Abstract
Objective
To investigate the underlying mechanisms of a decreased coronary flow reserve after myocardial infarction (MI) by analysing the characteristics of the diastolic hyperaemic coronary pressure–flow relationship.
Design
Prospective study.
Setting
Tertiary care hospital.
Patients
68 patients with a recent MI and 27 patients with stable angina pectoris (AP; control group).
Main outcome measures
The intercept with the pressure axis (the zero flow pressure or Pzf) and slope index of the pressure–flow relationship (SIPF) were calculated from the simultaneously recorded hyperaemic intracoronary blood flow velocity and aortic pressure after successful coronary stenting.
Results
A stepwise increase in Pzf from AP (14.6 (8.0) mm Hg), over non‐Q‐wave MI (22.5 (9.1) mm Hg), to Q‐wave MI (37.1 (12.9) mm Hg; p<0.001) was observed. Similar changes in Pzf were found in a reference artery perfusing the non‐infarcted myocardium. Multivariate analysis showed that in both regions the left ventricular end‐diastolic pressure (LVEDP) was the most important determinant of the Pzf. The SIPF was not statistically different in the treated vessel between patients with MI and AP, but was increased in MI patients with a markedly increased LVEDP.
Conclusions
After an MI, the coronary pressure–flow relationship is shifted to the right both in the infarcted and in the non‐infarcted remote myocardium, as shown by the increased Pzf. The correlation with Pzf suggests that elevated left ventricular filling pressures contribute to the impediment of myocardial perfusion in patients with infarction.
Although the aim of current treatment strategies for myocardial infarction (MI) is to restore perfusion at the tissue level, important structural and functional changes in the microcirculation are present after restoration of epicardial vessel patency.1 These changes result in a decrease in the maximum achievable hyperaemic flow or a reduced coronary flow reserve (CFR) in the infarct area.2,3,4 This impairment of the myocardial vasodilator capacity after MI has been reported to extend into the remote myocardium where a decreased CFR is observed as well.2
The hyperaemic diastolic coronary pressure–flow relationship and, in particular, its intercept with the pressure axis (the zero flow pressure or Pzf) have been extensively studied in animals.5,6 The advantage of this relationship over the CFR is that it provides a more comprehensive assessment of the properties of the microvascular compartment since it permits the assessment of coronary flow over a pressure range without the interference of cardiac contraction. Any decrease in the diastolic perfusion due to microvascular structural changes, microcirculatory functional alterations or effects of the surroundings (myocardial compartment) on the microvascular compartment results in a rightward displacement of the diastolic pressure–flow relationship in a pressure–flow diagram, and is reflected in an increase in the intercept pressure or Pzf.
In humans, the coronary pressure–flow relationship can be determined by simultaneous acquisition of the phasic coronary flow spectrum and perfusion pressure (fig 1). Using this technique, we aimed to compare the hyperaemic diastolic coronary pressure–flow relationship both in the treated and in the reference vessel between patients with a recent MI and patients with stable angina pectoris (AP) to gain more insight into the microvascular changes after MI. The intercept pressure or Pzf was used as a measure for the position of the diastolic pressure–flow relationship or, in other words, the amount of flow during late diastole.
Figure 1 (A) From top to bottom: flow spectrum, aortic pressure and triggered ECG (three cardiac cycles), which are superimposed and combined in a pressure flow loop (B). During late diastole (arrows), the diastolic pressure–flow relationship is determined by linear regression analysis (y = ax+b). The Pzf (zero flow pressure) is then equal to −b/a.
Methods
Patient selection
Ninety‐five patients with de novo single‐vessel coronary artery disease, who were scheduled for elective coronary intervention, were prospectively included in the study. Exclusion criteria were (1) previous cardiac surgery; (2) total occlusion of the infarct‐related vessel; (3) ECG evidence of hypertrophy; (4) visible collateral circulation on angiography; (5) significant valvular disease; and (6) cardiogenic shock.
