Abstract
In colonial species, recognition of offspring should be under strong selection. For accurate identification to occur offspring must emit individually distinctive signals and parents must be able to discriminate between signals. Female greater spear-nosed bats (Phyllostomus hastatus) roost in stable social groups and use infant vocalizations, termed isolation calls, to locate and identify their young. In this study, we investigate both the production and perception of isolation calls in P. hastatus. First, we measured acoustic features and found that after controlling for ontogenetic effects, sufficient variation exists between pups for isolation calls to function as individual signatures. Moreover, pups from the same social group emit calls with more similar spectral and spectro-temporal features than pups from different social groups, indicating that these features are likely heritable. We used psychoacoustic experiments in the laboratory to determine if adult females could discriminate between calls from pups in the same or different social group. Females discriminated between pups when faced with a template-matching task and their performance was correlated with the salience of spectral and spectro-temporal features. We found no difference in performance when females had to discriminate between pups from the same and different social groups. These results indicate that females should be able to accurately identify their young using isolation calls.
(Introduction)
The process of identifying offspring is expected to be under strong selection to insure that parental care is confined to related individuals. For animals living in large groups the probability of confusing related offspring with others can be high. Consequently, parent-offspring recognition systems have evolved in many colonial species (e. g. Trillmich 1981; Stoddard & Beecher 1983). For bats, offspring recognition can be a particularly vital but difficult task because mothers typically leave their pups behind in large colonies. Accurate offspring recognition requires fulfilment of two criteria: 1) offspring must emit individually distinctive signals and 2) parents must be able to discriminate between these signals (Beecher 1982). Here, we examine these two components of parent-offspring recognition in greater spear-nosed bats (Phyllostomus hastatus).
In Trinidad, West Indies, P. hastatas form stable social groups of eight to 40 adult females attended by one adult male (McCracken & Bradbury 1981). Unlike most other group-living mammals, females are typically unrelated to group members (McCracken & Bradbury 1981). Males have high reproductive control over harems (McCracken & Bradbury 1977) and socially mediated birth synchrony occurs within groups (Porter & Wilkinson 2001). Consequently, pups reside in clusters of predominantly paternal half-siblings of similar age from a single social group.
Most infant bats, including P. hastatus, produce frequency-modulated multi-harmonic vocalizations known as isolation calls which are used in parent-offspring recognition (Gould et al. 1973). In some species isolation calls contain enough information to serve as individual signatures (e.g. Thomson et al. 1985; Gelfand & McCracken 1986; Scherrer & Wilkinson 1993) and often change as pups age (reviewed in Altringham & Fenton 2003). In P. hastatus females sometimes visit and retrieve group members’ pups that have fallen to the cave floor (K. M. Bohn & G. S. Wilkinson pers. observ.). This observation raises the possibility that isolation calls may not contain sufficient information for a female to recognize her pup from others in her social group.
Females likely use a template-matching mechanism (Lacy & Sherman 1983) to recognize their own pups’ calls. By template matching, females compare incoming isolation calls to a signal template represented in memory. Offspring recognition should then depend on a female’s ability to form a template and discriminate among offspring signals. Psychoacoustic studies suggest that bats should be able to discriminate between isolation calls. For example, adult female Phyllostomus discolor can discriminate among frequency-modulated sounds similar to isolation calls (Esser & Lud 1997) and other studies have demonstrated maternal recognition of pup calls (Balcombe 1990; de Fanis & Jones 1996). No study has yet examined the acoustic features used by mothers to recognize their pups’ calls.
Here we present a comprehensive study of parent-offspring recognition in P. hastatus. First, we investigate signal production. We examine how isolation calls change with pup age and then control for age effects to assess how acoustic features vary among bats in different caves or social groups. Next, we use psychoacoustic experiments in the laboratory to examine signal perception. We test whether females can discriminate between pup isolation calls in a template-matching procedure, which mimics the perceptual task faced by females in the wild. We then determine which acoustic features are correlated with female performance and compare the results with the analysis of isolation call variation to infer potentially salient cues. Finally, in a second perceptual experiment we test whether call similarity within social groups affects female discrimination of individual pups.
METHODS
Isolation Call Recordings
We recorded isolation calls from infant P. hastatus at Guanapo and Tamana caves (McCracken & Bradbury 1981) in Trinidad, West Indies, in April and May, 2002 and 2004. In the evening non-volant pups were captured by hand for measuring and recording and then returned to their crèche before adult bats returned from foraging. We banded each pup with numbered stainless steel bands (National Band and Tag) and used calipers to measure forearm length (FA) to a tenth of a millimetre. We estimated age in days as 0.77 × FA − 24.6 (Stern & Kunz 1998). We recorded spontaneously emitted isolation calls from pups placed in a cardboard box (approximately 0.75 by 0.5 by 0.5 meters) lined with acoustic foam (Sonex). Calls were digitized at a sample rate of 250 kHz into Bat Sound Pro (Pettersson Electronik) on a laptop computer equipped with a data acquisition card (INEES, Daq508, 12 bits) using a bat detector as a high frequency microphone (Ultra Sound Advice, S-25) and an external amplifier (SHURE, FP-2).
Infant P. hastatus emit multiple types of isolation calls composed of different numbers of notes. Double-note calls are the simplest and most frequently emitted calls in P. hastatus and in many other species of bats (Gould et al. 1973). For both simplicity and consistency, we used double-note calls for all analyses and psychoacoustic experiments (see below).
