Abstract
Objective
To test the hypothesis that human embryonic stem cells (hESCs) can be guided to form new myocardium by transplantation into the normal or infarcted heart, and to assess the influence of hESC‐derived cardiomyocytes (hESCMs) on cardiac function in a rat model of myocardial infarction (MI).
Methods
Undifferentiated hESCs (0.5–1×106), human embryoid bodies (hEBs) (4–8 days; 0.5–1×106), 0.1 mm pieces of embryonic stem‐derived beating myocardial tissue, and phosphate‐buffered saline (control) were injected into the normal or infarcted myocardium of athymic nude rats (n = 58) by direct injection into the muscle or into preimplanted three‐dimensional alginate scaffold. By 2–4 weeks after transplantation, heart sections were examined to detect the human cells and differentiation with fluorescent in situ hybridisation, using DNA probes specific for human sex chromosomes and HLA‐DR or HLA‐ABC immunostaining.
Results
Microscopic examination showed transplanted human cells in the normal, and to a lesser extent in the infarcted myocardium (7/7 vs 2/6; p<0.05). The transplanted hESCs and hEBs rarely created new vessels and did not form new myocardium. Transplantation of hESCM tissue into normal heart produced islands of disorganised myofibres, fibrosis and, in a single case, a teratoma. However, transplantation of hESCMs into the infarcted myocardium did prevent post‐MI dysfunction and scar thinning.
Conclusions
Undifferentiated hESCs and hEBs are not directed to form new myocardium after transplantation into normal or infarcted heart and may create teratoma. Nevertheless, this study shows that hESC‐derived cardiomyocyte transplantation can attenuate post‐MI scar thinning and left ventricular dysfunction.
Keywords: heart, heart failure, myocardial infarction, regeneration, stem cells
Human embryonic stem cells (hESCs) are a promising source of cardiomyocytes for myocardial tissue repair. They are indefinitely expandable, pluripotent and capable of spontaneous differentiation into fetal cardiomyocytes.1,2,3 However, in vitro spontaneous differentiation of hESCs yields a relatively small number of cardiomyocytes.2 An alternative therapeutic approach is transplantation of undifferentiated hESCs into the heart. The rationale for this approach is the concept that the maintenance and differentiation of stem cells is controlled by their particular microenvironments, which are thought of as clusters of environmental cues affecting the state and behaviour of the cell.4,5 Hence, the heart might provide “guidance cues” that promote tissue‐specific differentiation of hESCs into cardiomyocytes, creating a new myocardium. Several studies have reported that mouse ESCs can develop into functional cardiomyocytes and improve cardiac function after transplantation into the infarcted myocardium of a mouse, rat or sheep.6,7,8,9,10,11,12 However, because mouse ESCs are more diverse in their myogenic differentiation tendency than hESCs,13,14 we sought to investigate whether hESCs could survive in normal and infarcted myocardium, and whether the heart environment would direct differentiation into mature cardiomyocytes. In addition, we investigated the fate and outcome of hESC‐derived cardiomyocytes (hESCMs) in the normal and infarcted heart, and assessed the influence on post‐myocardial infarction (MI) heart function.
Methods
Culture of human ESCs
We used hESC lines H9.2 and I6 (NIH approved lines). The culture of undifferentiated hESCs (passages 36–60) and the generation of human embryoid bodies (hEBs) were performed as previously described.2,15 Old hEBs (6–8 days) were plated on gelatin‐coated (0.1%; Sigma‐Aldrich, St. Louis, USA) 24‐well plates (Nunc, Roskilde, Denmark), 1–3 hEB per well. Daily microscopy observations were conducted to detect hEBs with spontaneous beating areas.
For cell transplantation, 0.5–1×106 cells/animal of the following cultures were used: (a) undifferentiated hESCs; (b) 4–8‐day‐old hEBs in suspension, disassociated with 0.5 mM EDTA supplemented with 1% fetal bovine serum; (c) 10–20‐day‐old beating myocardial tissue, dissected mechanically under inverted microscope imaging.
Rat model of MI, scaffold transplantation and cell transfer
Animal studies adhered to Tel‐Aviv University Guiding Principles in the Care and Use of Animals. Female athymic nude rats (HSD: RH‐Foxn1rnu, Harlen, Israel) were subjected to MI, sham operation or implantation of alginate scaffold on the ischaemic or intact myocardium as previously described.16,17
Cylindrical alginate scaffolds (5 mm diameter×1.0 mm height), with an average pore diameter of 100 µm, were prepared from sodium alginate (MVG; NovaMatrix, FMC BioPolymer, Drammen, Norway) and calcium gluconate as the cross‐linker according to a freeze‐dry technique.17,18 Scaffold transplantation was performed immediately after MI.17 One or two scaffolds were attached, each by one myocardial stitch to the heart.
