF-β or IL-10, had an effect on CD300a cell surface expression. The lack of a TGF-β mediated effect on CD300a expression by neutrophils is in contrast with the observed down-regulation of CD300a on T cells treated with TGF-β (data not shown). These data suggest that the CD300a inhibitory receptor may play an important role in controlling inflammation because its expression is up-regulated by inflammatory stimuli on human neutrophils.
Fig. 2.
CD300a is up-regulated on human neutrophils in response to pro-inflammatory agents. (A) Expression of CD300a on human neutrophils after treatment with LPS (1 μg/ml), GM-CSF (10 ng/ml), IFN-γ (100 ng/ml), IL-10 (40 ng/ml) or TGF-β (10 ng/ml) for 2 h. (B) Kinetic analysis of CD300a expression in the absence (dotted line) or presence (continuous line) of LPS (1 μg/ml). These data are representative of 3 independent experiments.
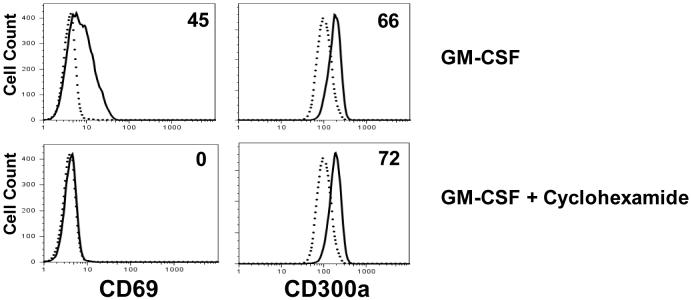
Next, we investigated the kinetics of the expression of this receptor in response to GM-CSF or LPS. In Figure 2B, we show that the increase in the cell surface expression of this receptor occurs very rapidly, by 30 min after stimulation with LPS. Similar results were observed when neutrophils were treated with GM-CSF (data not shown). Because of this fast increase in the CD300a cell surface expression, we reasoned that this could be the result of the translocation of an existing intracellular pool of this receptor. To investigate this, we checked the expression of CD300a in response to GM-CSF treatment in the presence and absence of the protein synthesis inhibitor cycloheximide. In Figure 3, we show that cycloheximide does not interfere with the increase in CD300a expression in response to GM-CSF stimulation, indicating that the up-regulation of cell surface CD300a does not require new synthesis. As a positive control, we show that de novo expression of CD69 in response to GM-CSF is blocked by the presence of cycloheximide indicating that CD69 expression involves new protein synthesis (Atzeni et al., 2002).
Fig. 3.
Expression of CD300a on neutrophils in response to GM-CSF in the absence or presence of cycloheximide (50 μg/ml). Cells were treated with GM-CSF with or without cycloheximide for 2 h and the expression of CD300a and CD69 were measured. Expression in the absence of GM-CSF is represented by dotted line and in the presence by the continuous line. Numbers in the histograms reflect the increase in the mean fluorescence intensity (MFI) (in percentage) of CD69 and CD300a expression compared with untreated cells. These data are representative of 3 independent experiments.