Isolation Call Measurements
We used SIGNAL (version 3.0, Engineering Design) to band-pass filter isolation calls between 5 kHz and 85 kHz, normalize amplitudes by dividing each signal by its peak amplitude, and measure 12 call features (Fig. 1). We measured three temporal, eight spectral and one spectro-temporal feature, the relative location of the frequency minimum of the first note (MNT1) using the formula MNT1 = (end time − time of minimum frequency)/end time. In addition to these measurements, for perceptual experiments, we used SIGNAL to calculate spectral cross-correlations between call pairs for each note (COR1, COR2). The spectral cross-correlation procedure slides two spectral contours across each other and calculates the maximum correlation between the two signals (Beeman 1996). Spectrograms were constructed using a transform length of 512 points, resulting in a temporal resolution of 2 ms and frequency resolution of 500 Hz. The time between transforms was set at 0.15 ms, so that all signals had the same number of transformations per second but different total number of transforms.
Figure 1.
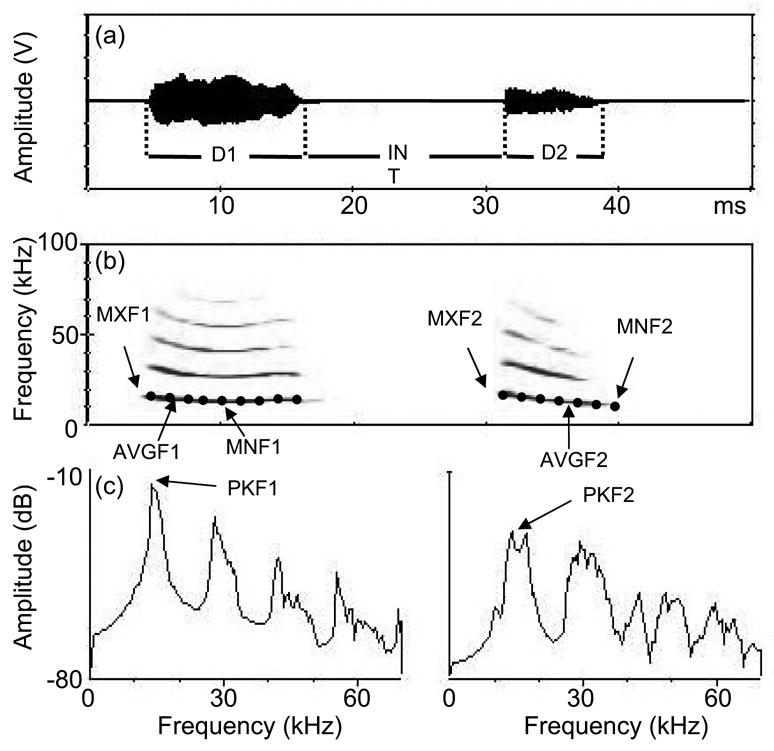
(a) Oscillogram, (b) spectrogram, and (c) power spectra of a typical double-note isolation call. Measurements taken are first note duration (D1), second note duration (D2), interval between notes (INT), average frequency of the first (AVGF1) and second (AVGF2) notes, minimum frequency of the first (MNF1) and second (MNF2) notes, maximum frequency of the first (MXF1) and second (MXF2) notes, and peak frequency of the first (PKF1) and second (PKF2) notes. Large dots represent spectral contours, which were averaged over the duration of the calls to calculate AVGF1 and AVGF2. Spectral contours were calculated by determining the peak frequency at each point in time of the call (Beeman 1996).
Subjects
For the psychoacoustic experiments we used five (Experiment 1) and four (Experiment 2) adult female P. hastatus. The subjects were captured in Tamana Cave in 1993, except for one bat that was born in captivity in 1996. During the study, bats were housed in a cage (3.3 × 2.7 × 2.4 m) kept in a room maintained on an 8L:16D cycle at approximately 24° C and 30% humidity. Bat weights were maintained between 60 and 70 g during experiments (minimum of 90% free-fed body weight) by feeding them a diet of fruit and marmoset food (Premium Nutritional Products). During experiments bats were rewarded with mealworms and fruit.
Psychoacoustic Apparatus and Procedures
All experiments were conducted in a single-walled acoustic chamber (Industrial Acoustics Company, Inc) lined with acoustic foam (Sonex). Bats were trained and tested using a V-shaped platform enclosed in a hardware-cloth cage (Bohn et al. 2004). A modified go/no-go procedure was used for both experiments (Suthers & Summers 1980). During experimental trials, bats were trained to either a) stay at the top of the platform (“no-go” trial) or b) run to the end of the right arm of the platform (“go” trial). Bats were rewarded at the starting position for correctly staying during “no-go” trials and rewarded at the end of the right arm of the platform for correctly responding during “go” trials. For both experiments (see below) bat performance was assessed by two measurements: percent of responses that were correct and response latency. Response latency was measured as the difference between the onset of a stimulus train and a bat’s departure time using a real-time processor (Tucker Davis Technologies, RP 2.1). Bat departure times were recorded automatically using an infrared light-emitting diode (LED) and matching photosensor located at the top of the platform and triggered whenever the bat left the starting position.
Playback Stimuli and Calibration
Isolation calls were played directly from a computer equipped with SIGNAL and a 250 kHz DA board (Data Translation, DT5727), band-pass filtered at 5 and 85 kHz (Krohn-Hite, 3550), amplified (Harman Kardan, AVR 100), and sent to a loudspeaker (Pioneer, PT-R), which was 1 m from the subject’s starting position. Stimuli were recorded daily onto a laptop computer and inspected for distortion. We recorded a calibration tone daily with a piston phone (Brüel and Kjaer type 4231) and adjusted amplitudes so that all stimuli were 75 dB SPL at the bats’ starting position. For both psychoacoustic experiments, stimuli presentations were determined from measurements of natural calling behaviour. Each call set was separated by 900 ms of silence and contained three calls each separated by 60 ms of silence (Figs. 2 and 3).
Figure 2.
