Abstract
Background
Real‐time three‐dimensional echocardiography (RT3DE) is an alternative modality to tissue Doppler imaging (TDI) for assessment of intraventricular dyssynchrony but its role is yet to be defined.
Objectives
To (1) compare RT3DE and TDI for assessment of intraventricular dyssynchrony; (2) determine whether the two techniques agreed regarding the magnitude of dyssynchrony and identification of the site of maximal mechanical delay; and (3) investigate the reason for disagreement.
Patients
100 patients with ischaemic cardiomyopathy.
Setting
Tertiary referral cardiac unit.
Main outcome measures
Dispersion in time interval from QRS onset to peak sustained systolic tissue velocity by TDI (SD‐TTV) and to minimal systolic volume by RT3DE (SD‐T3D) between 12 ventricular segments.
Results
RT3DE image quality was adequate for measurement of SD‐T3D in 77 (77%) patients. In the whole population, SD‐TTV was 40 (20) ms and SD‐T3D was 8.3% (3.4%). RT3DE identified a smaller proportion of patients as having significant dyssynchrony than TDI (49 (64%) patients vs 32 (42%) patients; p<0.01). The correlation between SD‐TTV and SD‐T3D was poor (r = 0.11, p = NS). There was concordance between TDI and RT3DE in identifying the site of maximal mechanical delay in 12 (16%) patients. Validating the two techniques with anatomical M‐mode (AMM) as a parameter of radial timing revealed better agreement with RT3DE than with TDI (χ2 = 11.8, p = 0.001).
Conclusion
In patients with ischaemic cardiomyopathy, TDI and RT3DE show poor agreement for evaluating the magnitude of intraventricular dyssynchrony and the site of maximal mechanical delay. This may partly relate to their respective assessment of longitudinal versus radial timing.
In the selection of suitable patients for cardiac resynchronisation therapy, the current practice is to use ECG criteria to identify patients with a high likelihood of intraventricular dyssynchrony. Although QRS duration has been widely used in clinical trials of resynchronisation therapy in heart failure, such an approach is likely to represent a suboptimal means of identifying intraventricular dyssynchrony, one of the reasons for the documented appreciable non‐responder rate to resynchronisation therapy.1 Other methods of accurately identifying dyssynchrony are being explored. Several recent studies have evaluated echocardiographic indices of intraventricular dyssynchrony.2,3,4,5,6,7,8,9,10,11,12 Although these measures seem to be more predictive of the clinical response to resynchronisation therapy than QRS duration, no prospective clinical trial of resynchronisation therapy has used echocardiographic dyssynchrony as the principal criterion for patient selection.
The need for a reliable method for assessment of dyssynchrony is being increasingly appreciated,13 because of the large number of patients with heart failure who have marked intraventricular conduction disturbance and because of awareness of the detrimental effects of dyssynchrony in a broader spectrum of patients. Relevant groups include patients without overt heart failure undergoing pacemaker14 or cardiac defibrillator15 implantation for standard indications, those with modest heart failure symptomatology and those with less obvious ECG conduction disturbance.16
Of the available echocardiographic modalities to characterise dyssynchrony, there is greatest evidence for the use of tissue Doppler imaging (TDI). This method offers the advantage of excellent temporal resolution, but its application is limited by unfamiliarity and signal noise. Furthermore, conventional tissue velocity‐based TDI is angle dependent and only provides information about timing in the longitudinal plane, potentially providing an incomplete assessment of dyssynchrony.17 Real‐time three‐dimensional echocardiography (RT3DE) is an alternative modality, but its specific role in the assessment of dyssynchrony is yet to be defined. Unlike TDI, RT3DE can provide a simultaneous evaluation of dyssynchrony in all segments and identify the maximum delay in walls that may not be adequately visualised by two‐dimensional imaging. Use of the three‐dimensional dataset provides a parameter that encompasses the radial, circumferential and longitudinal timing of the different segments of the left ventricle.12 Potential limitations of RT3DE for assessment of dyssynchrony include imperfect image quality and relatively low temporal resolution, which may compromise sensitivity.
These two techniques are increasingly available, but there are limited data on assessment of dyssynchrony by RT3DE and inadequate information about how such an assessment compares to TDI. We performed a direct comparison of RT3DE and TDI for the assessment of intraventricular dyssynchrony. We aimed to determine to what extent dyssynchrony, as assessed by RT3DE, would be reflected in a TDI measurement and whether the two techniques could be used interchangeably to identify the site of maximal mechanical delay. We hypothesised that any disagreement between the techniques may indicate differential assessments of longitudinal and radial timing.
