Abstract
Objectives
To investigate the impact of arterial remodelling on long‐term clinical outcome after stent implantation in patients with acute coronary syndrome (ACS).
Methods
134 patients with ACS were enrolled. External elastic membrane (EEM) cross‐sectional area (CSA) and lumen CSA were measured. Plaque and media CSA was calculated as EEM minus lumen CSA. Final minimal stent area (MSA) was also measured after stenting. Positive remodelling (PR) was defined as the ratio of the EEM CSA at the target lesion to that at the proximal reference of >1.05, and intermediate or negative remodelling (IR/NR) was defined as that of ⩽1.05.
Results
Although final MSA was similar, target lesion revascularisation (TLR) rates at 2 years were significantly higher in patients with PR (33.7%) than in those with IR/NR (13.7%; p = 0.01). In addition, non‐TLR rates were also significantly higher in patients with PR (42.2%) than in those with IR/NR (23.5%; p = 0.03). Cardiac event‐free survival (for events such as death, myocardial infarction, TLR and non‐TLR) was significantly lower in patients with PR than in those with IR/NR (log rank, p = 0.001). By multivariate logistic regression analysis, PR (χ2 6.57, OR 2.70; 95% CI, 1.27 to 5.78; p = 0.01) and plaque rupture (χ2 4.17, OR 2.38; 95% CI, 1.04 to 5.45; p = 0.04) were independent predictors of cardiac events.
Conclusion
In patients with ACS, PR and intravascular ultrasound findings that may correspond with plaque rupture predict cardiac events including both TLR and non‐TLR at 2 years.
Previous intravascular ultrasound (IVUS) studies have shown the relationship between arterial remodelling and clinical presentation. Positive remodelling (PR) or compensatory enlargement of the culprit lesion is a predominant pattern of lesion remodelling in patients with acute coronary syndrome (ACS)1,2,3 as compared with stable angina pectoris, where negative arterial remodelling or vessel shrinkage plays an important part in lumen compromise.
Several IVUS studies have shown that arterial remodelling may affect clinical restenosis of the target lesion after non‐stent intervention as well as stenting in patients with angina pectoris—that is, lesions with PR have higher clinical restenosis rates.4,5,6 In addition, an IVUS study has suggested that PR may be related to non‐culprit lesion progression in stable angina pectoris.5 However, the impact of arterial remodelling on long‐term clinical outcome including both target and non‐target lesion revascularisation in patients with ACS has not been investigated.
Accordingly, we aimed to assess the impact of pre‐interventional remodelling patterns on long‐term (2 years) clinical outcome after IVUS‐guided stent implantation in patients with ACS.
Methods
Patient and lesion criteria
From January 2001 to June 2003, 179 patients with ACS (135 acute myocardial infarction (AMI), 44 unstable angina pectoris) were successfully treated with IVUS‐guided bare metal stent implantation at Bell Land General Hospital, Sakai, Japan. The diagnosis of AMI was defined as (1) continuous chest pain lasting for >30 min, (2) ST elevation >2.0 mm in ⩾2 contiguous precordial ECG leads and (3) an increase in serum creatine phosphokinase >3 times the normal value. The diagnosis of unstable angina pectoris was defined as (1) symptoms of ischaemia that were increasing or occurred at rest, with the last episode occurring no more than 24 h before admission, and (2) ischaemic changes as assessed by ECG (defined as ST‐segment depression or transient ST‐segment elevation >0.05 mV, or T‐wave inversion of 0.2 mV in two contiguous leads). Patients' exclusion criteria included history of angina pectoris, myocardial infarction or bypass surgery. Lesion exclusion criteria included ostial lesions, severe target lesion calcification, bifurcation lesions and restenotic lesions.
Angiographic and IVUS procedure
Coronary angiography was performed following the standard femoral or radial approach. All patients received intravenous heparin (100 U/kg) before the procedures. Diagnostic angiography was performed after intracoronary nitroglycerin (200 μg) or isosorbide dinitrate (2 mg) administration. IVUS imaging was performed at baseline and repeated after percutaneous coronary intervention (PCI). By using an automated pullback device, IVUS pullback imaging was performed at a rate of 0.5 mm/s. After diagnostic IVUS examination, PCI was performed in a usual manner to achieve TIMI‐3 flow using bare‐metal stents. After PCI, patients were maintained on a regimen of aspirin (81–100 mg daily) and ticlopidine (200 mg daily) for at least 4 weeks. In this study population, glycoprotein IIb/IIIa inhibitors were not used during or after PCI.