3.3. CD300a down-modulates neutrophil function
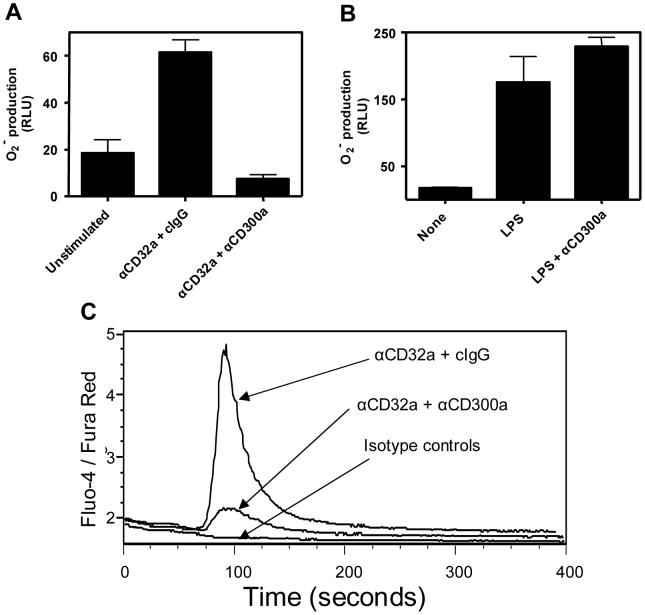
Next, we investigated the role of CD300a in neutrophil function. To do this, we examined ROS production in response to co-ligation of CD300a with either CD32a (FcγRIIa) or TLR-4. Signaling mediated through CD32a (FcγRIIa) is dependent on the ITAM motif within its intracytoplasmic tail (Ravetch and Bolland, 2001), while TLR-4 signals are mediated through the MyD88 pathway (Fitzgerald and Chen, 2006). Figure 4A shows that ROS production in response to CD32a (FcγRIIa) aggregation with the IV.3 mAb is significantly inhibited if the activating CD32a (FcγRIIa) receptor and the inhibitory CD300a receptor are co-ligated. Thus, these results show that ITIM generated signals are able to down-regulate ITAM initiated inflammatory signals. On the other hand, as shown in Figure 4B, TLR-4 signals are not inhibited by the ligation of CD300a with specific mAb. Similarly, ligation of the CD300a receptor with specific mAb did not inhibit fMLP mediated ROS production, suggesting that CD300a does not inhibit signaling mediated through a G-protein coupled receptor (data not shown). These results suggest that CD300a signals are selective in their down-modulation of inflammatory signals. Because Ca2+ increase is indispensable for ROS production in response to FcγRIIa ligation (Edberg et al., 1998), we investigated if CD300a inhibits FcγRIIa induced ROS production by a mechanism that affects Ca2+ flux. Figure 4C clearly shows that co-ligation of FcγRIIa with CD300a results in a significant decrease in intracellular Ca2+ flux indicating that at least one mechanism involved in the inhibitory effect of the CD300a receptor is the down-regulation of the Ca2+ flux by ITAM mediated signals.
Fig. 4.
CD300a modulates CD32a (FcγRIIa) signaling in neutrophils. (A) Neutrophils were left untreated or incubated with anti-CD32a (0.1 μg/ml) mAb plus anti-CD300a (2.5 μg/ml) mAb or plus isotype control (2.5 μg/ml) for 15-30 min. Then, 50 μl of cells were transferred to 96 well plates at a concentration of 2 × 106/ml. Pre-warmed Diogenes and goat anti-mouse Fab’ at 40 μg/ml were added to the cell suspension for a final volume of 100μl. ROS production was measured for one hour. Bars represent the cumulative ROS production over the hour and standard deviations of triplicates are shown. (B) Same type of experiment as reported in Fig. 4A but LPS (1 μg/ml) was added instead of anti-CD32a mAb. (C) Freshly isolated neutrophils were resuspended in a buffer composed of HBSS with 5% of fetal calf serum at 10 × 106 cells/ml. Then, cells were labeled with Fluo-4 (2 μg/ml) and Fura Red (5 μg/ml) (Invitrogen) for 30 min in a 30 °C water bath. To establish a baseline, cells were first acquired using a FACSort for 30 sec at which point the primary antibodies, anti-CD32a, anti-CD300a and/or isotype control mAb, were added at the same concentration as in the superoxide production assay. Then, at 60 sec the primary antibodies were cross-linked with goat anti-mouse Fab’ at 40μg/ml and fluorescence was measured in real time. Data are representative of 3 independent experiments.
4. Discussion
In this report, we have analyzed the expression and function of the CD300a inhibitory receptor on human neutrophils. We have shown that this receptor is expressed on human peripheral blood neutrophils and that its expression is developmentally regulated. Moreover, pro-inflammatory stimuli induced a fast translocation of a ready to go intracellular pool of the receptor to the cell surface. Finally, we have shown that CD300a is able to inhibit CD32a (FcγRIIa) mediated ROS production by inhibiting Ca2+ flux, and that the CD300a inhibitory signal selectively inactivates activation receptor signaling.