Methods
Study design
We studied the assessment of intraventricular dyssynchrony in 100 unselected patients with ischaemic cardiomyopathy. Diagnosis of ischaemic cardiomyopathy was based on documented previous myocardial infarction and/or significant epicardial stenoses at coronary angiography performed for routine clinical indications. Inclusion was irrespective of the extent of left ventricular systolic dysfunction or QRS appearance on the surface ECG. Patients were excluded if they had atrial fibrillation, atrioventricular block or paced rhythm. Routine demographic and clinical data were collected. ECG recordings were studied for measurement of QRS morphology and duration. RT3DE and TDI were performed on the same day, and the data on magnitude of dyssynchrony and site of maximal mechanical delay were compared between the two techniques. Since there are potentially important differences in the way these methods measure dyssynchrony, we sought to validate the identification of the site of maximal mechanical delay by each technique against the results obtained from anatomical M‐mode (AMM) measurements. This helped us to distinguish between longitudinal and radial measurements of ventricular timing. AMM was chosen because of its high frame rate.18 All participating patients gave informed consent and use of the data was approved by the hospital's human research ethics committee.
Colour tissue Doppler
Imaging was performed in the left lateral decubitus position using a standard commercial system (Vingmed System 7, General Electric‐Vingmed, Milwaukee, Wisconsin, USA). Transducer position and angulation were adjusted to minimise the angle between the insonating beam and the left ventricular long axis. Colour tissue Doppler images from apical four‐, two‐ and three‐chamber views were recorded as cine loops triggered to the QRS complex and saved to a magneto‐optical disc. To allow accurate comparison with the RT3DE images, care was taken to ensure that images were acquired with attention paid to correct alignment based on mitral annular points, septum and apex. Gain settings, filters and pulse repetition frequency were adjusted to optimise colour saturation and frame rate.
Recordings were analysed offline (Echopac, General Electric‐Vingmed) by a single experienced reader blinded to the results of the RT3DE data. A 5 mm×5 mm sample volume was positioned at the centre of the basal and mid‐portions of the septal, lateral, inferior, anterior, posterior and anteroseptal myocardial segments. From the generated regional myocardial velocity curves, the time interval between the onset of the QRS complex and peak sustained systolic tissue velocity (TTV) was measured (fig 1) in the 12 segments. The standard deviation in this interval between all 12 segments (SD‐TTV) was calculated as an index of intraventricular dyssynchrony and, to determine the prevalence of dyssynchrony, the data were compared with established criteria for defining significant dyssynchrony.7
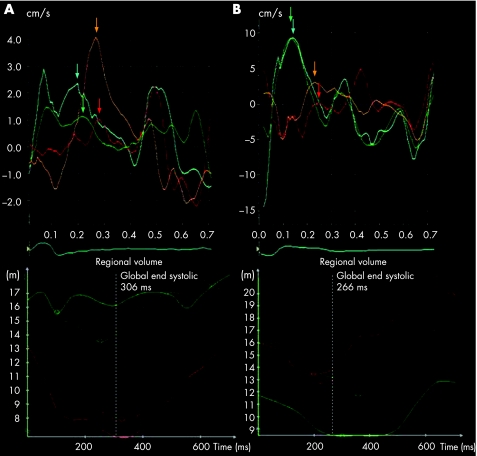
Figure 1 (A) Tissue velocity curves (tissue Doppler imaging (TDI), upper panel) and time volume curves (real‐time three‐dimensional echocardiography (RT3DE), lower panel) in a patient in whom there was agreement in the site of maximal mechanical delay. For TDI the curves are generated by placing the sample volume in the basal septal (cyan), mid‐septal (green), basal lateral (orange) and mid‐lateral (red) segments in the apical four‐chamber view, and for RT3DE by displaying the time–volume curves for the corresponding segments in the software report (basal/mid‐septum in green and basal/mid‐lateral in red). Both techniques indicate delay in the lateral wall relative to the septal wall. (B) Tissue velocity curves (upper panel) and time–volume curves (lower panel) in a patient in whom there was disagreement between the techniques regarding the site of maximal mechanical delay. The TDI curves show that systolic tissue velocity is reached earlier in the septal wall than in the lateral wall, but the RT3DE data show a contrasting pattern of septal delay. Colour of curves for both TDI and RT3DE as in (A).