Overall, 58 athymic nude rats were included in the survival, differentiation and functional experiments. Twelve rats died after the surgical procedure to induce MI. Thus, the final analysis was performed on 46 rats. At 7–10 days after MI, sham operation or alginate scaffold implantation, rats were treated with injections of cells or phosphate‐buffered saline (PBS) into the normal, infarcted or preimplanted alginate scaffold, in the following modes (table 1):
Table 1 Treatment subgroups and models in the experiments.
| Experiment and cell type | Transplantation | |
|---|---|---|
| Normal heart (n = 19) | Infarcted heart (n = 27) | |
| A: Histology: Differentiation of transplanted cells | ||
| Cells: hESCs (n = 4), hEBs (n = 6) | 5 | 5 |
| Scaffold + cells: hESCs (n = 3), hEBs (n = 4) | 4 | 3 |
| Control: PBS (n = 4), PBS + scaffold (n = 2) | 3 | 3 |
| B: Histology: Fate and outcome of transplanted hESCMs into intact rat heart | ||
| hESCMs | 7 | |
| C: Functional study: Transplantation of hESCMs after MI | ||
| hESCMs (n = 8), PBS (n = 8) | 16 | |
hEBs, human embryoid bodies; hESC, human embryonic stem cells; hESCM, human embryonic stem cell‐derived cardiomyocytes; MI, myocardial infarction; PBS, phosphate‐buffered saline.
The histology study (table 1, experiment A) included 23 rats treated with undifferentiated hESCs, hEBs and PBS:
hESCs (0.5–1×106) were injected into normal (n = 2) or infarcted (n = 2) myocardium, onto implanted scaffolds on normal (n = 2) or infarcted myocardium (n = 1), overall seven hearts.
hEBs (4–8 days; 0.5–1×106) were injected into normal (n = 3) or infarcted (n = 3) myocardium, onto implanted scaffolds on normal (n = 2) or infarcted myocardium (n = 2), overall 10 hearts.
PBS (control) was injected into normal (n = 2) or infarcted (n = 2) myocardium, onto implanted scaffolds on normal (n = 1) or infarcted myocardium (n = 1), overall six hearts.
In addition, seven athymic nude rats were included in the experiment designed to test the survival, differentiation, and integration of hESCMs in the heart (table 1, experiment B). Beating hESC‐derived outgrowth of 0.1 mm2 chunks were injected into normal rat heart.
Another group of 16 animals was included in an experiment aimed at exploring the effect of injected beating hESCMs on remodelling and function of the infarcted heart (table 1, experiment C).
Histology, fluorescent in situ hybridisation and immunohistochemistry
hESCs were deposited onto positively charged glass slides by centrifugation (Cytospin 3; Shandon, Manchester, UK). Cytospin slides were processed for two‐colour fluorescent in situ hybridisation (FISH) and subsequent nuclear staining with diamidinophenylindole. By 3–4 weeks after transplantation, the hearts were removed and representative sections were embedded in optimal cutting temperature embedding compound fixative and snap frozen. Frozen sections (5 µm) were used for FISH and HLA‐ABC (DakoCytomation, Glostrup, Denmark) immunostaining, which reacts against mature human but not rat cells. The remaining sections were fixed in formalin and embedded in paraffin.
For FISH, sections on slides were fixed in methanol:acetic acid (3:1), washed in PBS and dehydrated in 70%, 90% and 100% ethanol. Sections were denatured in 70% deionised formamide (Sigma), dehydrated. Denatured human XY‐chromosome probe mixture labelled with green and orange fluorescent dye, respectively, was added (Vysis, Downers Grove, Illinois, USA). After hybridisation at 42°C, sections were mounted in Vectashield anti‐fade with diamidinophenylindole (Vector Laboratories, Burlingame, California, USA).
Representative paraffin‐embedded sections (5 µm) were immunostained with anti‐HLA‐DR, which reacts against mature human but not rat cells; anti‐slow myosin heavy chain (MHC) (Sigma); anti‐fast MHC (Zymed‐Invitrogen, Invitrogen Corporation, Carlsbad, California, USA), anti‐actinin (Sigma), anti‐troponin T (Sigma) and anti‐α‐smooth muscle actin (Sigma) antibodies.