Human neutrophils have been shown to express other ITIM containing inhibitory receptors (Liu et al., 2002; Mingari et al., 2001; Richard et al., 2002; Skubitz et al., 2000; Verbrugge et al., 2006; Wu et al., 2005). CD305 (also known as LAIR-1) is expressed at very low levels on peripheral blood neutrophils, and its expression, like CD300a, is also up-regulated after stimulation of mature neutrophils with pro-inflammatory agents, although it is not known if the up-regulation of LAIR-1 expression is a consequence of the translocation of a pre-existing intracellular pool to the plasma membrane or new synthesis of the receptor is required (Verbrugge et al., 2006). On the other hand, expression of the CD305 inhibitory receptor by the pro-myeloid HL-60 cell line is down-regulated after differentiation into neutrophils (Verbrugge et al., 2006). Another ITIM containing inhibitory receptor expressed on human neutrophils is CLECSF6, a member of the C-type lectin superfamily. It is down-modulated after treatment of neutrophils with pro-inflammatory agents (Richard et al., 2003; Richard et al., 2002). It is interesting to note that these three ITIM containing inhibitory receptors, CD300a, CD305 and CLECSF6, have different patterns of expression, suggesting that they could function at different time points during neutrophil differentiation and activation. More studies are required to address this possibility.
CD31 (or PECAM-1) (Wu et al., 2005), CD66a (or CEACAM1 or BGPa) (Skubitz et al., 2000), SIRPα (Liu et al., 2002), p75/AIRM1 and CD33 (Mingari et al., 2001) are other ITIM containing inhibitory receptors expressed on human mature neutrophils. The expression levels of these receptors are also regulated during neutrophil activation, but studies on the function of these receptors has focused more on their role in adhesion and chemotaxis of neutrophils than on the inhibition of activating signals (Liu et al., 2002; Skubitz et al., 2000; Wu et al., 2005). In fact, it is very important to point out that, to our knowledge, our study is the first one showing an inhibitory function for an ITIM containing receptor in mature human neutrophils.
Our results suggest that CD300a potentially plays an important and possibly unique role in modulating the effect of pro-inflammatory stimuli in neutrophil responses. The fact that an intracellular pool of CD300a is maintained for fast translocation to the plasma membrane speaks to the importance of this receptor for modulating neutrophil function. In this regard, CD300a is like CTLA-4 receptor that is stored in intracellular compartments and is translocated to the cell surface in response to activating signals (Thompson and Allison, 1997). Signaling by this receptor is very important for down-modulating T cell responses, as evidenced by the fact that its absence results in multiorgan destruction and massive lymphoproliferation (Tivol et al., 1995). The fast up-regulation of CD300a in response to pro-inflammatory stimuli, but not anti-inflammatory agents, and our data showing that its inhibitory capabilities may be selective, could be of importance for designing therapies to specifically treat inflammatory based diseases.
Acknowledgments
This work is supported by the intramural program of the NIAID. We thank Steven Burgess, Gul’nar Fattakhova, Alina Marusina, Madhan Masilamani and Sriram Narayanan for their critical reading of the manuscript.