Quantitative coronary angiography
Angiography was analysed by an analyst (HT) blinded to the clinical and IVUS information. Angiographic frames were digitised and analysed using an automated edge‐detection algorithm (CMS‐QCA, MEDIS, Leiden, The Netherlands). The minimal lumen diameter (MLD) inside and outside the stent and reference diameter were used to calculate the percent diameter stenosis (%DS) before and after PCI.
Ultrasound imaging protocol
A commercially available system (CVIS/Boston Scientific Corporation, San Jose, California, USA) was used for IVUS examination. The system consisted of a single‐element 40 MHz transducer mounted on the tip of a flexible shaft and rotating at 1800 rpm within a 2.6 French rapid exchange/common distal lumen imaging sheath. Ultrasound images were recorded on a half‐inch, high‐resolution Super‐VHS videotape for off‐line quantitative analysis.
Quantitative and qualitative coronary ultrasound analysis
All ultrasound images were reviewed and evaluated for both qualitative and quantitative parameters by an analyst (HO) blinded to the clinical and angiographical information. The images were digitised to perform morphometric analysis with commercially available planimetry software (NetraIVUS, ScImage, California, USA). Morphometric parameters consisted of external elastic membrane (EEM), lumen and stent cross‐sectional areas (CSAs). The EEM CSA was defined as the area within the media/adventitial border (ie, including lumen, plaque and media). Plaque plus media (P+M) CSA was calculated as EEM CSA minus lumen or stent CSA.7
Morphological parameters consisted of plaque type, presence or absence of thrombus, and plaque rupture. Plaque was divided into one of three types: fibrous, fibrofatty or calcified. Fibrous plaque was defined to be as bright as or brighter than the adventitia without shadowing. Fibrofatty plaque was defined as less bright than the adventitia. Calcified plaque was defined as brighter than the adventitia, with acoustic shadowing. Intracoronary thrombus was defined as (1) a distinct hypoechoic mass, brightly speckled plaque, channelling within the plaque, evacuated plaque cavity, or (2) a detached mobile mass. Plaque rupture was defined as the presence of a cavity that communicated with the lumen with an overlying residual fibrous cap fragment.8
IVUS measurements were done at three cross sections in the target segment: the tightest segment; the proximal and distal reference segments (defined as the location in the native vessel with the least amount of disease within 10 mm of the tightest segment and before to the emergence of any major side branches).
Patterns of arterial remodelling were classified into two categories: (1) PR was defined as the ratio of the EEM CSA at the target lesion to that at the proximal reference of >1.05, and (2) intermediate or negative remodeling (IR/NR) was defined as the ratio of the EEM CSA at the target lesion to that at the proximal reference of ⩽1.05.2
Follow‐up
In‐hospital (<30 days) clinical events included death, myocardial infarction, heart failure and fatal arrhythmia (ventricular tachycardia or ventricular fibrillation).
Long‐term clinical events including death, myocardial infarction, target lesion revascularisation (TLR) and non‐TLR were also obtained at 2 years following the index PCI procedure. TLR was defined as clinically driven repeat revascularisation (either repeat PCI or coronary bypass graft surgery) of the initially treated target lesion, including stented segments and peri‐stent segments 5 mm from both proximal and distal stent edges. Non‐TLR was defined as clinically driven revascularisation of the lesions other than the target lesion.
Cardiac events were death, myocardial infarction, TLR and non‐TLR during a 2‐year follow‐up. Angiographical follow‐up was performed at 6 months. In selected patients, IVUS imaging was also performed at follow‐up.
Statistical analysis
Quantitative data were presented as mean (SD), and qualitative data were presented as frequencies. Continuous variables were compared using two‐tailed unpaired t tests. Binary variables were examined using Fisher's exact and χ2 tests. To identify predictors of clinical events, multivariate logistic models were used. Variables entered into the logistic models were those with a univariate probability value of p<0.15. Cumulative event‐free survival curves during follow‐up in patients with PR versus those with IR/NR were obtained by the Kaplan–Meier method using a log‐rank test. Significance was set at a value of p ⩽0.05. All statistical analyses were performed using the Statview V.5.0 (SAS Institute).
Results
Clinical characteristics
A total of 134 patients with ACS (103 with AMI, 31 with unstable angina pectoris) with pre‐intervention and post‐intervention IVUS imaging were selected and enrolled in this study. There were 105 men and 29 women, with a mean (SD) age of 64 (10) years. Pre‐intervention PR was present in 83 (62%) lesions and IR/NR in 51 (38%) of 134 lesions. Table 1 shows the clinical characteristics of each group. There were no significant differences in age, gender, hyperlipidaemia and family history between the PR and IR/NR groups. PR had a trend towards higher incidence of diabetes mellitus (p = 0.08) and significantly lower incidence of smoking (p = 0.002).