Echocardiography to evaluate remodelling and contractility
Transthoracic echocardiography was performed on all animals (n = 16) within 24 hours after MI (baseline echocardiogram) and 1 month later.16 Echocardiograms were performed with a commercially available echocardiography system equipped with 12 MHz phased‐array transducer (Hewlett Packard, Andover, Massachusetts, USA). We measured left ventricular (LV) anterior wall thickness; maximal LV end‐diastolic dimension; minimal LV end‐systolic dimension in two‐dimensional imaging; and fractional shortening as a measure of systolic function, which was calculated as
FS (%) = ((LVIDd−LVIDs)/LVIDd) × 100
where LVID indicates LV internal dimension, s is systole, and d is diastole. All measurements were averaged for three consecutive cardiac cycles and were performed by an experienced technician who was unaware of the treatment group.
Statistical analysis
Results are reported as mean (SEM). Differences between groups were compared with the Student t test for unpaired data. The Wilcoxon signed‐rank test was used to compare the animals' follow‐up changes from their baseline values. The difference in cell survival between normal and infarcted heart was assessed by the Fisher exact test (GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego California, USA). Probability values were two sided and considered significant when p<0.05.
Results
Overall, 58 athymic nude rats were included in the analysis. Table 1 describes the subgroups included in the experiments aimed at evaluating survival and differentiation of hESCs after transplantation into normal and infarcted hearts. Seven athymic nude rats were included in the experiment designed to test survival and integration of hESCMs into normal rat heart. Another group comprised 16 animals included in a preliminary experiment designed to explore the effect of the hESC‐derived cardiomyocytes on remodelling and function of the infarcted heart. Four animals, previously treated with hESCMs, were later diagnosed as infected by mycoplasma and were therefore excluded from the final analysis. Thus, the final functional analysis was carried out in 12 animals (four cell‐treated and eight controls).
Fate and outcome of transplanted embryonic cells in the heart
The presence of human cells was confirmed using DNA probes specific for human X or Y chromosomes and immunostaining for HLA‐DR or HLA‐ABC. FISH staining identified X (green signal) and Y (red signal) chromosomes in cultured undifferentiated hESCs (fig 1A). The human X and Y chromosome probes did not hybridise to the rat cells. Immunostaining showed that undifferentiated hESCs expressed neither HLA‐DR nor HLA‐ABC. However, the EB cells that contained beating cardiomyocytes were positive for HLA staining (fig 1B) and α‐smooth muscle actin (fig 1C).
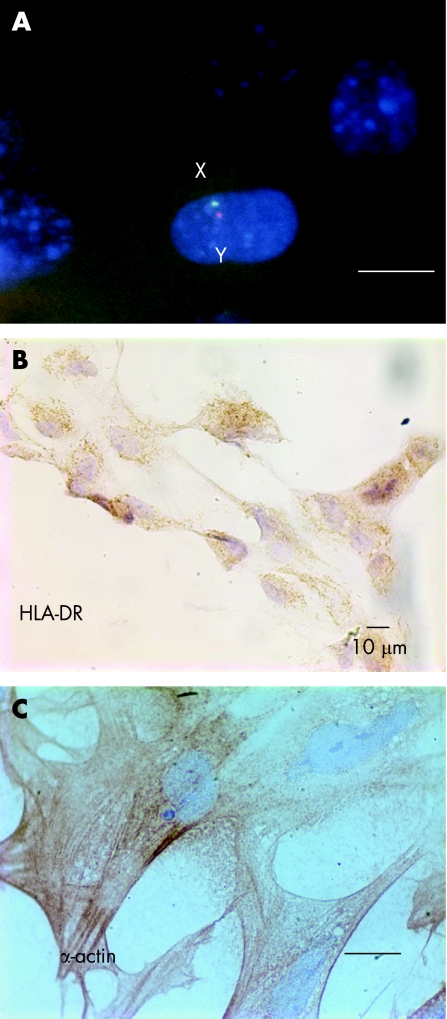
Figure 1 Cultured human embryonic stem cells (hESCs) and their derivatives. (A) Fluorescence in situ hybridisation staining identified X (green signal) and Y (red signal) chromosomes in an hESC from line H9.2 embryonic stem (ES) nuclei stained with diamidinophenylindole. Original magnification ×1000; scale bar 10 µm. (B) Cultured ES‐derived cardiomyocytes stained positive for HLA‐DR. Original magnification ×400; scale bar 10 µm. (C) Cultured ES‐derived cardiomyocytes stained positive for smooth muscle actin. Original magnification ×1000; scale bar 10 µm.