Real‐time three‐dimensional echocardiography
RT3DE was performed under the same conditions using a Philips Sonos 7500 (Andover, Massachusetts, USA) with a 3.5 mHz transducer. Full‐volume datasets (four cardiac cycles) were gathered from an apical window with the patient in the same position, using a breath‐hold of approximately 10 s. Opacification of the left ventricle with echocardiographic contrast was not used.
Data were analysed off‐line using Tomtec software (Untersclessheim, Germany) by an operator blinded to the TDI analysis. After designating the frames for end‐diastolic and end‐systolic volume, annular and apical landmarks were defined. Care was taken to align equivalent septal, annular and apical points, thereby aligning the septum in the heart's circumferential and longitudinal axes. Automatic border detection was corrected as appropriate. Tracking of the border through the cardiac cycle was used to construct 12 regional time–volume curves analogous to the myocardial velocity curves for the TDI analysis. The interval between QRS onset and minimal volume (T3D) was calculated (fig 1). The standard deviation between all segments (SD‐T3D) was calculated as a measure of intraventricular dyssynchrony.12 To determine the prevalence of dyssynchrony, the data were compared with established criteria for defining significant regional delay12 and, rather than being expressed in milliseconds, SD‐T3D was expressed as a percentage of the cardiac cycle. The regional left ventricular volume data were also used to derive left ventricular ejection fraction. Chamber volumes were corrected for body size by indexing to body surface area.
AMM analysis
Measurements were made off‐line using two‐dimensional grey‐scale images recorded in the apical four‐, two‐ and three‐chamber views. These were acquired alongside the TDI recordings. In each view, the M‐mode cursor was positioned at 90° to the long axis of the left ventricle to give a short‐axis section across the chamber. Analysis involved measurement of the time interval from QRS onset to peak myocardial incursion in systole (TAMM). This was performed at both basal and mid‐ventricular levels for each of the six walls to provide information that could be compared with the TDI and RT3DE data. For identification of the site of maximal mechanical delay, the average TAMM of the basal and mid‐segments for each of the six walls was calculated.
Statistical analysis
Continuous variables are expressed as mean (SD) and categorical variables as frequencies and percentages. Continuous variables were compared with Student's unpaired t test and categorical variables compared with the χ2 test. The relationship between TDI and RT3DE variables of dyssynchrony was examined using the Pearson correlation coefficient. On the basis of previous work, significant dyssynchrony was defined by a cut‐off value of 32 ms for SD‐TTV7 and 8.3% for SD‐T3D.12 Regression analysis with a general linear model was used to identify variables predictive of significant dyssynchrony. For comparison of wall timing between techniques, the average TTV, T3D and TAMM for the basal and mid‐segments for each of the six walls was calculated. A p value of <0.05 was considered significant.
Results
Feasibility of imaging and population characteristics
A total of 100 patients underwent both TDI and RT3DE. Colour tissue Doppler images were considered to be adequate for analysis in all patients. However, satisfactory myocardial velocity curves for all 12 segments could not be derived in 39 (51%) patients. The number of segments per patient with missing data was 0.8 (1.0). The mid‐anterior segment was the common site of difficulty. Since the number of segments per patient with missing data was limited, patients with incomplete data were not excluded from the analysis. Translational artefacts were reported in the RT3DE images of five patients, precluding the analysis of left ventricular volume. In a further 18 patients, the RT3DE images were suboptimal for other reasons and endocardial border delineation was deemed inadequate for accurate assessment of left ventricular volume. These 23 patients were excluded from the analysis. Table 1 shows the clinical characteristics of the remaining 77 patients constituting the study population. Regarding RT3DE, there were 34 patients in whom the image quality was deemed to be optimal/good, 26 patients with fair images and a further 17 patients with images that were poor but adequate.