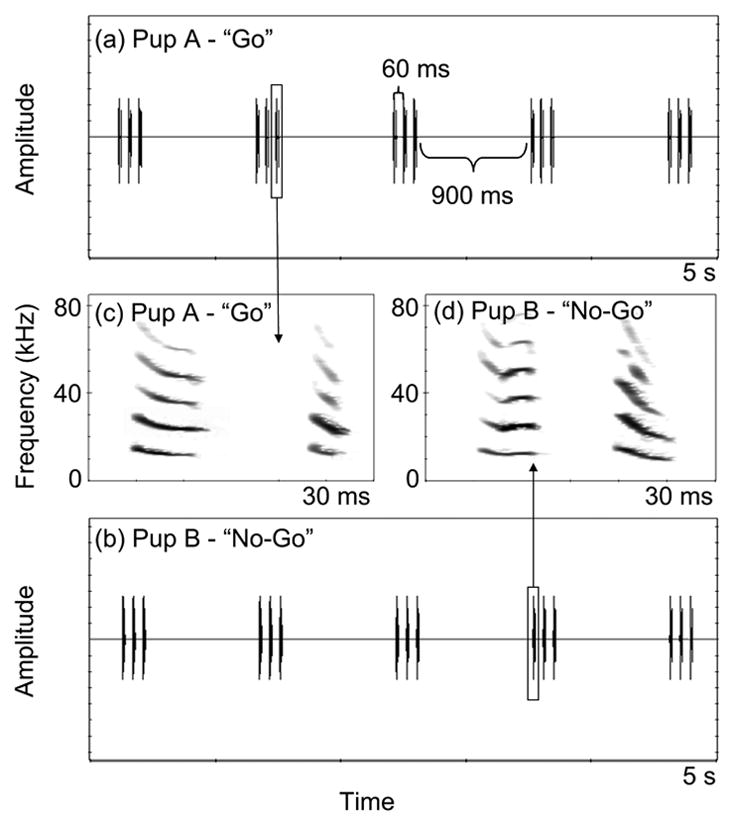
Stimuli used for experiment 1 on pup discrimination. (a) “Go” trial of five call sets with 60 ms between calls and 900 ms between sets; all calls are from a single pup, “Pup A”. (b) “No-go” trial, all calls are from a single pup, “Pup B”. (c) and (d) spectrograms of single isolation calls from Pup A and Pup B, respectively.
Figure 3.

Stimuli used for experiment 2 on group discrimination. (a) “Go” trials consisted of three, four, or five background call sets from one pup (Pup A), two calls from a target pup (Pup B), and one call from the first pup (Pup A). Subjects were rewarded for responding during the last three call sets. (b) “No-go” trials consisted of six, seven or eight call sets. Subjects were rewarded for staying during the entire trial. (c) Example call set for Pup A. (d) Example call set for Pup B.
Experiment 1: Pup Discrimination
The goals of this experiment were to determine whether bats could discriminate between isolation calls emitted by different pups and identify the acoustic features that were correlated with performance. Only calls recorded from Guanapo pups in 2002 were used in this experiment. Calls from sixteen pup pairs were selected at random without replacement but the difference in age between pups was no greater than 2 days for each pair. Two isolation calls were used for each pup and presented in random order. For each pup pair, the calls from one pup were arbitrarily assigned as a “no-go” stimulus and the other was a “go” stimulus (Fig. 2). This procedure required signal recognition by the subjects because females had to store calls in memory to determine which call was associated with which behaviour. Before testing, bats were trained using the same procedure on calls from two pairs of pups whose ages differed by at least 10-days of age and whose calls were the most different in temporal, spectral and spectro-temporal features. Calls used for training were not used in experimental trials.
Each testing day consisted of 30 trials per subject. Trials were randomized but no more than three trials of one type occurred consecutively (Gellerman 1933). For the first five trials of each day, we presented mealworms at the end of the platform (“go” stimulus) or at the top of the platform (“no-go” stimulus) to show the subjects the correct responses. For the remaining 25 trials bats were only rewarded at the end of trials if they performed correctly. Responses and response latencies were only recorded for the last 25 trials. Each stimulus pair was used for 2 days of testing with 1 day without testing between pairs. For each 2-day testing period the day with the best performance (the highest percent correct responses) for each bat was used for analyses. Equipment malfunction resulted in the loss of five response latency estimates.
Experiment 2: Group Discrimination
The goal of this experiment was to determine whether social group membership affected discrimination of pup isolation calls. For this experiment we used a modified Alternating Sound Task (Dooling & Okanoya 1995). During “go” trials bats were trained to stay at the starting position while call sets from one pup were played (the background) and move to the end of the ramp when call sets from a different pup (the target) were alternated with the background (Fig. 3). During “no-go” trials only the background pup was played. Using this procedure all stimuli were presented in each test session, which controlled for day-to-day variation in the subjects’ performance.
Bats were trained using calls from two pairs of differently aged pups from different social groups. Calls used for training were not used in experimental trials. For testing, calls from twelve different pups served as background and were each paired with calls from two different target pups: one pup from the same group as the background pup and one pup from a different group than the background pup. Pairs were selected at random without replacement as long as the difference in ages of the background and target pups was no greater than 2 days. We incorporated within pup variation in double note calls by randomly combining four to five calls from each pup into the stimulus sets daily.
Bats were tested using calls from all 24 “go” pairs and twelve “no-go” pairs on each of 9 days. All pairs were presented nine times, three times at each repetition level or number of initial background sets. The number of call sets was randomized across days. “No-go” and “go” trials were randomized within days but no more than five trials of one type could occur consecutively. If a bat made an early start during a “go” trial, that stimulus was added to the end of the day’s trials to insure a clear response was recorded for each day. For response latencies, one data point was missing because one bat did not respond correctly during any of the three trials.