Sixty‐eight patients had had a recent (3–12 days) MI, which is defined as (1) an episode of thoracic pain; (2) a rise in troponins; and (3) ST‐segment changes.7 Of the 68 patients with infarction, 48 patients developed a Q‐wave on their ECG (Q‐wave MI) and 20 patients did not (non‐Q‐wave MI). The presence of Q‐waves on the ECG was established according to the Minnesota code (code 1.1.1–1.2.8).8 Patients with ST‐segment elevation MI had been treated with thrombolysis.
Twenty‐seven patients with stable AP, who had no signs of previous infarction, were included as a control group.
The study complied with the Declaration of Helsinki. All patients gave informed consent and the protocol was approved by the ethics committee of the Antwerp University Hospital, Edegen, Belgium.
Measurement protocol
All patients were treated with intravenous heparin (5000 U), and a continuous intravenous infusion of nitrates was started before the catheterisation procedure. Cardiac catheterisation was performed in all patients by percutaneous femoral approach. A 6 Fr guiding catheter was used for the guidance of the balloon catheter. Predilatation and subsequent stenting were carried out. After obtaining an angiographically optimal result, coronary flow velocity was measured by a Doppler crystal‐tipped angioplasty guidewire (Flowire, Volcano Therapeutics, Rancho Cordova, California, USA), which was connected to a 15 MHz pulsed‐Doppler velocimeter (FloMap, Volcano Therapeutics). This guidewire was positioned 1 cm behind the stented lesion and manipulated until an optimal flow signal was reached. After stabilisation of the signals, coronary flow velocity and aortic pressure at the tip of the guiding catheter were continuously recorded at rest and throughout induction and decline of maximum hyperaemia after an intracoronary bolus of 40 μg of adenosine. Subsequently, the Doppler crystal‐tipped guidewire was positioned in the mid part of an unobstructed reference vessel perfusing the non‐infarcted myocardium, and the same measurements were performed.
The left ventricular end‐diastolic pressure (LVEDP) was measured after the flow velocity measurements. Ejection fraction was determined by contrast ventriculography.
Data analysis
The signals of aortic pressure, coronary blood flow velocity, ECG and the in‐phase and quadrature outputs of the Doppler were digitised by a data acquisition system (Wavebook 512, IOtech, Cleveland, Ohio USA) at a sampling rate of 31 250 Hz. With a custom‐written program in Matlab (Mathworks, Natick, Massachusetts, USA), the signals were analysed off‐line. A power spectrum was recomputed by a complex Fast Fourier Transform and the maximum velocity envelope was automatically derived. Three cardiac cycles with tracings of aortic pressure, coronary blood flow velocity and ECG were selected and superimposed (fig 1).
A pressure–flow loop was constructed using the pressure and flow data. The Pzf and the slope of the diastolic pressure–flow relationship (slope index of the pressure–flow relationship or SIPF) were obtained by linear regression applied to the simultaneously acquired pressure and flow velocity recordings during late diastole (fig 1). This method is based on the description given by Di Mario et al,9 who advised analysis of the diastolic pressure–flow relationship during late diastole as originally proposed by Mancini et al.10,11 With this method, intraclass correlation coefficients for intra‐ and interobserver variability close to 0.9 were obtained for the determination of the SIPF and the Pzf (n = 25).
In a preliminary validation study (n = 26), pressure–flow relationships constructed with an intracoronary pressure (Wavewire, Volcano Therapeutics) and aortic pressure were compared. The use of aortic pressure recorded at the tip of the guiding catheter yielded results similar to the intracoronary measurements: the mean difference and limits of agreement (SD) for the SIPF were –0.0045 (0.0052) cm/mm Hg and for the Pzf 0.40 (3.48) mm Hg.
The CFR was calculated as the ratio of average peak velocity (cm/s) in hyperaemic to baseline conditions. Besides the systolic, mean and diastolic pressures, the average pressure and the corresponding flow velocity of the diastolic interval, used for the construction of the pressure–flow relationship, were determined.
Statistics
Patients' characteristics of the three study groups were compared with a χ2 test for categorical variables and with analysis of variance for continuous variables. Differences in characteristics of the pressure–flow relationship and flow velocities were assessed by analysis of variance. When overall significance was found, a post hoc test (Bonferroni correction) was performed to assess differences between the measurements of the three patient groups. Differences were considered statistically significant if p<0.05.