Table 1 Clinical characteristics of the study population (n = 77).
| Demographics | |
| Age (years) | 64 (11) |
| Gender (male) | 69 (90%) |
| Clinical data | |
| Diabetes mellitus | 18 (23%) |
| Hypertension | 29 (38%) |
| Previous coronary revascularisation | 24 (31%) |
| Heart failure | 58 (75%) |
| NYHA class | 1.8 (0.8) |
| Angina pectoris | 53 (69%) |
| β‐blocker therapy | 58 (75%) |
| ACE inhibitor/angiotensin receptor blocker therapy | 62 (81%) |
| Coronary disease | |
| Coronary angiography | 60 (78%) |
| Single‐vessel disease | 19/60 (31%) |
| Multi‐vessel disease | 41/60 (69%) |
| ECG | |
| QRS duration (ms) | 109 (26) |
| QRS duration >100 ms | 36 (47%) |
| QRS duration >120 ms | 22 (29%) |
| LBBB | 18 (23%) |
| 2D echocardiography | |
| LVEDVI (ml) | 91 (35) |
| LVESVI (ml) | 59 (30) |
| LVEF (%) | 37 (12) |
LBBB, left bundle branch block; LVEDVI, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end‐systolic volume index; NYHA, New York Heart Association.
A total of 68 (88%) patients were taking at least one drug to retard/prevent progressive left ventricular dysfunction (β blocker, ACE inhibitor or angiotensin receptor blocker). Most patients had significant left ventricular systolic dysfunction, with 49 (64%) having a left ventricular ejection fraction <40%. Most patients had undergone coronary angiography for routine clinical indications and this most often demonstrated multivessel disease.
Segmental timing and magnitude of intraventricular dyssynchrony by the two techniques
Across all segments, the values for TTV were significantly less than T3D. In the septal wall, TTV was 153 (42) ms compared with a T3D of 374 (98) ms. In the lateral wall, TTV was 202 (60) ms compared with a T3D of 352 (93) ms. The respective values for the inferior and anterior walls were 165 (46) vs 358 (79) ms and 165 (57) vs 344 (93) ms. For the posterior and anteroseptal walls, the respective values were 187 (56) vs 339 (68) ms and 151 (38) vs 385 (119) ms.
In the whole population, SD‐TTV was 40 (20) ms and SD‐T3D was 8.3% (3.4%). Applying the previously defined cut‐off values for the two techniques, TDI identified a greater proportion of patients as having significant dyssynchrony than did RT3DE (49 (64%) vs 32 (42%) patients; p<0.01).
Regression analysis revealed that none of the clinical variables (including presence of heart failure symptoms, New York Heart Association class, diabetes or QRS duration) were predictive of significant dyssynchrony by either technique. With TDI, the most important univariate correlate with significant dyssynchrony was left ventricular end‐diastolic volume index (F = 7.36, p = 0.008), followed by left ventricular end‐systolic volume index (F = 5.54, p = 0.021). The most important univariate correlate for significant dyssynchrony by RT3DE was left ventricular ejection fraction (F = 7.51, p = 0.008), followed by left ventricular end‐systolic volume index (F = 6.70, p = 0.012).
Patients identified as having significant dyssynchrony by TDI had a similar QRS duration to those who had less dyssynchrony (113 (26) vs 103 (24) ms, p = NS). Patients identified as having significant dyssynchrony by RT3DE also had a similar QRS duration to those who had less dyssynchrony (115 (29) vs 105 (23) ms, p = NS).
Analysis of the magnitude of dyssynchrony by the two techniques using all 12 segments revealed a poor correlation between SD‐TTV and SD‐T3D (r = 0.11, p = NS). We also analysed the data using only the six basal segments and found a similarly poor correlation between SD‐TTV and SD‐T3D (r = 0.05). Separate analysis of patients grouped according to image quality showed that the correlation between TDI and RT3DE for the magnitude of dyssynchrony was poor regardless of image quality. For the group with optimum/good image quality r = 0.21, for those patients with fair image quality, r = 0.03 and for those with poor image quality, r = 0.02.
Identification of site of maximal mechanical delay
The site of maximal mechanical delay varied according to the technique used (fig 2). In 11 patients the site of maximal mechanical delay by TDI was ambiguous because of similar TTV values for multiple walls. The most common site of maximal mechanical delay by TDI was the lateral wall (27 (35%) patients), followed by the posterior wall (15 (19%) patients). Using RT3DE, maximal mechanical delay was most commonly seen in the anteroseptal wall (22 (29%) patients) followed by the septal wall (18 (23%) patients). There was concordance between TDI and RT3DE in identification of the site of maximal mechanical delay in only 12 (16%) patients.