Statistical Analyses
Variables were examined for normality using normal-probability plots and Shapiro-Wilk’s tests. Any variable deviating from normality was transformed to satisfy assumptions for parametric tests (see Appendix for summary of transformations used). Statistical significance was evaluated using two-tailed tests with α = 0.05. Analyses were performed in either JMP 5.0 or SAS 9.1 (SAS Institute Inc).
For analysis of isolation call variation, we used pups if we had at least four double-note calls with high signal-to-noise ratio and at least four pups from a social group. First, we used the average of each measurement for each pup and tested whether age, sex, or age by sex interactions affected isolation call features using a multivariate analysis of variance (MANOVA). Next, to reduce the number of variables and control for colinearity, we used a principal components analysis (PCA) and varimax factor rotation on age-corrected residuals for the measurements on each call. Factors with eigenvalues greater than 1.0 were used in a MANOVA with cave, group, and pup as nested random effects (PROC GLM, SAS Institute Inc.). We used restricted maximum likelihood to calculate variance component estimates (VCEs), i.e. the proportion of variation in the PCA factors explained at each nesting level. We then calculated repeatability where repeatability = VCEpup/(VCEpup + VCEcall).
For experiment 1, we calculated the average daily percent correct response for each bat, compared these values with confidence intervals for “guessing” (50%), and conducted binomial tests on the pooled responses to all 400 trials. For each call feature, we calculated the absolute difference between call pairs. PCA and varimax factor rotations were calculated on call differences. To test if call differences correlated with performance, we used a logistic regression (PROC GLIMMIX, SAS) to examine response outcome (correct or incorrect) and an ANCOVA to analyze response latency for factors with eigenvalues greater than one. For both analyses a full model was tested with bat as a random factor, order of pair presentation as a repeated measure, extracted factors as predictors and all bat by factor interactions. The repeated measures variance components did not differ from zero, and all interaction effects were non-significant. Thus, these terms were removed from the final model.
For experiment 2, we calculated the percentage of correct responses for each individual. In this experiment, unlike in experiment 1, the probability of responding correctly by chance was not 50%. In order to respond correctly, the bat not only had to “go” or “not go” but also had to depart within acceptable time windows depending on the number of initial background repetitions. For each of the possible time windows, we calculated the proportion of trials that would result in a correct response if the bat had departed. These calculations resulted in a chance probability of 45% correct.
Because there was only one “no-go” stimulus for both the within and between social group pairs, we only used “go” trials for testing whether group membership affected discrimination. For “go” trials, we counted the number of correct responses and the average response latency for each bat-stimulus-repetition combination. We then used a logistic regression (PROC GLIMMIX, SAS) on response outcome and an ANOVA on response latency. For both variables, a full model was first analyzed with pup pair as a random block, number of background repetitions, group (same or different), and number of background repetitions by group interaction as fixed effects, and bat and bat by group interaction as random effects. For both variables, interaction terms were non-significant and consequently removed from the final model.
RESULTS
Isolation Call Variation
Pup age had a significant effect on call features (MANOVA, Wilk’s Lambda = 0.477, df = 12, 47, P < 0.0001). Call features were not affected by sex and there was no interaction between sex and age (Wilk’s Lambda = 0.894 and 0.897 respectively, df = 12, 47, P = 0.92 and 0.93 respectively). Age had a negative effect on temporal features (D1 and D2) and a positive effect on spectral features (PKF1, PKF2, and MXF2, Table 1). Spectrotemporal features were not affected by age (Fig. 4).
Table 1.
Results of regressions of age on average call variables for 63 pups. Intercepts and slopes from transformed variables (see Appendix)
| Variable | F | Intercept | Slope |
|---|---|---|---|
| Temporal | |||
| D1 | 6.91* | 13.7 | −0.96 |
| D2 | 4.66* | 0.90 | −0.03 |
| INT | 3.75 | 3.59 | −0.16 |
| Spectral-Note 1 | |||
| MNF1 | 1.29 | 133,000 | 4240 |
| MXF1 | 3.68 | 14.1 | 0.28 |
| AVGF1 | 2.41 | 151,000 | 5,800 |
| PKF1 | 15.60*** | 11.3 | 0.64 |
| Spectral-Note 2 | |||
| MNF2 | 2.12 | 0.10 | 0.001 |
| MXF2 | 5.12* | 220,000 | 9,990 |
| AVGF2 | 3.19 | 157,000 | 6,900 |
| PKF2 | 8.65** | 12.5 | 0.54 |
| Spectro-temporal | |||
| MNT1 | 0.32 | 0.46 | 0.020 |
P < 0.05;
P < 0.01;
P < 0.001
Figure 4.

Oscillogram (top) and spectrogram (bottom) for isolation calls from a pup just after parturition and at 10 days of age. Note the spectral shape remains constant while duration decreases (first note durations = 10 and 6 ms) and frequency increases (first note peak frequencies = 11.9 and 14.1 kHz).
Residuals from regressions of call features on age were used for the remaining analyses on isolation call variation. Factor analysis produced four factors, which together explained 81% of the variation in the twelve call measurements. Spectral features loaded into the first factor, D2 loaded into the second factor, MNT1 and INT loaded into the third factor and D1 loaded into the fourth factor (Table 2). There was significant variation among pups (Wilk’s Lambda = 0.01, df = 220, 973, P < 0.0001) and social groups (Wilk’s Lambda = 0.49, df = 24, 183, P = 0.02) but not among caves (Wilk’s Lambda = 0.92, df = 4, 3, P = 0.99). While all factors contributed to differences among pups, only the first factor (spectral features) and third factor (spectro-temporal features) contributed to differences among social groups (Table 3).