Table 1 Clinical characteristics.
| All (n = 134) | AMI (n = 103) | |||||
|---|---|---|---|---|---|---|
| PR (n = 83) | IR/NR (n = 51) | p Value | PR (n = 68) | IR/NR (n = 35) | p Value | |
| Mean (SD) age (years) | 63.0 (8.0) | 64.2 (7.2) | NS | 62.3 (10.0) | 64.3 (10.0) | NS |
| Sex, M/F | 66/17 | 12/1 | NS | 55/13 | 26/9 | NS |
| Risk factors | ||||||
| Diabetes mellitus, n (%) | 37 (45) | 15 (29) | 0.08 | 30 (44) | 10 (29) | NS |
| Hyperlipidaemia, n (%) | 41 (49) | 24 (42) | NS | 29 (43) | 13 (37) | NS |
| Hypertension, n (%) | 42 (51) | 22 (43) | NS | 33 (49) | 12 (34) | NS |
| Smoking, n (%) | 29 (35) | 32 (63) | 0.002 | 25 (37) | 21 (60) | 0.02 |
| Family history, n (%) | 7 (8) | 5 (10) | NS | 6 (9) | 1 (3) | NS |
| AMI, n (%) | 68 (82) | 35 (69) | 0.08 | 68 (100) | 35 (100) | NS |
| Unstable angina pectoris, n (%) | 15 (18) | 16 (31) | 0.08 | 0 (0) | 0 (0) | NS |
AMI, acute myocardial infarction; F, female; IR, intermediate remodelling; M, male; NR, negative remodelling; PR, positive remodelling.
Baseline high‐sensitivity C reactive protein (mean (SD) 1.8 (2.5) vs 1.4 (3.4) mg/l) and blood glucose levels (126.1 (56.3) vs 111.9 (34.8) mg/dl) were similar between the PR and IR/NR groups (both p = NS). On the other hand, low‐density lipoprotein‐cholesterol level showed a trend towards higher values in the PR group than in the IR/NR group (136.1 (33.7) vs 124.3 (26.7) mg/dl, p = 0.07), although the difference did not reach statistical significance. Peak creatine phosphokinase levels during the initial hospitalisation did not differ between the groups (2741.0 (2448.0) vs 2191.5 (2275.0) IU/l, p = NS).
At the 2 year follow‐up, aspirin was prescribed in 77 patients (93%) of the PR group and 44 (88%) of the IR/NR group (p = NS). Statins were used in 20 (24%) patients of the PR group and in 13 (25%) of the IR/NR group (p = NS).
Quantitative coronary angiography results at baseline
Table 2 shows the quantitative coronary angiography results. There were no significant differences in pre‐intervention reference vessel size, MLD, pre‐intervention %DS and lesion length. Similarly, reference vessel size, MLD and %DS after intervention did not differ between the two groups.
Table 2 Quantitative coronary angiography results.
| All (n = 134) | AMI (n = 103) | ||||||
|---|---|---|---|---|---|---|---|
| PR (n = 83) | IR/NR (n = 51) | p Value | PR (n = 68) | IR/NR (n = 35) | p Value | ||
| Target vessel | |||||||
| LAD/LCX/RCA | 44/12/27 | 39/6/6 | 0.02 | 35/8/25 | 26/3/6 | NS | |
| Pre‐intervention | |||||||
| Reference size, mm | 2.8 (0.4) | 2.8 (0.3) | NS | 2.8 (0.5) | 2.8 (0.4) | NS | |
| Lesion MLD, mm | 0.3 (0.2) | 0.2 (0.4) | NS | 0.2 (0.3) | 0.2 (0.3) | NS | |
| Lesion DS, % | 87 (12) | 86 (15) | NS | 90 (17) | 91 (18) | NS | |
| Lesion length, mm | 9.9 (3.6) | 9.8 (4.6) | NS | 9.5 (4.7) | 9.2 (6.2) | NS | |
| Post‐intervention | |||||||
| Reference size, mm | 3.2 (0.4) | 3.1 (0.3) | NS | 3.3 (0.5) | 3.2 (0.3) | NS | |
| MLD, mm | 2.8 (0.5) | 2.8 (0.4) | NS | 2.8 (0.5) | 2.9 (0.4) | NS | |
| DS, % | 12 (8) | 11 (6) | NS | 13 (10) | 10 (8) | NS | |
AMI, acute myocardial infarction; DS, diameter stenosis; IR, intermediate remodelling; LAD, left anterior descending artery; LCX, left circumflex artery; MLD, minimal lumen diameter; NR, negative remodelling; PR, positive remodelling; RCA, right coronary artery.