To identify the determinants of the Pzf, the relationship between Pzf and haemodynamic variables was examined by stepwise linear regression analysis (F to enter = 4).
All results are presented as mean (SD).
Results
Patients' characteristics
Table 1 presents the patients' characteristics. The three groups were comparable for body mass index, age and cardiovascular risk factors. Patients were more often treated with ACE inhibitors after infarction. Angiographic characteristics were similar for the three groups.
Table 1 Patients' characteristics.
| AP (n = 27) | Non‐Q‐wave MI (n = 20) | Q‐wave MI (n = 48) | p Value | |
|---|---|---|---|---|
| Mean (SD) age (years) | 64.4 (11.2) | 57.4 (12.6) | 61.9 (11.3) | >0.05 |
| Mean (SD) BMI (kg/m2) | 27.6 (3.9) | 25.8 (5.4) | 27.0 (4.1) | >0.05 |
| Male:female | 17:10 | 13:7 | 40:8 | >0.05 |
| Hypercholesterolaemia, n (%) | 23 (85) | 15 (75) | 33 (69) | >0.05 |
| Smoking, n (%) | 13 (48) | 15 (75) | 27 (56) | >0.05 |
| Diabetes mellitus, n (%) | 8 (30) | 2 (10) | 5 (10) | >0.05 |
| Arterial hypertension, n (%) | 14 (52) | 10 (50) | 17 (35) | >0.05 |
| Familial history, n (%) | 16 (59) | 15 (75) | 26 (54) | >0.05 |
| ACE inhibitors, n (%) | 10 (37) | 6 (30) | 34 (71) | 0.001 |
| β‐Adrenergic receptor antagonist, n (%) | 15 (56) | 14 (70) | 36 (75) | >0.05 |
| Statins, n (%) | 16 (59) | 8 (40) | 27 (56) | >0.05 |
| Reference artery | >0.05 | |||
| LAD | 6 | 6 | 6 | |
| LCX | 15 | 9 | 25 | |
| Treated artery | >0.05 | |||
| LAD, n (%) | 19 (70) | 10 (50) | 28 (58) | |
| LCX, n (%) | 5 (19) | 5 (25) | 6 (13) | |
| RCA, n (%) | 3 (11) | 5 (25) | 14 (29) | |
| Mean (SD) diameter stenosis after stenting (%) | 11.0 (6.6) | 13.1 (7.4) | 9.6 (6.4) | >0.05 |
| Mean (SD) LVEDP (mm Hg) | 20.7 (6.5) | 21.6 (5.1)* | 28.2 (7.7)† | <0.001 |
| Mean (SD) ejection fraction (%) | 69.8 (15.2) | 67.4 (10.9)* | 55.4 (12.3)‡ | <0.001 |
| Baseline | ||||
| Mean (SD) heart rate (/min) | 63.1 (8.5) | 62.5 (16.3)* | 71.7 (11.5)† | 0.001 |
| Mean (SD) systolic BP (mm Hg) | 136.7 (24.9) | 124.2 (18.2) | 125.4 (23.2) | >0.05 |
| Mean (SD) diastolic BP (mm Hg) | 70.4 (14.0) | 68.2 (10.3) | 72.1 (10.5) | >0.05 |
| Mean (SD) BP (mm Hg) | 97.9 (16.5) | 91.0 (10.2) | 94.6 (14.5) | >0.05 |
| Hyperaemia | ||||
| Mean (SD) heart rate (/min) | 63.7 (8.6) | 63.5 (14.2)* | 72.6 (10.5)† | 0.001 |
| Mean (SD) systolic BP (mm Hg) | 134.3 (25.3) | 119.6(17.3) | 124.3 (22.8) | >0.05 |
| Mean (SD) diastolic BP (mm Hg) | 67.1 (13.7) | 64.3 (11.3) | 69.6 (10.9) | >0.05 |
| Mean BP (mm Hg) | 93.7 (17.6) | 85.7 (12.0) | 92.0 (15.1) | >0.05 |
AP, patients with stable angina pectoris, BMI, body mass index; BP, blood pressure; LAD, left anterior descending; LCX, left circumflex artery; LVEDP, left ventricular end‐diastolic pressure; non‐Q wave MI, patients with non‐Q‐wave myocardial infarction; Q‐wave MI, patients with Q‐wave myocardial infarction; RCA, right coronary artery.