FISH staining was carried out in a subgroup of 10 hearts, from experiment A (table 1), treated with either direct injection of hESCs (n = 4), hEBs (n = 4) or PBS (n = 2) into the normal (n = 5) or infarcted heart (n = 5). Only four hearts, two treated with an hEB injection into infarcted heart (fig 2A) and two with an hESC injection into normal heart, exhibited XX‐positive signals. To track the implanted human cells, immunostaining with HLA‐DR or HLA‐ABC antibodies was carried out in 22 of 23 hearts included in experiment A (table 1). Microscopic examination showed that 13 of the 16 hearts treated with hESCs or hEBs were colonised by donor cells that stained positive (brown colour), suggesting partial differentiation into adult phenotype (fig 2). Because of the small number of animals in each group, we were unable to determine the superiority of hESCs over hEBs or vice versa for cell survival, differentiation or integration. Analysis of 13 representative mid‐sections from hearts treated with either hESCs or hEBs and immunostained for HLA‐ABC, indicated better survival of hESCs and EBs in normal hearts than in infarcted hearts: (7/7 vs 2/6; p = 0.02). Only hEBs survived in the infarcted myocardium (without scaffold).
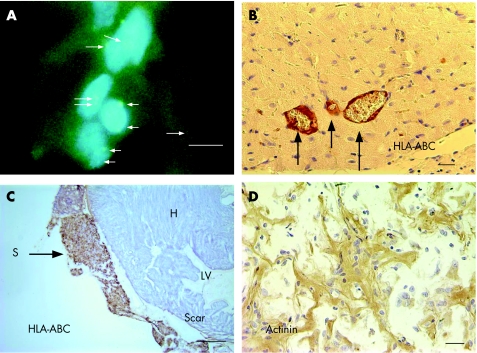
Figure 2 (A) At 3 weeks after injection, human embryoid bodies (hEBs) in the scar tissue are identified by positive FISH green signals for the X chromosome probes (arrows). The hEBs were injected 7 days after myocardial infarction. Original magnification ×1000; scale bar 10 µm. (B) Microscopic examination of normal heart treated with hEBs. Immunostaining for HLA‐ABC identified positive staining at the vessel walls (arrows), suggesting that the donor cells gave rise to new endothelial and smooth muscle cells (×400). Scale bar 20 µm. (C) Photograph of heart at week 3 after implantation of alginate scaffold (S) on infarcted heart and 2 weeks after human embryonic stem cell transfer. Immunostaining for HLA‐ABC identified viable graft that stained positive brown for the human cells (arrow), whereas rat myocardium (H) was stained negative. Scale bar = 500 µm. (D) Higher magnification of the biograft immunostained with anti‐actinin antibodies. The engrafted hEBs within scaffold remnant on infarcted heart stained positively (brown colour) for the myogenic protein, but appeared immature. Scale bar 20 µm.
In two cases, HLA‐DR immunostaining showed that hESCs injected into normal heart were incorporated into vessel walls (fig 2B). We found neither well‐differentiated human cardiomyocytes nor endothelial cells in the scar. Colocalisation of HLA‐DR and slow MHC was examined in serial micrograph sections. None of the hearts, either normal or infarcted, in either the hESC‐ or hEB‐treated groups, exhibited the presence of cardiomyocytes expressing HLA‐DR. This indicates that the cardiomyocytes in the treated hearts did not originate from a human source.
The use of alginate scaffold did not promote myogenic differentiation or organisation of the implanted cells. The scaffold did not evoke inflammatory response in the normal heart and was well integrated within the infarcted heart. Examination of hearts at 3 weeks after scaffold implantation on normal and infarcted heart, and 2 weeks after hESC transfer, identified viable cell graft within scaffold remnants that were stained positive for HLA‐ABC and HLA‐DR (fig 2C). The host myocardium was not stained with anti‐HLA. Some seeded EBs within the scaffold, albeit morphologically immature and disorganised, were stained positive with anti‐actinin antibody (fig 2D).
hES‐Derived cardiomyocyte transplantation
In vitro, hESCMs exhibited typical morphology of embryonic cardiomyocytes with myofibrils and expression of fetal protein smooth muscle actin and were HLA‐DR (fig 1). Implantation of beating hESCMs into the normal myocardium of seven rats produced islands of cells that were stained positive for α‐smooth muscle actin, human troponin T and HLA‐DR and exhibited typical striation, but were isolated from the host heart by scar tissue (fig 3A).