Values are represented as mean (SD).
IVUS results at baseline
Table 3 shows the qualitative and quantitative IVUS results. Before PCI, plaque type was not different between the two groups. Intracoronary thrombus and IVUS findings that may correspond to plaque rupture were similarly found in both groups.
Table 3 Morphological and quantitative coronary ultrasound results.
| All (n = 134) | AMI (n = 103) | |||||
|---|---|---|---|---|---|---|
| PR (n = 83) | IR/NR (n = 51) | p Value | PR (n = 68) | IR/NR (n = 35) | p Value | |
| Pre‐intervention | ||||||
| Plaque type | NS | NS | ||||
| Fibrous, n (%) | 50 (60) | 27 (53) | 46 (68) | 17 (49) | ||
| Fibrofatty, n (%) | 28 (34) | 17 (33) | 20 (29) | 14 (40) | ||
| Calcified, n (%) | 5 (6) | 7 (14) | 2 (3) | 4 (11) | ||
| Thrombus, n (%) | 29 (35) | 16 (31) | NS | 27 (40) | 15 (43) | NS |
| Plaque rupture, n (%) | 22 (27) | 11 (22) | NS | 21 (31) | 11 (31) | NS |
| Lesion EEM CSA, mm2 | 18.6 (6.0) | 14.5 (4.5) | 0.002 | 19.2 (5.3) | 15.2 (4.2) | 0.002 |
| Lesion P+M CSA, mm2 | 16.2 (5.8) | 12.4 (4.5) | 0.003 | 16.7 (5.4) | 12.9 (4.4) | 0.006 |
| Lesion lumen CSA, mm2 | 2.2 (0.4) | 2.1 (0.6) | NS | 2.2 (0.6) | 2.1 (0.6) | NS |
| Proximal EEM CSA, mm2 | 15.7 (4.3) | 17.5 (5.4) | NS | 16.2 (3.9) | 18.6 (5.5) | 0.01 |
| Proximal P+M CSA, mm2 | 8.7 (2.6) | 8.9 (3.0) | NS | 8.9 (2.8) | 9.5 (3.6) | NS |
| Proximal lumen CSA, mm2 | 7.0 (2.5) | 8.6 (3.8) | 0.02 | 7.3 (2.3) | 9.1 (3.6) | 0.0002 |
| Distal EEM CSA, mm2 | 12.9 (4.6) | 12.3 (3.9) | NS | 13.3 (4.4) | 13.2 (3.8) | NS |
| Distal P+M CSA, mm2 | 6.3 (3.1) | 5.9 (2.6) | NS | 6.7 (3.5) | 6.3 (2.6) | NS |
| Distal lumen CSA, mm2 | 6.3 (2.8) | 6.3 (2.2) | NS | 6.4 (2.3) | 6.8 (2.2) | NS |
| Post‐intervention | ||||||
| Minimal stent area, mm2 | 8.1 (2.5) | 8.0 (2.6) | NS | 8.2 (2.2) | 8.3 (2.0) | NS |
AMI, acute myocardial infarction; CSA, cross‐sectional area; EEM, external elastic membrane; IR, intermediate remodelling; MSA, minimal stent area; NR, negative remodelling; P+M, plaque plus media; PR, positive remodelling.
Values are represented as mean (SD) unless otherwise specified.
Patients with PR had significantly larger EEM and P+M CSAs than those with IR/NR. However, lumen CSA was not different between the two groups. After PCI, MSA was similar between the two groups.
In‐hospital and long‐term clinical follow‐up
In‐hospital (<30 days) clinical events including death (2.4% vs 2.0%), myocardial infarction (2.4% vs 0%), congestive heart failure (3.6% vs 3.9%) and fatal arrhythmia (8.4% vs 3.9%) were similarly documented between the PR and IR/NR groups (all p = NS).
Angiographical follow‐up at 6 months was performed in 118 of 134 (88%) patients. Angiographical restenosis was documented in 31 of 118 (26%) patients. Angiographical restenosis rate at 6 months was significantly higher in patients with PR than in those with IR/NR (34% vs 14%, p = 0.02).