*p<0.01 non‐Q‐wave MI versus Q‐wave MI.
†p<0.001 Q‐wave MI versus AP.
‡p<0.01 Q‐wave MI versus AP.
The LVEDP was significantly higher in patients with a Q‐wave MI than in those with stable AP and in those with a non‐Q‐wave MI. The ejection fraction was significantly lower in patients with a Q‐wave MI than in the controls and the patients with a non‐Q‐wave MI.
In baseline and hyperaemic conditions, the heart rate was significantly higher in patients with a Q‐wave MI than in the controls and the patients with a non‐Q‐wave MI (table 1). The systolic, diastolic and mean blood pressures were not statistically different for the three groups. Induction of hyperaemia had no effect on the heart rate, but decreased the systolic, diastolic and mean blood pressure to a small degree (p<0.01). This difference was present in all three patient groups (p>0.05).
Coronary blood flow velocity data after stenting
Figures 2A and B show the coronary blood flow velocities and the CFR recorded in the treated vessel after stenting for the three patient groups, respectively. In the treated vessel, the basal coronary blood flow velocity after stenting was comparable for the three groups (fig 2A). The hyperaemic flow velocity was significantly higher in the AP and non‐Q‐wave MI group than in the Q‐wave MI group (both p<0.01; fig 2A). As a consequence, the CFR was significantly lower in the Q‐wave MI group than in the AP and non‐Q‐wave MI group (both p<0.01; fig 2B).
Figure 2 Intracoronary flow measurements in baseline and hyperaemic conditions. (A) Baseline average peak velocity (bAPV) and hyperaemic average peak velocity (hAPV) in the treated artery. (B) Coronary flow reserve (CFR) in the treated artery. (C) bAPV and hAPV in the reference artery. (D) coronary flow reserve (CFR) in the reference artery. *p<0.05; **p<0.01. AP, patients with stable angina pectoris, Non‐QMI, patients with a non‐Q‐wave myocardial infarction; QMI, patients with Q‐wave myocardial infarction.
In a normal reference vessel, a gradual increase in baseline flow velocity from AP over patients with a non‐Q‐wave MI to Q‐wave MI was found (p<0.05 AP vs Q‐wave MI group; fig 2C). The hyperaemic velocities were not different for the three groups (fig 2C). The CFR of the reference vessel was significantly lower in the patients with a Q‐wave MI than in those with stable AP and with a non‐Q‐wave MI (fig 2D).
Diastolic pressure–flow relationship
The characteristics of in the diastolic coronary pressure–flow relationship showed a close parallelism between the treated and the reference arteries (fig 3). Pzf measured during hyperaemia was significantly higher both in the treated and in the reference vessels in the patients with a Q‐wave MI (p<0.01 vs AP), whereas an intermediate value was obtained in the patients with a non‐Q‐wave MI (fig 3A). The SIPF in the treated vessel tended to be higher in patients with a Q‐wave MI than in patients with stable AP. The SIPF of MI patients with a Pzf ⩾30 mm Hg was significantly higher than in MI patients with a Pzf <30 mm Hg (p<0.05) and in paients with stable AP (p<0.05; fig 4). In the reference artery, the SIPF was significantly higher in patients with a Q‐wave MI than in patients with stable AP (p<0.01; fig 3B).
Figure 3 Diastolic pressure–flow relationship for the three patient groups. (A) Zero flow pressure (Pzf) in treated and reference vessels. (B) Slope index of diastolic pressure–flow relationship (SIPF). *p<0.05; **p<0.01. AP, patients with angina pectoris, non‐QMI, patients with a non‐Q‐wave myocardial infarction; QMI, patients with a Q‐wave myocardial infarction.
Figure 4 Slope index of the diastolic pressure–flow relationship (SIPF) in the treated vessel for patients with stable angina pectoris (AP) myocardial infarction, patients with a zero flow pressure (Pzf) <30 mm Hg and myocardial infarction patients with Pzf ⩾30 mm Hg. *p<0.05.