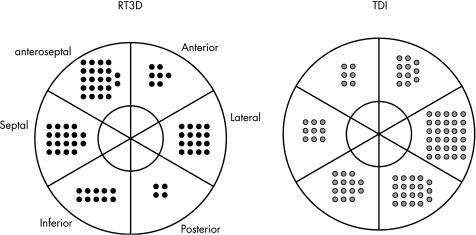
Figure 2 Site of maximal mechanical delay by segment according to technique. Note that for tissue Doppler imaging (TDI) maximal delay was simultaneous in more than one segment, in which case more than one site is shown.
Reproducibility of measurements
For RT3DE, variability was assessed by the random selection of 10 patients and measurement of T3D. Interobserver variability was 18%. For intraobserver variability we used the same ten patients, with results derived from the first observer being compared with results from the same observer with an interval between measurements of at least 6 months. Intraobserver variability was 14%. We have previously assessed interobserver and intraobserver variability for TDI measures of dyssynchrony in our laboratory. For TTV, interobserver variability was 24% and intraobserver variability was 13%.
Longitudinal versus radial timing: comparison with AMM
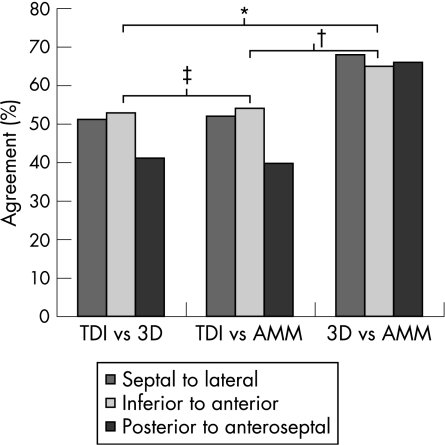
Figure 3 shows the agreement between each of the three techniques (TDI, RT3DE and AMM) for determining opposing wall mechanical delay. There was poor agreement between TDI and RT3DE and between TDI and AMM in all three apical views. The agreement between RT3DE and AMM was significantly better (χ2 = 16.2, p<0.001).
Figure 3 Percentage agreement between echocardiographic techniques in identifying walls with greater mechanical delay. Data are displayed comparing the timing of opposing walls in each of the three apical views. *χ2 = 13.6, p<0.001; †χ2 = 11.8, p = 0.001; ‡χ2 = 0.027, p = 0.869. AMM, anatomic M‐mode; 3D, three dimensional; TDI, tissue Doppler imaging.
Discussion
In this study we have shown that, in patients with ischaemic cardiomyopathy, TDI and RT3DE provide distinctly different measures of ventricular timing and cannot be considered directly comparable for assessing intraventricular dyssynchrony. Important discrepancies are observed between the two techniques both for assessing the magnitude of dyssynchrony and for identifying the site of maximal mechanical delay. These may be partly due to differences in measurement of longitudinal and radial timing.
Echocardiographic techniques available for assessment of intraventricular dyssynchrony
Advances in echocardiography have meant that there are now a number of proposed methods for assessing intraventricular dyssynchrony.2,3,4,5,6,7,8,9,10,11,12 The predominant focus has been on TDI indices, and responders to resynchronisation therapy responders can be more accurately identified using this method than using ECG QRS duration.7 It is not yet clear which of the proposed TDI parameters will be most predictive of resynchronisation therapy response in clinical practice but ongoing work is dealing with this question.19
RT3DE has emerged as a competing technique for TDI and is increasingly available, but there has been only one report of its application for assessment of dyssynchrony.12 The rationale for the use of RT3DE is that in a perfectly synchronous left ventricle, all segments should achieve a minimum volume simultaneously. Variation in this timing between segments indicates the presence of dyssynchrony. There are a number of attractive aspects to the use of RT3DE for the definition of dyssynchrony. In a single cardiac cycle, it offers the potential to identify mechanical delay in walls that may not be adequately visualised by two‐dimensional imaging except in off‐axis views. Furthermore, RT3DE provides information about longitudinal timing of the left ventricular segments (the only data supplied by conventional TDI) and encompasses elements of radial and circumferential function that are undoubtedly important components of left ventricular function.17,20
Comparison of TDI with RT3DE for assessment of the magnitude of intraventricular dyssynchrony
Peak tissue velocity is reached in early systole and corresponds to peak systolic ventricular pressure. Physiologically, this interval differs markedly from the interval to minimal systolic volume measured by RT3DE. The latter is an end‐systolic event as ventricular pressure is already falling. It seems likely that disturbances of electrical activation may affect these systolic indices differently. Therefore, an index of dyssynchrony based on the dispersion of the interval data between segments will not be equivalent between RT3DE and TDI. Furthermore, it is unclear at this stage whether tissue measures of dyssynchrony should be preferred over regional volumetric changes. A comparison of the relative merits of the two techniques for identifying reverse remodelling or clinical response after resynchronisation therapy has not been performed. Comparison of measures of dyssynchrony based on previous independent reports is made more complex because interval data quoted for RT3DE has been adjusted for heart rate whereas this is not the case for TDI.