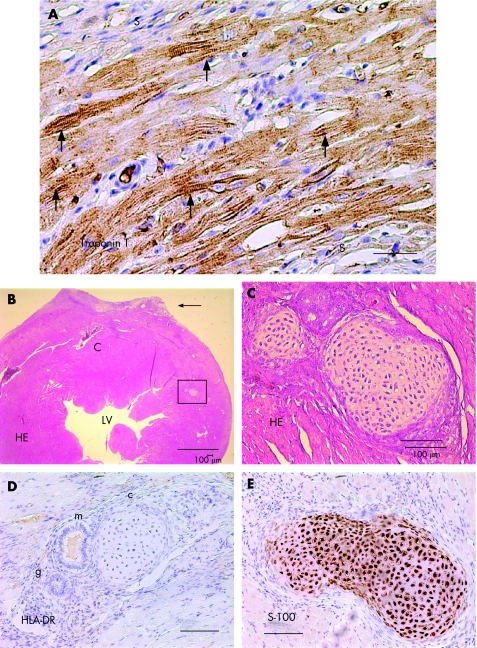
Figure 3 Transplantation of beating ES‐derived cardiomyocytes into the normal heart. Normal heart section at week 4 after injection of human embryonic stem cell (hESC)‐derived beating tissue. (A) Immunostaining for human cardiac troponin T identified islands of disorganised myofibres composed of hESC‐derived cardiomyocytes with early sarcomeric formation (arrows) at the site of injection. The cells were isolated from the host myocardium by scar (S) tissue. Original magnification ×400; scale bar 10 µm. (B) Haematoxylin and eosin staining showed an area of left ventricular (LV) free wall deformation (arrow) fibrosis and large vascular cysts (C) at the site of injection. The rectangle designates an island of ectopic tissue. Scale bar 500 µm. (C) Higher magnification of the rectangle in (B) showed an island of cartilage near the site of injection (arrows; ×200). Scale bar = 100 µm. (D) Higher magnification showed another area of respiratory epithelium with ciliated columnar and mucin‐producing goblet cells (m), non‐ciliated columnar gland (g), stratified squamous epithelium, and distinct focus of cartilage (c), near the site of hESC‐derived cardiomyocyte injection (×200). Scale bar 100 µm. (E) Positive immunostaining with anti‐S‐100 protein (brown colour) confirms cartilage formation at the cell transplantation site (×200). Scale bar 100 µm.
In one case, we observed an area of fibrosis, giant cysts filled with red blood cells, areas of respiratory epithelium with ciliated columnar and mucin‐producing goblet cells, non‐ciliated columnar gland, stratified squamous epithelium, and distinct focus of cartilage, stained positive for F100 protein near the site of hESCM injection (fig 3).
Echocardiography functional study
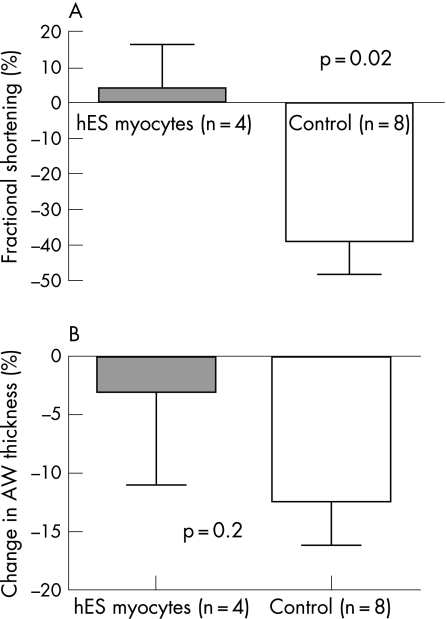
Transplantation of beating hESCMs attenuated the classic course of LV systolic dysfunction complicating extensive anterior MI. During 1 month of follow‐up, fractional shortening was relatively increased by 4 (12)% (from 34 (9)% to 37 (13)%) in cell‐treated animals and decreased by 39 (10)% (38 (4)% to 23 (5)%) in controls (p = 0.02; fig 4). In addition, hESCMs attenuated scar thinning as scar thickness was decreased by 3 (8)% in cell‐treated animals and by 12 (4)% in controls (p = 0.2; Figure 4). However, both hESC‐treated animals and controls developed significant LV dilatation: from 0.61 (0.02) to 0.76 (0.04) cm and 0.61 (0.02) to 0.76 (0.03) cm, respectively (p<0.05). The hearts were processed for histological analysis; the engrafted hESCMs were not detected in the scar tissue by HLA immunostaining 3 weeks after injection.
Figure 4 Transplantation of beating human embryonic stem cell (hESC)‐derived cardiomyocytes (hESCMs) attenuated the classic course of left ventricular systolic dysfunction complicating extensive anterior myocardial infarction. During 1 month of follow‐up, fractional shortening was increased by 4 (12)% in cell‐treated animals and decreased by 39 (10%) in controls (A). In addition, hESCMs attenuated scar thinning as scar thickness was decreased by 3 (8)% in cell‐treated animals and by 12 (4)% in controls (p = 0.2; B).