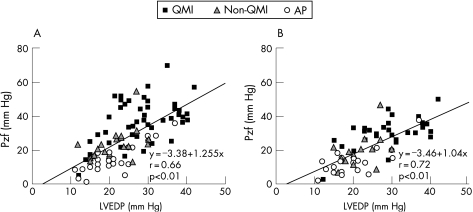
The LVEDP and CFR were found by stepwise regression analysis to be significant independent determinants of the Pzf both in the treated and in the reference arteries (table 2). Figure 5A and B shows the strong relationship between the LVEDP and the Pzf for the treated artery and for the reference artery, respectively.
Table 2 Stepwise regression analysis: determinants of the zero flow pressure in treated and reference vessel.
| F to enter | ||
|---|---|---|
| Treated vessel | Reference vessel | |
| LVEDP | 54.203 | 48.536 |
| CFR | 12.035 | 5.238 |
| Diastolic flow velocity (hyperaemia*) | 3.807 | 0.332 |
| Diastolic BP (hyperaemia) | 3.767 | 0.308 |
| Heart rate (hyperaemia) | 3.539 | 0.657 |
| hAPV | 3.478 | 0.324 |
| Mean BP (hyperaemia*) | 2.136 | 0.957 |
| Systolic BP (hyperaemia) | 2.054 | 2.306 |
| Maximal flow velocity (hyperaemia) | 0.736 | 0.516 |
| Mean BP (hyperaemia) | 0.122 | 0.957 |
BP, blood pressure; CFR, coronary flow reserve; hAPV, hyperaemic average peak velocity; LVEDP, left ventricular end‐diastolic pressure.
* Interval for the construction of the diastolic pressure–flow relationship.
Figure 5 Graph showing the relationship between the left ventricular end‐diastolic pressure (LVEDP) and the zero flow pressure (Pzf) in the treated (A) and the reference arteries (B). AP, patients with angina pectoris; non‐QMI, patients with a non‐Q‐wave myocardial infarction; QMI, patients with a Q‐wave myocardial infarction.
Discussion
In the present study, the effect of MI on coronary haemodynamics was studied by comparing the diastolic hyperaemic coronary pressure–flow relationships between patients with angina and patients with a recent MI. The two major findings of this study are that (1) in patients with a recent MI the hyperaemic diastolic coronary pressure–flow relationship is displaced to the right both in the infarcted and in the non‐infarcted myocardium and (2) the associated increase in Pzf is most strongly related with the LVEDP, suggesting that diastolic left ventricular dysfunction contributes to the impaired hyperaemic coronary flow in patients with MI.
Several other factors have been shown in previous investigations to decrease the hyperaemic flow after MI. Brief periods of ischaemia can decrease the reactivity of the resistance vessels to external hyperaemic stimuli (vascular stunning).13 After longer periods of ischaemia, complete reperfusion of the ischaemic area is not possible owing to microvascular obstruction (no reflow phenomenon).14 Finally, neurohormonal activation after infarction with the release of several vasoconstrictive substances may interfere with the reactivity of the resistance vessels.15 As a result of these functional and structural changes, lower hyperaemic flow ensues.
In this study, patients with a Q‐wave MI also had lower hyperaemic coronary flow velocities than patients with AP. This lower flow level in the infarcted area will shift the pressure–flow relationship to the right, and this partially explains the higher Pzf in the coronary artery perfusing the infarcted myocardium. Figure 6 shows this schematically. As demonstrated in this figure, the position of the diastolic pressure–flow relationship and thus the Pzf can be used as a measure for myocardial perfusion during late diastole.
Figure 6 Schematic presentation of the changes in the diastole pressure–flow relationship in the treated and the reference vessels. For controls (full line) and patients with myocardial infarction (MI) (dashed line), the pressure–flow loops and the diastolic pressure–flow relationship are plotted. In the treated vessel of patients with infarction, a lower hyperaemic flow will result in an increased value of the zero flow measure (Pzf). Moreover, in patients with MI with a Pzf ⩾30 mm Hg, a steeper slope was seen (dotted line). In the reference vessel, a steeper slope was found in patients with MI. The hatched areas indicate the lower hyperaemic flow level in patients with infarction during diastole.