Table 2.
Factor loadings after PCA and varimax rotation of isolation calls for analysis of call variation
| Variable | Factor 1 | Factor 2 | Factor 3 | Factor 4 |
|---|---|---|---|---|
| Temporal | ||||
| D1 | −0.07 | 0.02 | 0.12 | 0.93 |
| D2 | −0.03 | −0.92 | −0.03 | 0.01 |
| INT | 0.01 | 0.48 | −0.69 | 0.39 |
| Spectral-Note 1 | ||||
| MNF1 | 0.82 | −0.26 | −0.13 | −0.21 |
| MXF1 | 0.76 | −0.10 | −0.16 | 0.49 |
| AVGF1 | 0.90 | −0.28 | −0.19 | −0.10 |
| PKF1 | 0.77 | −0.08 | −0.34 | 0.03 |
| Spectral-Note 2 | ||||
| MNF2 | 0.79 | 0.35 | 0.06 | −0.17 |
| MXF2 | 0.87 | −0.03 | 0.05 | 0.16 |
| AVGF2 | 0.90 | 0.25 | 0.08 | −0.02 |
| PKF2 | 0.77 | 0.20 | 0.07 | −0.03 |
| Spectro-temporal | ||||
| MNT1 | −0.07 | 0.17 | 0.83 | 0.28 |
Call features with loadings of 0.70 or greater in bold.
Table 3.
Univariate F-tests from nested ANOVAs on PCA factors from Table 2
| Group
|
Pup
|
Call
|
||||
|---|---|---|---|---|---|---|
| Estimate | F | VCE† | F | VCE† | VCE† | Repeatability |
| N | 8 | 63 | 309 | |||
| Factor 1 | 3.12* | 0.17 | 19.68*** | 0.66 | 0.17 | 0.80 |
| Factor 2 | 0.94 | 0.00 | 8.58*** | 0.61 | 0.39 | 0.61 |
| Factor 3 | 2.59* | 0.10 | 7.37*** | 0.50 | 0.40 | 0.56 |
| Factor 4 | 0.27 | 0.00 | 10.15*** | 0.63 | 0.37 | 0.63 |
P < 0.05;
P < 0.001
Variance component estimates (VCE) show the proportion of variation explained by differences among groups within caves, pups within groups, and calls within pups.
Experiment 1: Pup Discrimination
On average the five bats performed between 78% and 83% correct responses per day, well above the 95% confidence interval for guessing (Fig. 5a). Across all pairs of call presentations, all five bats performed above 50% (binomial tests, N = 400, all P < 0.0001). A PCA on acoustic differences between call pairs resulted in four rotated factors, which together explained 79% of the variation. The first factor was correlated with spectral differences, the second factor with spectral and spectro-temporal differences, the third factor with temporal differences, and the fourth factor with differences in the spectro-temporal feature MNT1 (Table 4).
Figure 5.

Mean (± SE) percent correct responses by bat and day for (a) pup discrimination (experiment 1, N = 16 days) and (b) group discrimination (experiment 2, N = 9 days). Dashed lines indicate 95% upper confidence interval for guessing (50% experiment 1, 45% experiment 2).
Table 4.
Factor loadings after PCA and varimax rotation on isolation call variables measured in experiment 1
| Variable | Factor 1 | Factor 2 | Factor 3 | Factor 4 |
|---|---|---|---|---|
| Temporal | ||||
| D1 | −0.31 | 0.59 | 0.57 | 0.01 |
| D2 | −0.19 | 0.18 | 0.73 | 0.34 |
| INT | 0.15 | −0.20 | 0.70 | −0.48 |
| Spectral-Note 1 | ||||
| MNF1 | −0.21 | −0.90 | 0.06 | 0.01 |
| MXF1 | −0.86 | −0.06 | 0.04 | −0.02 |
| AVGF1 | −0.57 | −0.74 | 0.06 | −0.05 |
| PKF1 | −0.81 | −0.18 | −0.28 | −0.27 |
| Spectral-Note 2 | ||||
| MNF2 | −0.70 | 0.24 | 0.26 | 0.39 |
| MXF2 | −0.53 | −0.60 | −0.20 | −0.18 |
| AVGF2 | −0.69 | −0.25 | 0.32 | 0.30 |
| PKF2 | −0.62 | −0.45 | 0.28 | 0.26 |
| Spectro-temporal | ||||
| MNT1 | 0.00 | −0.13 | 0.00 | 0.86 |
| COR1 | 0.08 | −0.79 | −0.38 | 0.22 |
| COR2 | 0.01 | −0.12 | −0.92 | 0.01 |
Bats did not differ in the number of correct responses (logistic regression: χ42 = 6.95, P = 0.14). Only the second rotated factor predicted whether or not bats responded correctly (Table 5). Response latency did differ between bats (ANCOVA: F4,67 = 3.22, P = 0.02). Only the first factor had a significant effect on response latency (Table 5). Thus, spectral and spectro-temporal features correlated with female discrimination of isolation calls (Tables 4 and 5).
Table 5.
Results from a logistic regression on correct responses and an ANOVA on response latencies in experiment 1
| Variable | Factor Description | Response Outcome† | Response Latency§ |
|---|---|---|---|
| Factor 1 | Spectral | 0.43 | 5.09* |
| Factor 2 | Spectral & Spectro-temporal | 7.01** | 0.85 |
| Factor 3 | Temporal & Spectro-temporal | 0.35 | 0.53 |
| Factor 4 | Spectro-temporal | 1.29 | 0.07 |
χ2, df = 1
F statistic, df = 1, 67
P < 0.05,
P<0.01
Experiment 2: Group Discrimination
Each of the four bats averaged close to 80% correct responses each test day (Fig. 5b). Across all trials their performance was greater than the guessing value of 45% (binomial test, N = 324, all P < 0.0001). The proportion of correct responses differed across bats(χ32 = 13.73, P = 0.003) and number of background repetitions ((χ22 = 12.76, P = 0.002). We detected no difference in the proportion of “go” responses (percent correct) when bats listened to calls of pups from the same social group and those from a different group ((χ12 = 1.37, P = 0.24, Fig. 6a).