Abbreviations
- ADCC
antibody dependent cellular cytotoxicity
- FcγR
Fc gamma receptor
- GM-CSF
granulocyte macrophage colony-stimulating factor
- IFN-γ
interferon gamma
- ITAM
immune receptor tyrosine-based activating motif
- ITIM
immune receptor tyrosine-based inhibitory motif
- LPS
lipopolysaccharide
- mAb
monoclonal antibody
- MFI
mean fluorescence intensity
- NK
natural killer
- PLC
phospholipase C
- RA
all trans-retinoic acid
- RLU
relative light units
- ROS
reactive oxygen species
- SHP
src homology 2 domain (SH2)-containing tyrosine phosphatase
- SHIP
SH2-containing inositide phosphatase
- TGF-β
transforming growth factor beta
- TLR
toll-like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atzeni F, Schena M, Ongari AM, Carrabba M, Bonara P, Minonzio F, Capsoni F. Induction of CD69 activation molecule on human neutrophils by GM-CSF, IFN-gamma, and IFN-alpha. Cell Immunol. 2002;220:20–9. doi: 10.1016/s0008-8749(03)00002-9. [DOI] [PubMed] [Google Scholar]
- Bachelet I, Munitz A, Levi-Schaffer F. Abrogation of allergic reactions by a bispecific antibody fragment linking IgE to CD300a. J Allergy Clin Immunol. 2006;117:1314–20. doi: 10.1016/j.jaci.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Bachelet I, Munitz A, Moretta A, Moretta L, Levi-Schaffer F. The inhibitory receptor IRp60 (CD300a) is expressed and functional on human mast cells. J Immunol. 2005;175:7989–95. doi: 10.4049/jimmunol.175.12.7989. [DOI] [PubMed] [Google Scholar]
- Breitman TR, Selonick SE, Collins SJ. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980;77:2936–40. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante CD, Fledel-Alon A, Williamson S, Nielsen R, Hubisz MT, Glanowski S, Tanenbaum DM, White TJ, Sninsky JJ, Hernandez RD, Civello D, Adams MD, Cargill M, Clark AG. Natural selection on protein-coding genes in the human genome. Nature. 2005;437:1153–7. doi: 10.1038/nature04240. [DOI] [PubMed] [Google Scholar]
- Cantoni C, Bottino C, Augugliaro R, Morelli L, Marcenaro E, Castriconi R, Vitale M, Pende D, Sivori S, Millo R, Biassoni R, Moretta L, Moretta A. Molecular and functional characterization of IRp60, a member of the immunoglobulin superfamily that functions as an inhibitory receptor in human NK cells. Eur J Immunol. 1999;29:3148–59. doi: 10.1002/(SICI)1521-4141(199910)29:10<3148::AID-IMMU3148>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Edberg JC, Moon JJ, Chang DJ, Kimberly RP. Differential regulation of human neutrophil FcgammaRIIa (CD32) and FcgammaRIIIb (CD16)-induced Ca2+ transients. J Biol Chem. 1998;273:8071–9. doi: 10.1074/jbc.273.14.8071. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Chen ZJ. Sorting out Toll signals. Cell. 2006;125:834–6. doi: 10.1016/j.cell.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Ghazizadeh S, Bolen JB, Fleit HB. Physical and functional association of Src-related protein tyrosine kinases with Fc gamma RII in monocytic THP-1 cells. J Biol Chem. 1994;269:8878–84. [PubMed] [Google Scholar]
- Liu Y, Buhring HJ, Zen K, Burst SL, Schnell FJ, Williams IR, Parkos CA. Signal regulatory protein (SIRPalpha), a cellular ligand for CD47, regulates neutrophil transmigration. J Biol Chem. 2002;277:10028–36. doi: 10.1074/jbc.M109720200. [DOI] [PubMed] [Google Scholar]
- Maasho K, Masilamani M, Valas R, Basu S, Coligan JE, Borrego F. The inhibitory leukocyte-associated Ig-like receptor-1 (LAIR-1) is expressed at high levels by human naive T cells and inhibits TCR mediated activation. Mol Immunol. 2005;42:1521–30. doi: 10.1016/j.molimm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Mingari MC, Vitale C, Romagnani C, Falco M, Moretta L. p75/AIRM1 and CD33, two sialoadhesin receptors that regulate the proliferation or the survival of normal and leukemic myeloid cells. Immunol Rev. 2001;181:260–8. doi: 10.1034/j.1600-065x.2001.1810122.x. [DOI] [PubMed] [Google Scholar]