Not all the characteristics of the pressure–flow relationship in patients with infarction can, however, be explained by the above‐described phenomena. Also, in the remote myocardium, higher Pzf values were found in patients with infarction. Moreover, the steepness of the slope of the diastolic pressure–flow relationship was increased in patients with infarction with a markedly increased LVEDP (fig 6). These two findings indicate a restriction of the late diastolic coronary perfusion, which cannot be related to the above‐described, ischaemia‐induced changes in structural and functional properties of microcirculation.
The most likely explanation for the steeper slope and higher Pzf is that myocardial perfusion is also influenced by the mechanics of the myocardial muscle during late diastole. The interaction between the myocardial and the microvascular compartment is well known from the deceleration or even reversal of coronary flow during cardiac contraction, but cardiac mechanics exert their effect also during diastole.16 The intramyocardial vascular capacitance decreases in dogs, when the left ventricular diastolic pressure is raised by intraventricular balloon insufflation.17 Stiffening of the cardiac muscle by hypoxic perfusion without increase in the left ventricular diastolic pressure has the same effect.17 Most likely, the combination of higher intraventricular pressure and increased muscle stiffness decreases the intramyocardial vascular capacitance of patients with a Q‐wave MI. In other words, the changes in the diastolic function and its effect on the left ventricular wall can lead to a compression of the intramyocardial vascular compartment. This restriction of the intramyocardial capacitance limits coronary flow during late diastole and causes a steeper slope of the pressure–flow relationship.
Further supporting evidence comes from animal studies. An increased the left ventricular diastolic pressure limits diastolic perfusion in animals as shown by the shift of the coronary pressure–flow relationship as shown by the rightward displacement of the coronary.18,19 Some believe that this impeding effect is related to the transmitted intraventricular pressure.18,19 On the other hand, myocardial tissue stretching can increase the resistance and Pzf of the embedded vasculature on its own.20 The close proximity of the vascular and the myocardial compartments makes intramyocardial vessels thus susceptible to geometrical deformations and resistance changes. As a consequence, the direct mechanical interactions between the microcirculation and the surrounding tissues are important determinants of the coronary pressure–flow relationship.
Some findings in this study corroborate this conclusion. The LVEDP was the most important determinant of the Pzf in the infarcted myocardium (table 2). This indicates that a higher intraventricular pressure building has a more restrictive influence on the late diastolic coronary flow. Figure 5A shows the strong relationship between the LVEDP and the Pzf. The scatter of the data can be explained by the fact that besides the left ventricular filling pressure, the maximum flow level determines the position of the diastolic pressure–flow relationship, and thus the value of the Pzf. This is also manifested in the stepwise regression analysis, which showed that the second, independent determinant of the Pzf was the CFR.
Another argument is that an increased SIPF and a higher Pzf were also found in the remote myocardium. Again, the LVEDP was the most important determinant. The underlying mechanism of the increased Pzf thus does not seem to be directly related to the local process of infarction, but is present in the whole ventricle.
In summary, the functional and structural microvascular changes after MI will lead to a lower microcirculatory reactivity and hence a lower hyperaemic flow. The diastolic left ventricular dysfunction leads to a further restriction of the microvascular compartment.
Clinical implications
This study points out that the effects of ischaemia and subsequent infarction are not limited to the infarct area. Microvascular disturbances in the infarct area have been demonstrated in numerous previous studies.2,3 The vasomotor response in myocardium remote from the infarcted area has been reported as abnormal in some studies,2,21,22 whereas other studies could not find any influence of infarction on remote myocardial segments.23,24 This study confirms the presence of a lower CFR in patients with MI in both regions and adds diastolic dysfunction as an additional contributing factor, as any limitation of the hyperaemic flow will decrease the CFR.