Discussion
The major new findings of this study suggest that hESCs at various levels of differentiation can survive transplantation in the normal, and to a lesser extent the infarcted, myocardium. The cells are developed into confluent connective tissue when seeded in three‐dimensional alginate scaffold. However, by using human sex chromosome probes and HLA as a marker for human cells, we found that hESCs did not give rise to new myocardium. The experiments designed to test the safety and efficacy of hESCM transplantation showed grafts composed of disorganised myocardial fibres and in one case a teratoma. Nevertheless, this study shows, for the first time, that injection of hESCMs into the infarcted myocardium does preserve LV function.
Previous reports on ES transplantation into the heart
Mouse undifferentiated ES cells can develop into functional cardiomyocytes and improve cardiac function after transplantation into the infarcted myocardium of mouse, rat or sheep.6,7,8,9,10,11,12 Data on the outcome of an hESC graft in normal and infarcted hearts are sporadic. Several studies have been reported in which hESC‐derived cardiomyocytes were injected into animal models of human disease of slow heart rate.19,20 In animal models of atrioventricular block, for example, the implanted hESCs became “pacemakers” and accelerated heart rate.
Most recently Laflamme et al21 reported on injection of hESCMs into the intact LV free wall of athymic rats. Although initial grafts were predominantly epithelial, non‐cardiac elements were lost over time, and grafts consisted predominantly of cardiomyocytes by 4 weeks. In that study, no teratomatous elements were seen. The cardiac implants exhibited substantial angiogenesis, both recipient and graft derived. Importantly, there was greater proliferation in human cardiomyocytes than previously seen in rodent‐derived cardiomyocytes: 14.4% of graft cardiomyocytes expressed the proliferation marker Ki‐67 at 4 weeks after transplantation. This proliferation was associated with a sevenfold increase in graft size over a 4‐week interval. The authors concluded that hESCs can form human myocardium in the rat heart.21
Our findings, even though less favourable, extend those findings and suggest that although the hESCMs do not create “new myocardium”, hESCM transplantation does prevent functional deterioration of the injured heart. In our study, however, the implanted hESCMs did not integrate and were isolated from the host myocardium by reactive fibrosis and appeared immature and disorganised (fig 3), as we previously found with rat and human fetal cardiomyocytes.16,22 This finding is in agreement with a previous report,16 and suggests that the engrafted cells may have some non‐specific paracrine, passive physical effect that increases scar thickening and which might prevent the typical course of LV dysfunction.23
Surprisingly, the only case of teratoma formation was seen when we attempted to transplant beating embryonic cardiomyocytes into normal heart. The most likely explanation is that in the present experiment, the beating tissue included either undifferentiated ES or precursor cells that were differentiated into ectopic tissues such as cartilage and respiratory glands. This finding of teratoma raises concern about the safety of current approach of hESCM selection.
We chose to deliver the cells 7 days after MI to avoid cell loss due to intense inflammation and washout at the infarct zone.24,25,26,27 The time of cell delivery in the present study might be more relevant to cell therapy for patients with MI and allow baseline evaluation of myocardial damage and viability as well as donor and recipient HLA matching.
The mechanism by which the implanted hESCMs prevent functional deterioration of the infarcted heart needs to be established. Most of the implanted cells do not survive or differentiate into mature cardiomyocytes that integrate with the host myocardium. Stem cells can improve functional recovery of infarcted or failing myocardium by various potential mechanisms, including indirect improvement by paracrine factors released by the cells that may inhibit cardiac apoptosis, affect remodelling, or enhance endogenous repair by tissue‐resident progenitor cells or enhancement of neovascularisation.28 Several recent works support the concept that stem cells may protect the myocardium without directly participating in myocardial regeneration.29,30 Other reports have suggested that the therapeutic effects of cells on LV remodelling and function might be independent of implanted cell survival,29,30 transdifferentiation,16,29,30 or scar vessel density.16,30
The implanted cells prevent LV deterioration but not LV remodelling. A similar discrepancy was seen in previous animal works,24 and preliminary clinical trials.31 Possibly, prevention of scar thinning, as seen in the present study, is the mechanism. By thickening the scar, wall stress is reduced (Laplace law) and the degree of outward motion of the infarct that occurs during systole (paradoxical systolic bulging) is reduced. As a result, although the cells do not prevent LV diastolic dilatation, there is a lower end‐systolic dimension in the treated group that is translated to better LV fractional shortening. This is a significant effect since one of the most important predictors of mortality in patients with MI is the degree of LV systolic dilatation.32
Limitations
We are aware of several limitations in our study. First, we investigated human ES transplantation into the heart of immunocompromised athymic rats. The results obtained in this xenotransplantation model might be less favourable than transplantation of ES cells from the same species (syngeneic animals). However, previous reports using an athymic rat or mouse model for human disease, demonstrated the potential of human progenitor cells to differentiate into mature human cells after transplantation.33,34 Second, the experiments included several subgroups with a relatively small number of animals that did not allow for in‐depth comparison among groups. However, no new myocardium was created in any of the 17 normal or infarcted hearts treated with undifferentiated hESCs or hEBs.