- Munitz A, Bachelet I, Eliashar R, Moretta A, Moretta L, Levi-Schaffer F. The inhibitory receptor IRp60 (CD300a) suppresses the effects of IL-5, GM-CSF, and eotaxin on human peripheral blood eosinophils. Blood. 2006a;107:1996–2003. doi: 10.1182/blood-2005-07-2926. [DOI] [PubMed] [Google Scholar]
- Munitz A, Bachelet I, Levi-Schaffer F. Reversal of airway inflammation and remodeling in asthma by a bispecific antibody fragment linking CCR3 to CD300a. J Allergy Clin Immunol. 2006b;118:1082–9. doi: 10.1016/j.jaci.2006.07.041. [DOI] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–82. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Nielsen R, Bustamante C, Clark AG, Glanowski S, Sackton TB, Hubisz MJ, Fledel-Alon A, Tanenbaum DM, Civello D, White TJ, J J S, Adams MD, Cargill M. A scan for positively selected genes in the genomes of humans and chimpanzees. PLoS Biol. 2005;3:e170. doi: 10.1371/journal.pbio.0030170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada JM, Lopez LG, Buron MI, Alcain FJ, Borrego F, Velde JP, Blanco I, Bouillon R, Navas P. Ascorbate increases the 1,25 dihydroxyvitamin D3-induced monocytic differentiation of HL-60 cells. Calcif Tissue Int. 1996;59:277–82. doi: 10.1007/s002239900123. [DOI] [PubMed] [Google Scholar]
- Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–90. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- Richard M, Thibault N, Veilleux P, Breton R, Beaulieu AD. The ITIM-bearing CLECSF6 (DCIR) is down-modulated in neutrophils by neutrophil activating agents. Biochem Biophys Res Commun. 2003;310:767–73. doi: 10.1016/j.bbrc.2003.09.077. [DOI] [PubMed] [Google Scholar]
- Richard M, Veilleux P, Rouleau M, Paquin R, Beaulieu AD. The expression pattern of the ITIM-bearing lectin CLECSF6 in neutrophils suggests a key role in the control of inflammation. J Leukoc Biol. 2002;71:871–80. [PubMed] [Google Scholar]
- Selvaraj P, Fifadara N, Nagarajan S, Cimino A, Wang G. Functional regulation of human neutrophil Fc gamma receptors. Immunol Res. 2004;29:219–30. doi: 10.1385/IR:29:1-3:219. [DOI] [PubMed] [Google Scholar]
- Shen Z, Lin CT, Unkeless JC. Correlations among tyrosine phosphorylation of Shc, p72syk, PLC-gamma 1, and [Ca2+]i flux in Fc gamma RIIA signaling. J Immunol. 1994;152:3017–23. [PubMed] [Google Scholar]
- Skubitz KM, Campbell KD, Skubitz AP. Synthetic peptides of CD66a stimulate neutrophil adhesion to endothelial cells. J Immunol. 2000;164:4257–64. doi: 10.4049/jimmunol.164.8.4257. [DOI] [PubMed] [Google Scholar]
- Speckman RA, Wright Daw JA, Helms C, Duan S, Cao L, Taillon-Miller P, Kwok PY, Menter A, Bowcock AM. Novel immunoglobulin superfamily gene cluster, mapping to a region of human chromosome 17q25, linked to psoriasis susceptibility. Hum Genet. 2003;112:34–41. doi: 10.1007/s00439-002-0851-y. [DOI] [PubMed] [Google Scholar]
- Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–50. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–7. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- Verbrugge A, de Ruiter T, Geest C, Coffer PJ, Meyaard L. Differential expression of leukocyte-associated Ig-like receptor-1 during neutrophil differentiation and activation. J Leukoc Biol. 2006;79:828–36. doi: 10.1189/jlb.0705370. [DOI] [PubMed] [Google Scholar]
- Wu Y, Stabach P, Michaud M, Madri JA. Neutrophils lacking platelet-endothelial cell adhesion molecule-1 exhibit loss of directionality and motility in CXCR2-mediated chemotaxis. J Immunol. 2005;175:3484–91. doi: 10.4049/jimmunol.175.6.3484. [DOI] [PubMed] [Google Scholar]