This study further elucidates the relationship between alterations in ventricular function after infarction and coronary flow by showing that diastolic dysfunction restricts late coronary flow. This finding may have prognostic and pathophysiological implications. As the Pzf is an index of both diastolic and microvascular function, it may well prove to be a strong predictive factor after infarction. In a recent study, it was shown to be a better predictor of functional recovery than the CFR.25 Furthermore, the Pzf may be very valuable for studies on ventricular remodelling. As an expression of the interaction between the myocardial and vascular compartments, it could provide us with useful information on the forces that are active in the myocardial wall and thus on the remodelling process after infarction.
Limitations
All the patients, who were included in this study had undergone different degrees of myocardial ischaemic damage. Patients with myocardial necrosis, as shown by the positive troponin values, were subdivided according to the presence of a Q‐wave on their ECG.7 The division of patients into Q‐wave MI and non‐Q‐wave MI groups can be questioned. However, in a recent MRI study, patients with a Q‐wave MI were shown to have larger infarctions and more left ventricular dysfunction than patients with a non‐Q‐wave MI.26 Also, in this study, patients with a Q‐wave MI had a lower ejection fraction and higher end‐diastolic pressures than the other patient groups. Even if this distinction is not considered robust, it was clearly shown that, irrespective of clinical presentation, the LVEDP is the most important determinant of the Pzf.
The left ventricular pressures were not measured simultaneously during the acquisition of the coronary pressure and flow data. This would require a second arterial puncture, which was not accepted by the ethics committee of our hospital.
Conclusion
Restoration of tissue perfusion after MI is important for the prognosis of the patient. The effects of MI are, however, not limited to the infarct area. Even during diastole, when the predominant portion of perfusion occurs normally, the changes in left ventricular mechanics after infarction restrict coronary flow both in the infarct and in the remote areas. This restriction of perfusion may not only be a sign of the downward spiral of the detrimental remodelling process but may also contribute to it.
Abbreviations
AP - angina pectoris
CFR - coronary flow reserve
LVEDP - left ventricular end‐diastolic pressure
MI - myocardial infarction
Pzf - zero flow pressure
SIPF - slope index of the pressure–flow relationship
Footnotes
PLVH is a PhD Fellow of the Research Foundation—Flanders (Fonds voor Wetenschappelijk Onderzoek—Vlaanderen, Egmontstraat 5, 1000 Brussels, Belgium).
Competing interests: None.
References
- 1.Gibson C M. Has my patient achieved adequate myocardial reperfusion? Circulation 2003108504–507. [DOI] [PubMed] [Google Scholar]
- 2.Uren N G, Crake T, Lefroy D C.et al Reduced coronary vasodilator function in infarcted and normal myocardium after myocardial infarction. N Engl J Med 1994331222–227. [DOI] [PubMed] [Google Scholar]
- 3.Suryapranata H, Zijlstra F, MacLeod D.et al Predictive value of reactive hyperemic response on reperfusion on recovery of regional myocardial function after coronary angioplasty in acute myocardial infarction. Circulation 1994891109–1117. [DOI] [PubMed] [Google Scholar]
- 4.Claeys M J, Vrints C J, Bosmans J.et al Coronary flow reserve during coronary angioplasty in patients with a recent myocardial infarction: relation to stenosis and myocardial viability. J Am Coll Cardiol 1996281712–1719. [DOI] [PubMed] [Google Scholar]
- 5.Spaan J A. Coronary diastolic pressure‐flow relation and zero flow pressure explained on the basis of intramyocardial compliance. Circ Res 198556293–309. [DOI] [PubMed] [Google Scholar]
- 6.Klocke F J, Mates R E, Canty J M., Jret al Coronary pressure‐flow relationships. Controversial issues and probable implications. Circ Res 198556310–323. [DOI] [PubMed] [Google Scholar]
- 7.Alpert J S, Thygesen K, Antman E.et al Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 200036959–969. [DOI] [PubMed] [Google Scholar]