Motion of left ventricular regional segments during the cardiac cycle is complex. With conventional TDI, the practicalities of transducer orientation restrict assessment to the longitudinal plane. Longitudinal measurement of dyssynchrony has a smaller range and more variability than circumferential measures,17 highlighting a potential limitation in the clinical applicability of such an approach. The recent finding that radial tissue timing based on speckle tracking (a new means of measuring myocardial shortening and lengthening based on tissue characteristics from grey‐scale images) offers alternative information about dyssynchrony further supports this hypothesis.11 Notably, the authors found that the small subgroup of patients with radial dyssynchrony but no longitudinal dyssynchrony showed a favourable response to resynchronisation therapy. Whether composite information about radial and circumferential left ventricular timing can be translated into improved selection of appropriate candidates for resynchronisation therapy in clinical practice requires further clarification.
Comparison of TDI with RT3DE for determining the site of maximal mechanical delay
Inclusion of patients with ischaemic left ventricular dysfunction provided us with an opportunity to address this question in a heterogeneous population since these patients have variable proportions and sites of viable and scarred myocardium. Identification of the lateral wall as the site of maximal mechanical delay by TDI contrasted with anteroseptal wall delay with RT3DE. The reason for this poor agreement is unclear. The ischaemic cause of left ventricular dysfunction in this population may be relevant since the loss of apical torsion that can occur with apical infarction21 may affect radial and longitudinal measures of dyssynchrony differently. The notable heterogeneity in the site of maximal mechanical delay in our patient population is consistent with previous findings in ischaemic cardiomyopathy.22 We wonder whether anteroapical akinesis due to infarction may cause difficulties in determining the time to minimal volume in these remodelled segments of the ventricle.
Clinical implications and future directions
The issue of variation in measurement between techniques is not unique to the measurement of dyssynchrony. Similar disparities have been described with other echocardiographic parameters, most notably that of left ventricular systolic function.23,24 Since measuring dyssynchrony is more complex than measuring left ventricular ejection fraction, it is not surprising that a level of disagreement exists between methods. As with ejection fraction, efforts should aim to understand the reasons for the disagreement. Both the techniques studied here have inherent problems. In addition to suboptimal endocardial definition and translational artefacts, RT3DE has the problem of an automated centre‐point that can critically affect the calculation of the regional time–volume curve data. With conventional velocity‐based TDI, curves are affected by the effects of tethering, although the application of speckle tracking has suggested that strain‐rate imaging may have an important role.11 One of the difficulties regarding assessment of dyssynchrony is lack of consensus on a convenient gold standard. Use of clinical responses to resynchronisation therapy in order to determine the more appropriate technique to describe dyssynchrony is flawed by the confounding influences of scar tissue and frequent lack of concordance between pacing site location and site of maximal mechanical delay. A more direct parameter of segmental myocardial timing is necessary. Considering its high temporal resolution and ability to accurately sample specific segments, we selected AMM as the most appropriate method in this study. Two‐dimensional strain was a possible alternative, but we were concerned about limitations imposed by an inferior frame rate.
Patients with greater dyssynchrony are most likely to be considered for resynchronisation therapy. Differences between RT3DE and TDI for assessing the magnitude of dyssynchrony may influence which patients are selected. Of the cross section of patients considered for resynchronisation therapy, it will be important to determine whether RT3DE and TDI identify different patients as likely responders. To adequately address this issue, more data on the variability and reproducibility of both techniques, preferably from a range of centres, are required. The importance of left ventricular lead location in association with site of maximal mechanical delay is becoming increasingly recognised. There is evidence that if a patient is paced at a site distant from that of maximal mechanical delay, the response to resynchronisation therapy is likely to be suboptimal.11 Echocardiographic data play an important part in determining where the maximal delay is located, and the discrepancies in our study between RT3DE and TDI in this regard merit further investigation.