Implications and future research
The major findings of the present study suggest that undifferentiated hESCs or hEB cells are not directed to form new myocardium when injected into both normal and infarcted heart with or without scaffold. Injection of hESCMs into LV free wall created islands of myofibres in normal heart and prevented LV dysfunction of infarcted heart. The present study highlights two of the major concerns about the application of hESCs for myocardial repair: (a) a low level of differentiation into relevant cells and (b) the risk of teratoma. Our study suggests that careful selection of cardiomyocytes from hESCs before transplantation is mandatory for the safety and success of hESC‐based therapy for myocardial repair. Future advanced methods for cardiomyocyte selection, guided differentiation, and immune tolerance would promote the potential application of hESCs as a viable source of cells for myocardial repair. The advantage of this approach compared with other most recently discovered adult cardiac progenitors, needs further research.
Acknowledgements
Supported by a grant from Israeli Ministry of Science (JL, SC, JIE)
Abbreviations
ES - embryonic stem
FISH - fluorescent in situ hybridisation
hEBs - human embryoid bodies
hESCs - human embryonic stem cells
hESCMs - hESC‐derived cardiomyocytes
LV - left ventricular
MI - myocardial infarction
MHC - myosin heavy chain
PBS - phosphate‐buffered saline
Footnotes
Conflict of interest: None declared.
References
- 1.Thomson J A, Itskovitz‐Eldor J, Shapiro S S.et al Embryonic stem cell lines derived from human blastocysts. Science 19982821145–1147. [DOI] [PubMed] [Google Scholar]
- 2.Kehat I, Kenyagin‐Karsenti D, Snir M.et al Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest 2001108407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gepstein L. Derivation and potential applications of human embryonic stem cells. Circ Res 200291866–876. [DOI] [PubMed] [Google Scholar]
- 4.Czyz J, Wobus A. Embryonic stem cell differentiation: the role of extracellular factors. Differentiation 200168167–174. [DOI] [PubMed] [Google Scholar]
- 5.Mummery C, Ward‐Van Oostwaard D, Doevendans P.et al Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm‐like cells. Circulation 20031072733–2740. [DOI] [PubMed] [Google Scholar]
- 6.Behfar A, Zingman L V, Hodgson D M.et al Stem cell differentiation requires a paracrine pathway in the heart. FASEB J 2002161558–1566. [DOI] [PubMed] [Google Scholar]
- 7.Min J Y, Yang Y, Converso K L.et al Transplantation of embryonic stem cells improves cardiac function in postinfarcted rats. J Appl Physiol 200292288–296. [DOI] [PubMed] [Google Scholar]
- 8.Min J Y, Yang Y, Sullivan M F.et al Long‐term improvement of cardiac function in rats after infarction by transplantation of embryonic stem cells. J Thorac Cardiovasc Surg 2003125361–369. [DOI] [PubMed] [Google Scholar]
- 9.Kofidis T, de Bruin J L, Hoyt G.et al Myocardial restoration with embryonic stem cell bioartificial tissue transplantation. J Heart Lung Transplant 200524737–744. [DOI] [PubMed] [Google Scholar]
- 10.Hodgson D M, Behfar A, Zingman L V.et al Stable benefit of embryonic stem cell therapy in myocardial infarction. Am J Physiol Heart Circ Physiol 2004287H471–H479. [DOI] [PubMed] [Google Scholar]
- 11.Himes N, Min J Y, Lee R.et al In vivo MRI of embryonic stem cells in a mouse model of myocardial infarction. Magn Reson Med 2004521214–1219. [DOI] [PubMed] [Google Scholar]
- 12.Menard C, Hagege A A, Agbulut O.et al Transplantation of cardiac‐committed mouse embryonic stem cells to infarcted sheep myocardium: a preclinical study. Lancet 20053661005–1012. [DOI] [PubMed] [Google Scholar]
- 13.Ginis I, Luo Y, Miura T.et al Differences between human and mouse embryonic stem cells. Dev Biol 2004269360–380. [DOI] [PubMed] [Google Scholar]
- 14.Wei H, Juhasz O, Li J.et al Embryonic stem cells and cardiomyocyte differentiation: phenotypic and molecular analyses. J Cell Mol Med 20059804–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerecht‐Nir S, Ziskind A, Cohen S.et al Human embryonic stem cells as an in vitro model for human vascular development and the induction of vascular differentiation. Lab Invest 2003831811–1820. [DOI] [PubMed] [Google Scholar]