- 8.Rose G A, Blackburn H.Cardiovascular survey methods. Geneva: World Health Organization, 1968 [PubMed]
- 9.Di Mario C, Krams R, Gil R.et al Slope of the instantaneous hyperemic diastolic coronary flow velocity‐pressure relation. A new index for assessment of the physiological significance of coronary stenosis in humans. Circulation 1994901215–1224. [DOI] [PubMed] [Google Scholar]
- 10.Mancini G B, McGillem M J, DeBoe S F.et al The diastolic hyperemic flow versus pressure relation. A new index of coronary stenosis severity and flow reserve. Circulation 198980941–950. [DOI] [PubMed] [Google Scholar]
- 11.Mancini G B, Cleary R M, DeBoe S F.et al Instantaneous hyperemic flow‐versus‐pressure slope index. Microsphere validation of an alternative to measures of coronary reserve. Circulation 199184862–870. [DOI] [PubMed] [Google Scholar]
- 12.Cleary R M, Ayon D, Moore N B.et al Tachycardia, contractility and volume loading alter conventional indexes of coronary flow reserve, but not the instantaneous hyperemic flow versus pressure slope index. J Am Coll Cardiol 1992201261–1269. [DOI] [PubMed] [Google Scholar]
- 13.Bolli R, Triana J F, Jeroudi M O. Prolonged impairment of coronary vasodilation after reversible ischemia. Evidence for microvascular “stunning”. Circ Res 199067332–343. [DOI] [PubMed] [Google Scholar]
- 14.Kloner R A, Ganote C E, Jennings R B. The “no‐reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest 1974541496–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schafer U, Kurz T, Jain D.et al Impaired coronary flow and left ventricular dysfunction after mechanical recanalization in acute myocardial infarction: role of neurohumoral activation? Basic Res Cardiol 200297399–408. [DOI] [PubMed] [Google Scholar]
- 16.Masuyama T, Uematsu M, Doi Y.et al Abnormal coronary flow dynamics at rest and during tachycardia associated with impaired left ventricular relaxation in humans: implication for tachycardia‐induced myocardial ischemia. J Am Coll Cardiol 1994241625–1632. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe J, Levine M J, Bellotto F.et al Left ventricular diastolic chamber stiffness and intramyocardial coronary capacitance in isolated dog hearts. Circulation 1993882929–2940. [DOI] [PubMed] [Google Scholar]
- 18.Domenech R J. Regional diastolic coronary blood flow during diastolic ventricular hypertension. Cardiovasc Res 197812639–645. [DOI] [PubMed] [Google Scholar]
- 19.Jeremy R W, Hughes C F, Fletcher P J. Effects of left ventricular diastolic pressure on the pressure‐flow relation of the coronary circulation during physiological vasodilatation. Cardiovasc Res 198620922–930. [DOI] [PubMed] [Google Scholar]
- 20.Resar J, Livingston J Z, Halperin H R.et al Effect of wall stretch on coronary hemodynamics in isolated canine interventricular septum. Am J Physiol 1990259H1869–H1880. [DOI] [PubMed] [Google Scholar]
- 21.Daher E, Dione D P, Heller E N.et al Acute ischemic dysfunction alters coronary flow reserve in remote nonischemic regions: potential mechanical etiology identified in an acute canine model. J Nucl Cardiol 20007112–122. [DOI] [PubMed] [Google Scholar]
- 22.Joye J D, Lasorda D, Farah T.et al Coronary flow reserve in non‐infarct related arteries in patients with acute myocardial infarction: relationship to infarct size [abstract]. Circulation 199694I22 [Google Scholar]
- 23.Haas F, Nguyen N, Schad H.et al Effect on coronary artery flow reserve and resistance in the remote area after acute coronary artery occlusion in the pig model. J Nucl Cardiol 19996507–513. [DOI] [PubMed] [Google Scholar]
- 24.Pizzuto F, Voci P, Mariano E.et al Coronary flow reserve of the angiographically normal left anterior descending coronary artery in patients with remote coronary artery disease. Am J Cardiol 200494577–582. [DOI] [PubMed] [Google Scholar]
- 25.Shimada K, Sakanoue Y, Kobayashi Y.et al Assessment of myocardial viability using coronary zero flow pressure after successful angioplasty in patients with acute anterior myocardial infarction. Heart 20038971–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon J C, De Arenaza D P, Elkington A G.et al The pathologic basis of Q‐wave and non‐Q‐wave myocardial infarction: a cardiovascular magnetic resonance study. J Am Coll Cardiol 200444554–560. [DOI] [PubMed] [Google Scholar]