Study limitations
We did not use echocardiographic contrast agents to enhance endocardial definition. Since RT3DE image quality was suboptimal in a number of patients, it is likely that use of such agents would have increased the feasibility of studies and reduced the amount of off‐line manual editing required. Nonetheless, use of contrast agents does not solve image quality issues in all patients and translational artefacts remain a significant limitation.
The population studied comprised some patients who would not be considered to meet conventional criteria for resynchronisation therapy and this may have contributed to some of the heterogeneity of the results. However, it should be acknowledged that such treatment has been postulated in other clinical groups and we wanted to address the issue of echocardiographic assessment in a broader group of patients, some of whom had less severe ventricular disease.
In this study, an index of dyssynchrony (SD‐T3D) that was corrected for heart rate was compared with one (SD‐T3D) that was not corrected. This may have affected the comparison of the two techniques. No clear consensus has been reached regarding correcting for cycle length when assessing dyssynchrony, but patients in this study were studied after several minutes in the resting state and had a relatively narrow range of heart rate. Similarly, the choice of a cut‐off value for definition of significant dyssynchrony for each of the techniques was based on limited data. Nonetheless, for both TDI and RT3DE, we sought previously published data derived from normal people.
Unlike other recent studies we did not use tissue tracking to obtain radial timing. Short‐axis images were available only at mid‐ventricular level and we could not provide separate data for basal segments. Also, these images were not always on axis and often poorly optimised for TDI.
In comparing the site of maximal mechanical delay, we used the average timing of the basal and mid‐ventricular segments in each left ventricular wall. This is undoubtedly a simplisitic approach in these patients with coronary disease, in whom there may have been an heterogeneous distribution of timing along each wall.
Conclusions
RT3DE and TDI are increasingly available but provide different assessments of intraventricular dyssynchrony possibly because of differences in assessment of radial and longitudinal cardiac timing. Differences in magnitude of dyssynchrony between the techniques may influence the selection of appropriate candidates for resynchronisation therapy, and differences in the site of maximal mechanical delay could affect the selection of left ventricular pacing lead position. Further work will be required to define the relative merits of these techniques for the assessment of dyssynchrony.
Abbreviations
AMM - anatomical M‐mode
RT3DE - real‐time three‐dimensional echocardiography
SD‐TTV - standard deviation in time interval from QRS onset to peak sustained systolic tissue velocity across all segments
SD‐T3D - standard deviation in time interval from QRS onset to minimal systolic volume
TAMM - time interval from QRS onset to peak myocardial incursion in systole
TDI - tissue Doppler imaging
TTV - time interval from QRS onset to peak sustained systolic tissue velocity
T3D - time interval from QRS onset to minimal systolic volume
Footnotes
Funding: MIB is supported by a University of Queensland Postdoctoral Research Fellowship.
Competing interests: None.
Ethical approval: Use of the data for analysis was approved by the hospital's human research ethics committee.
References
- 1.Abraham W T, Fisher W G, Smith A L.et al MIRACLE Study Group. Cardiac resynchronization in chronic heart failure. N Engl J Med 20023461845–1853. [DOI] [PubMed] [Google Scholar]
- 2.Pitzalis M V, Iacoviello M, Romito R.et al Cardiac resynchronization therapy tailored by echocardiographic evaluation of ventricular asynchrony. J Am Coll Cardiol 2002401615–1622. [DOI] [PubMed] [Google Scholar]
- 3.Breithardt O A, Stellbrink C, Kramer A P.et al PATH‐CHF Study Group. Pacing therapies for congestive heart failure. Echocardiographic quantification of left ventricular asynchrony predicts an acute hemodynamic benefit of cardiac resynchronization therapy. J Am Coll Cardiol 200240536–545. [DOI] [PubMed] [Google Scholar]
- 4.Ansalone G, Giannantoni P, Ricci R.et al Doppler myocardial imaging in patients with heart failure receiving biventricular pacing treatment. Am Heart J 2001142881–896. [DOI] [PubMed] [Google Scholar]