Figure 6.

Group discrimination (experiment 2) results: (a) mean (± SE) percent correct response and (b) mean (± SE) latency to respond to calls of pup pairs from the same and different social groups.
Response latency also differed between bats (F3,269 = 7.79, P < 0.0001) and the number of background repetitions (F2, 269 = 5.72, P = 0.004). We did not detect a difference in response latency between calls of pups from the same and different social groups (F1, 269 = 3.31, P = 0.07, Fig. 6b); however response latency tended to be shorter in response to calls from different social groups.
DISCUSSION
Isolation Call Variation
In P. hastatus, isolation calls become shorter and increase in frequency with age. Such age-related changes likely result from vocal tract development (Suthers & Fattu 1973, Gould 1975). Nevertheless, the spectro-temporal shape of each pup’s isolation call appears to remain constant for at least the first week or two after parturition (Fig. 4), as has been noted for other species of bats (Scherrer & Wilkinson 1993).
After removing age effects, isolation calls exhibited significant differences between pups and social groups according to multivariate analyses. Among pups, all four acoustic factors were significant and explained between 50% and 66% of the variation in isolation calls. The factor associated with spectral features showed the greatest variation between pups and the least variation within pups, i.e. had the highest repeatability (Table 3). Among social groups, only non-temporal factors were significant, and explained 10% and 17% of the variation. Assuming that all pups within a group share a common father and have unrelated mothers, i.e. are paternal half-siblings (McCracken & Bradbury 1977), the heritability (percent of variation attributable to additive genetic effects) of call components can be estimated as four times the proportion of variance explained by social group. Thus, our analyses indicate that the first and third factors, i.e. spectral and spectral-temporal variables, have significant heritabilities of 0.68 and 0.40, respectively. The existence of heritable isolation calls in P. hastatus, as in other bat species (Scherrer & Wilkinson 1993), could facilitate individual and potentially sib-group recognition of pups by females.
Experiment 1: Isolation Call Discrimination
The bats successfully performed the first psychoacoustic experiment, which was designed to test a template-matching process. Unlike many psychoacoustic procedures, in which subjects only need to detect a difference in stimuli, in this experiment the bats had to store isolation calls in memory. The bats could have performed this task in two ways. Females could have compared calls from a trial with calls that they learned and stored in memory during the first five trials. Alternatively, females could have compared calls from a trial with those of the previous trial. In either case they had to compare a new call with one stored in memory.
Although we did not directly test which acoustic features are used for isolation call recognition, positive correlations between acoustic dissimilarity and discrimination performance indicate that spectral and spectro-temporal features were important in discrimination. These findings were consistent across bats, as shown by the lack of statistical interactions among bats, and extracted factors for percent correct and response latency data. On the other hand, temporal features of pup isolation calls were not correlated with performance, though they should carry useful information for identification. One possible explanation for this result is that isolation calls are emitted at high signal amplitudes in roosting sites, such as caves or hollow trees, where temporal features, such as call duration, likely suffer distortions from reverberation (Bradbury & Vehrencamp 1998). Thus, selection may have acted on the auditory system for enhanced discrimination of call features that suffer the least distortion during transmission.
Experiment 2: Group Discrimination
The multivariate analysis of isolation call features revealed that group mates emit calls with more similar spectral and spectro-temporal features than non-group mates. Experiment 1 showed that bats use these same features to discriminate pups. For experiment 2, we then examined whether this hierarchical signature system affected female discrimination of pups from the same or different group. For this experiment we incorporated between four and five isolation calls per pup. Consequently, bats had to discriminate between individuals even though calls varied within individuals. We found that group-level similarity did not affect discrimination ability as measured by the proportion of correct responses. This result was consistent across bats as shown by all bats performing above chance levels and the lack of statistical interactions between bats and social group. Our results indicate that although pups from the same social group have similar calls and call discrimination is correlated with call similarity, on average, calls differ enough between pups from the same social group for females to discriminate between them. Moreover, while the difference in percent correct responses to same and different social group calls was not statistically significant, performance tended to be higher for calls from different social groups, suggesting that percent correct performance may not be sufficiently sensitive to detect differences in perceptual encoding of social signals by bats performing in this task. Response latency appears to be a more sensitive measure of perceptual discrimination of calls from same and different social groups; however, this response measure yielded marginally non-significant (P = 0.07) differences.
Our findings from the group discrimination experiment demonstrate that P. hastatus can discriminate well among isolation calls of individuals from the same and different social groups but raise the possibility that group-similarity may affect isolation call recognition in a natural acoustic environment that is noisier than our anechoic chamber. On the other hand, our experiments were more difficult than what females face in the wild because we only used double-note calls, the simplest of isolation calls. Pups also emit isolation calls with three or more notes, which likely provide more information for discrimination. Furthermore, we confined age differences between pups to two days of age, which is at the lower end of age difference ranges within social groups in the wild (Porter & Wilkinson 2001). Thus, in this experiment the bats were likely faced with more similar calls than what would occur naturally. In this context, it is worth noting that critical ratio estimates for P. hastatus showed that they have highest frequency selectivity at the fundamental frequency of isolation calls (Bohn et al. 2004), which should enhance discrimination of spectral features of isolation calls. If P. hastatus use spectral and spectro-temporal features from multiple call types, they should be able to differentiate between most, if not all, pup calls in the wild.