- 16.Etzion S, Battler A, Barbash I M.et al Influence of embryonic cardiomyocyte transplantation on the progression of heart failure in a rat model of extensive myocardial infarction. J Mol Cell Cardiol 2001331321–1330. [DOI] [PubMed] [Google Scholar]
- 17.Leor J, Aboulafia‐Etzion S, Dar A.et al Bioengineered cardiac grafts: a new approach to repair the infarcted myocardium? Circulation 2000102III56–III61. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro L, Cohen S. Novel alginate sponges for cell culture and transplantation. Biomaterials 199718583–590. [DOI] [PubMed] [Google Scholar]
- 19.Xue T, Cho H C, Akar F G.et al Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell‐based pacemakers. Circulation 200511111–20. [DOI] [PubMed] [Google Scholar]
- 20.Kehat I, Khimovich L, Caspi O.et al Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol 2004221282–1289. [DOI] [PubMed] [Google Scholar]
- 21.Laflamme M A, Gold J, Xu C.et al Formation of human myocardium in the rat heart from human embryonic stem cells. Am J Pathol 2005167663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leor J, Patterson M, Quinones M J.et al Transplantation of fetal myocardial tissue into the infarcted myocardium of rat. A potential method for repair of infarcted myocardium? Circulation 199694II332–II336. [PubMed] [Google Scholar]
- 23.Chien K R, Moretti A, Laugwitz K L. Development. ES cells to the rescue. Science 2004306239–240. [DOI] [PubMed] [Google Scholar]
- 24.Leor J, Guetta E, Feinberg M S.et al Human umbilical cord blood‐derived CD133+ cells enhance function and repair of the infarcted myocardium. Stem Cells 200624772–780. [DOI] [PubMed] [Google Scholar]
- 25.Dow J, Simkhovich B Z, Kedes L.et al Washout of transplanted cells from the heart: a potential new hurdle for cell transplantation therapy. Cardiovasc Res 200567301–307. [DOI] [PubMed] [Google Scholar]
- 26.Reinecke H, Zhang M, Bartosek T.et al Survival, integration, and differentiation of cardiomyocyte grafts: a study in normal and injured rat hearts [in process citation]. Circulation 1999100193–202. [DOI] [PubMed] [Google Scholar]
- 27.Li R K, Mickle D A, Weisel R D.et al Optimal time for cardiomyocyte transplantation to maximize myocardial function after left ventricular injury. Ann Thorac Surg 2001721957–1963. [DOI] [PubMed] [Google Scholar]
- 28.Dimmeler S, Zeiher A M, Schneider M D. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest 2005115572–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balsam L B, Wagers A J, Christensen J L.et al Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature 2004428668–673. [DOI] [PubMed] [Google Scholar]
- 30.Agbulut O, Vandervelde S, Al Attar N.et al Comparison of human skeletal myoblasts and bone marrow‐derived CD133+ progenitors for the repair of infarcted myocardium. J Am Coll Cardiol 200444458–463. [DOI] [PubMed] [Google Scholar]
- 31.Wollert K C, Meyer G P, Lotz J.et al Intracoronary autologous bone‐marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 2004364141–148. [DOI] [PubMed] [Google Scholar]
- 32.Migrino R Q, Young J B, Ellis S G.et al End‐systolic volume index at 90 to 180 minutes into reperfusion therapy for acute myocardial infarction is a strong predictor of early and late mortality. The Global Utilization of Streptokinase and t‐PA for Occluded Coronary Arteries (GUSTO)‐I Angiographic Investigators. Circulation 199796116–121. [DOI] [PubMed] [Google Scholar]
- 33.Zhang S, Wang D, Estrov Z.et al Both cell fusion and transdifferentiation account for the transformation of human peripheral blood CD34‐positive cells into cardiomyocytes in vivo. Circulation 20041103803–3807. [DOI] [PubMed] [Google Scholar]
- 34.Yeh E T, Zhang S, Wu H D.et al Transdifferentiation of human peripheral blood CD34+‐enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation 20031082070–2073. [DOI] [PubMed] [Google Scholar]