- 5.Bax J J, Marwick T H, Molhoek S G.et al Left ventricular dysynchrony predicts benefit of cardiac resynchronization therapy in patients with end‐stage heart failure before pacemaker implantation. Am J Cardiol 2003921238–1240. [DOI] [PubMed] [Google Scholar]
- 6.Bax J J, Bleeker G B, Marwick T H.et al Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol 2004441834–1840. [DOI] [PubMed] [Google Scholar]
- 7.Yu C M, Fung J W, Zhang Q.et al Tissue Doppler imaging is superior to strain rate imaging and postsystolic shortening on the prediction of reverse remodeling in both ischemic and nonischemic heart failure after cardiac resynchronization therapy. Circulation 200411066–73. [DOI] [PubMed] [Google Scholar]
- 8.Sogaard P, Egeblad H, Kim W Y.et al Tissue Doppler imaging predicts improved systolic performance and reversed left ventricular remodeling during long‐term cardiac resynchronization therapy. J Am Coll Cardiol 200240723–730. [DOI] [PubMed] [Google Scholar]
- 9.Popovic Z B, Grimm R A, Perlic G.et al Noninvasive assessment of cardiac resynchronization therapy for congestive heart failure using myocardial strain and left ventricular peak power as parameters of myocardial synchrony and function. J Cardiovasc Electrophysiol 2002131203–1208. [DOI] [PubMed] [Google Scholar]
- 10.Gorcsan J I I I, Kanzaki H, Bazaz R.et al Usefulness of echocardiographic tissue synchronization imaging to predict acute response to cardiac resynchronization therapy. Am J Cardiol 2004931178–1181. [DOI] [PubMed] [Google Scholar]
- 11.Suffoletto M S, Dohi K, Cannesson M.et al Novel speckle‐tracking radial strain from routine black‐and‐white echocardiographic images to quantify dyssynchrony and predict response to cardiac resynchronization therapy. Circulation 2006113960–968. [DOI] [PubMed] [Google Scholar]
- 12.Kapetenakis S, Siva A, Corrigan N.et al Real‐time three‐dimensional echocardiography: a novel technique to quantify global left ventricular mechanical dyssynchrony. Circulation 2005112992–1000. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins N M, Petrie M C, MacDonald M R.et al Selecting patients for cardiac resynchronization therapy: electrical or mechanical dyssynchrony? Eur Heart J 2006271270–1281. [DOI] [PubMed] [Google Scholar]
- 14.Sweeney M O, Hellkamp A S. Heart failure during cardiac pacing. Circulation 20061132082–2088. [DOI] [PubMed] [Google Scholar]
- 15.Wilkoff B L, Cook J R, Epstein A E.et al DAVID Trial Investigators. Dual‐chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) trial, JAMA 20022883115–3123. [DOI] [PubMed] [Google Scholar]
- 16.Bader H, Garrigue S, Lafitte S.et al Intra‐left ventricular electromechanical asynchrony a new independent predictor of severe cardiac events in heart failure patients. J Am Coll Cardiol 200443248–256. [DOI] [PubMed] [Google Scholar]
- 17.Helm R H, Leclercq C, Faris O P.et al Cardiac dyssynchrony analysis using circumferential versus longitudinal strain implications for assessing cardiac resynchronization. Circulation 20051112760–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmes P P, Masuyama T, Yamamoto K.et al High‐frame‐rate tissue harmonic imaging enhances anatomic M‐mode sections of the left ventricle in short‐axis view. J Am Soc Echocardiogr 200013738–747. [DOI] [PubMed] [Google Scholar]
- 19.Yu C M, Abraham W T, Bax J.et al Predictors of response to cardiac resynchronization therapy (PROSPECT)‐study design. Am Heart J 2005149600–605. [DOI] [PubMed] [Google Scholar]
- 20.Waldman L K, Covell J W. Effects of ventricular pacing on finite deformation in canine left ventricles. Am J Physiol 1987252(Pt 2)H1023–H1030. [DOI] [PubMed] [Google Scholar]
- 21.Notomi Y, Lysyansky P, Setser R M.et al Measurement of ventricular torsion by two‐dimensional ultrasound speckle tracking imaging. J Am Coll Cardiol 2005452034–2041. [DOI] [PubMed] [Google Scholar]
- 22.Van de Veire N, De Sutter J, Van Camp G. Global and regional parameters of dyssynchrony in ischemic and nonischemic cardiomyopathy. Am J Cardiol 200595421–423. [DOI] [PubMed] [Google Scholar]
- 23.Bellenger N G, Burgess M, Ray S G.et al Comparison of left ventricular ejection fraction and volumes in heart failure by two‐dimensional echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance: are they interchangeable. Eur Heart J 2000211387–1396. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins C, Bricknell K, Hanekom L.et al Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real‐time three‐dimensional echocardiography. J Am Coll Cardiol 200444878–886. [DOI] [PubMed] [Google Scholar]