The high performance in isolation call discrimination found in P. hastatus is consistent with strong selection for accurate offspring recognition. P. hastatus give birth to only one pup per year, and infant mortality is high (Stern & Kunz 1998). Parental care is extensive because pups are altricial at birth and do not begin to fly until at least 6 weeks of age (Stern & Kunz 1998). Traits that increase the likelihood of infant survival will strongly impact female reproductive success. Isolation calls are likely crucial to pup survival in P. hastatus because non-volant pups emit these calls when they fall from roost sites (K. M. Bohn & G. S. Wilkinson pers. orbserv.). Isolation calls attract females who bring pups back to their roost sites. If non-volant pups are not retrieved, they do not survive. Presumably, females accrue some energetic costs by responding to other young as this may reduce care directed to filial young. Based on the evidence presented in this study, mistaken identity is unlikely to be a plausible explanation for recurrent cases of female P. hastatus remaining near or retrieving group mates’ fallen pups (K. M. Bohn & G. S. Wilkinson pers. orbserv.).
Parent-Offspring Recognition in Mammals
Infant vocalizations that are used in parent-offspring communication are common in mammals and often contain enough information for individual identification (e. g. rodents Tokumaru et al. 2004; seals, Job et al. 1995; primates, Hammerschmidt & Todt 1995; ungulates Terrazas et al. 2003, carnivores Sieber 1986). Although maternal recognition has been demonstrated in some species (e. g. seals, Insley 2001; pigs, Illmann et al. 2002; primates, Symmes & Biben 1985), a lack of evidence for maternal recognition has also been reported even when vocalizations contained signature information (seals, Job et al. 1995, McCulloch et al. 1999). In these cases it was impossible to determine whether similar responses to offspring and non-filial young were the result of perceptual or behavioural mechanisms. Here, we isolated the perceptual aspect of offspring recognition by performing experiments out of a natural context.
Our finding that spectral rather than temporal acoustic cues appear most important for offspring recognition may be part of a larger mammalian pattern. In many mammals, temporal features of infant vocalizations have greater intra-individual variation than spectral features and are therefore less informative for recognition (bats, Gelfand & McCracken 1986; seals, Insley 1992; primates Masataka & Symmes 1986). Unfortunately, few studies have examined the ability of female mammals to discriminate among infants using vocalizations; although, spectral and spectro-temporal cues were found to be important in offspring recognition in seals (Charrier et al. 2002). Given the widespread use of infant vocalizations in mammals, perception of these acoustic signals deserves further study.
Acknowledgments
The University of Maryland Animal Care and Use Committee approved all procedures in this research (protocol number R-01-09A). This material is based upon work supported by a dissertation improvement grant (IBN-0308642) from the National Science Foundation, a predoctoral institutional traineeship in Neuroethology from the National Institute of Mental Health (T32-MH020048) and a grant to the Center for Comparative and Evolutionary Biology of Hearing (P30-DC04664). Thanks to Katrina Smith, Jason South and Tameeka Williams for assistance in the field and Ed Smith for technical assistance in the lab.
Appendix
Data transformations for the multivariate analysis on isolation call variation and experiment 1. “None” indicates data were normally distributed. “NA” = not applicable indicates the variable was not in the analysis.
| Variable | Call Variation | Experiment 1 |
|---|---|---|
| D1 | none | log x |
| D2 | log x | none |
| INT | log x | |
| MNF1 | x2 | log x |
| MXF1 | x2 | |
| AVGF1 | x2 | log x |
| PKF1 | none | none |
| MNF2 | log x | |
| MXF2 | x2 | |
| AVGF2 | x2 | x2 |
| PKF2 | none | none |
| MNT1 | ||
| COR1 | NA | |
| COR2 | NA | none |
| Age | NA |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Altringham JD, Fenton MB. Sensory ecology and communication in the chiroptera. In: Kunz TH, Fenton MB, editors. Bat Ecology. Chicago: The University of Chicago Press; 2003. pp. 90–127. [Google Scholar]
- Balcombe JP. Vocal recognition of pups by mother Mexican free-tailed bats, Tadarida brasiliensis mexicana. Animal Behaviour. 1990;39:960–966. [Google Scholar]
- Beecher MD. Signature systems and kin recognition. American Zoologist. 1982;22:477–490. [Google Scholar]
- Beeman K. SIGNAL/RTSD User’s Guide. Belmont: Engineering Design; 1996. [Google Scholar]
- Bohn KM, Boughman JW, Wilkinson GS, Moss CF. Auditory sensitivity and frequency selectivity in greater spear-nosed bats suggest specializations for acoustic communication. Journal of Comparative Physiology A. 2004;190:185–192. doi: 10.1007/s00359-003-0485-0. [DOI] [PubMed] [Google Scholar]
- Bradbury JW, Vehrencamp SL. Principles of Animal Communication. Sunderland: Sinauer Associates; 1998. [Google Scholar]
- Charrier I, Mathevon N, Jouventin P. How does a seal mother recognize the voice of her pup? An experimental study of Arctocephalus tropicalis. Journal of Experimental Biology. 2002;205:603–612. doi: 10.1242/jeb.205.5.603. [DOI] [PubMed] [Google Scholar]
- de Fanis E, Jones G. Allomaternal care and recognition between mothers and young in pipistrelle bats (Pipistrellus pipistrellus) Journal of Zoology. 1996;240:781–787. [Google Scholar]
- Dooling RJ, Okanoya K. Pscyhophysical methods for assessing perceptual categories. In: Klump GM, Dooling RJ, Fay RR, Stebbins WC, editors. Methods in Comparative Psychoacoustics. Basel: Birkhåuser Verlag; 1995. pp. 307–318. [Google Scholar]
- Esser KH, Lud B. Discrimination of sinusoidally frequency-modulated sound signals mimicking species-specific communication calls in the FM-bat Phyllostomus discolor. Journal of Comparative Physiology A. 1997;180:513–522. doi: 10.1007/s003590050068. [DOI] [PubMed] [Google Scholar]
- Gelfand DL, McCracken GF. Individual variation in the isolation calls of Mexican free-tailed bat pups (Tadarida brasiliensis mexicana) Animal Behaviour. 1986;34:1078–1086. [Google Scholar]
- Gellerman LW. Chance orders of alternating stimuli in visual discrimination experiments. Journal of Genetic Psychology. 1933;42:206–208. [Google Scholar]
- Gould E. Experimental studies of ontogeny of ultrasonic vocalizations in bats. Developmental Psychobiology. 1975;8:333–346. doi: 10.1002/dev.420080407. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolf NK, Turner DC. Double-note communication calls in bats - occurrence in 3 families. Journal of Mammalogy. 1973;54:998–1001. [PubMed] [Google Scholar]
- Hammerschmidt K, Todt D. Individual differences in vocalisations of young barbary macaques (Macaca sylvanus): a multi-parametric analysis to identify critical cues in acoustic signalling. Behaviour. 1995;132:381–399. [Google Scholar]
- Illmann G, Schrader L, Spinka M, Sustr P. Acoustical mother-offspring recognition in pigs (Sus scrofa domestica) Behaviour. 2002;139:487–505. [Google Scholar]
- Insley SJ. Mother-offspring separation and acoustic stereotypy: a comparison of call morphology in two species of pinnipeds. Behaviour. 1992;120:103–122. [Google Scholar]
- Insley SJ. Mother-offspring vocal recognition in northern fur seals is mutual but asymmetrical. Animal Behaviour. 2001;61:129–137. doi: 10.1006/anbe.2000.1569. [DOI] [PubMed] [Google Scholar]
- Job DA, Boness DJ, Francis JM. Individual variation in nursing vocalizations of Hawaiian monk seal pups, Monachus schauinslandi (Phocidae, Pinnipedia), and lack of maternal recognition. Canadian Journal of Zoology. 1995;73:975–983. [Google Scholar]
- Lacy RC, Sherman PW. Kin recognition by phenotype matching. American Naturalist. 1983;121:489–512. [Google Scholar]
- Masataka N, Symmes D. Effect of separation distance on isolation call structure in squirrel monkeys (Saimiri sciureus) American Journal of Primatology. 1986;10:271–278. doi: 10.1002/ajp.1350100307. [DOI] [PubMed] [Google Scholar]
- McCracken GF, Bradbury JW. Paternity and genetic-heterogeneity in the polygynous bat, Phyllostomus hastatus. Science. 1977;198:303–306. doi: 10.1126/science.198.4314.303. [DOI] [PubMed] [Google Scholar]
- McCracken GF, Bradbury JW. Social organization and kinship in the polygynous bat Phyllostomus hastatus. Behavioral Ecology and Sociobiology. 1981;8:11–34. [Google Scholar]
- McCulloch S, Pomeroy PP, Slater PJB. Individually distinctive pup vocalizations fail to prevent allo-suckling in grey seals. Canadian Journal of Zoology. 1999;77:716–723. [Google Scholar]
- Porter TA, Wilkinson GS. Birth synchrony in greater spear-nosed bats (Phyllostomus hastatus) Journal of Zoology. 2001;253:383–390. [Google Scholar]
- Scherrer JA, Wilkinson GS. Evening bat isolation calls provide evidence for heritable signatures. Animal Behaviour. 1993;46:847–860. [Google Scholar]
- Sieber OJ. Acoustic recognition between mother and cubs in raccoons Procyon lotor. Behaviour. 1986;96:130–163. [Google Scholar]
- Stern AA, Kunz TH. Intraspecific variation in postnatal growth in the greater spear-nosed bat. Journal of Mammalogy. 1998;79:755–763. [Google Scholar]
- Stoddard PK, Beecher MD. Parental recognition of offspring in the cliff swallow. Auk. 1983;100:795–799. [Google Scholar]
- Suthers RA, Summers CA. Behavioral audiogram and masked thresholds of the megachiropteran echolocating bat, Rousettus. Journal of Comparative Physiology. 1980;136:227–233. [Google Scholar]
- Suthers RA, Fattu JM. Mechanisms of sound production by echo locating bats. American Zoologist. 1973;13:1215–1226. [Google Scholar]
- Symmes D, Biben M. Maternal recognition of individual infant squirrel monkeys from isolation call playbacks. American Journal of Primatology. 1985;9:39–46. doi: 10.1002/ajp.1350090105. [DOI] [PubMed] [Google Scholar]
- Terrazas A, Serafin N, Hernandez H, Nowak R, Poindron P. Early recognition of newborn goat kids by their mother: II. Auditory recognition and evidence of an individual acoustic signature in the neonate. Developmental Psychobiology. 2003;43:311–320. doi: 10.1002/dev.10139. [DOI] [PubMed] [Google Scholar]
- Thomson CE, Fenton MB, Barclay RMR. The role of infant isolation calls in mother infant reunions in the little brown bat, Myotis lucifugus (Chiroptera, Vespertilionidae) Canadian Journal of Zoology. 1985;63:1982–1988. [Google Scholar]
- Tokumaru RS, Ades C, Monticelli PF. Individual differences in infant guinea pig pups isolation whistles. Bioacoustics. 2004;14:197–208. [Google Scholar]
- Trillmich F. Mutual mother-pup recognition in Galapagos fur seals and sea lions - cues used and functional significance. Behaviour. 1981;78:21–42. [Google Scholar]