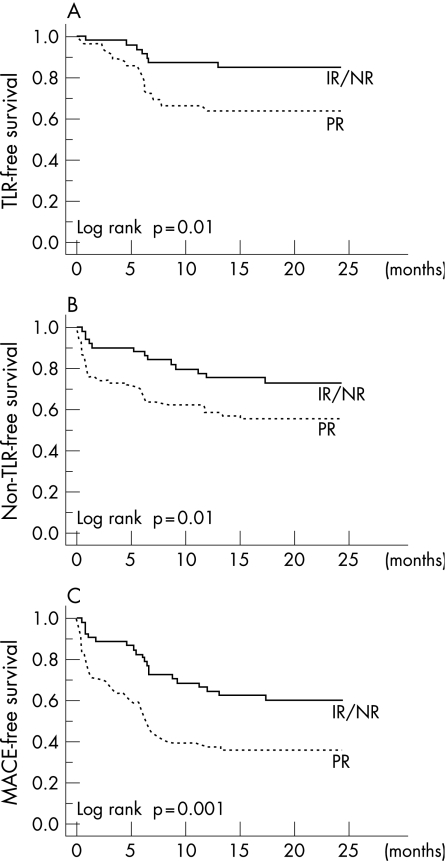
Table 4 summarises the long‐term clinical follow‐up data. Incidence of death and myocardial infarction tended to be higher in patients with PR than in those with IR/NR during 2 years after the index procedure, although the differences did not reach statistical significance. During follow‐up, TLR rate was significantly higher in patients with PR than in those with IR/NR (p = 0.01). The Kaplan–Meier plot of TLR‐free survival rate was significantly lower in patients with PR than in those with IR/NR (log rank, p = 0.02; fig 1A).
Table 4 Clinical events at 2 years.
| All (n = 134) | AMI (n = 103) | |||||||
|---|---|---|---|---|---|---|---|---|
| PR (n = 83) | IR/NR (n = 51) | p Value | PR (n = 68) | IR/NR (n = 35) | p Value | |||
| Death, n (%) | 4 (4.8) | 1 (2.0) | 0.4 | 4 (3.9) | 1 (2.9) | 0.5 | ||
| MI, n (%) | 3 (3.6) | 0 (0) | 0.17 | 2 (2.9) | 0 (0) | 0.3 | ||
| TLR, n (%) | 28 (33.7) | 7 (13.7) | 0.01 | 22 (32.4) | 5 (14.3) | 0.048 | ||
| PCI, n (%) | 26 (92.9) | 6 (85.7) | 20 (90.9) | 4 (80.0) | ||||
| CABG, n (%) | 2 (7.1) | 1 (14.3) | 2 (9.1) | 1 (20.0) | ||||
| Non‐TLR, n (%) | 35 (42.2) | 12 (23.5) | 0.03 | 27 (39.7) | 8 (22.9) | 0.09 | ||
| PCI, n (%) | 33 (94.3) | 11 (91.6) | 25 (92.6) | 7 (87.5) | ||||
| CABG, n (%) | 2 (5.7) | 1 (8.4) | 2 (6.4) | 1 (12.5) | ||||
| Cardiac events, n (%) | 51 (61.4) | 18 (35.3) | 0.003 | 43 (63.2) | 13 (37.1) | 0.01 | ||
AMI, acute myocardial infarction; CABG, coronary artery bypass graft; IR, intermediate remodelling; NR, negative remodelling; PCI, percutaneous coronary intervention; PR, positive remodelling; TLR, target lesion revascularization.
Figure 1 (A) Target lesion revascularisation (TLR)‐free survival. The Kaplan–Meier curve showed that TLR‐free survival was significantly lower in patients with positive remodelling (PR). (B) Non‐TLR‐free survival. The Kaplan–Meier curve demonstrated a significantly lower non‐TLR‐free survival rate in patients with PR. (C) Cardiac event‐free survival. The Kaplan–Meier curve showed that cardiac event‐free survival was significantly lower in patients with PR. IR, intermediate remodelling; MACE, major adverse cardiac event; NR, negative remodelling.
In addition, non‐TLR rate was significantly higher in patients with PR than in those with IR/NR (p = 0.03). The Kaplan–Meier plot of non‐TLR‐free survival rate was significantly lower in patients with PR than in those with IR/NR (log‐rank, p = 0.02; fig 1B).
As a result, the rate of cardiac events was significantly higher in patients with PR than in those with IR/NR (p = 0.003). The Kaplan–Meier plot of cardiac event‐free survival rate was significantly lower in patients with PR than in those with IR/NR (log‐rank, p = 0.02; fig 1C).
Univariate predictors (p <0.15) of TLR were PR (p = 0.01) and post‐intervention minimal stent area (p = 0.01). Multivariate logistic regression analysis, yielded PR (χ2 6.29, odds ratio (OR) 3.77; 95% CI 1.34 to 10.63; p = 0.01) and post‐intervention minimal stent area (χ2 6.33, OR 0.76; 95% CI 0.62 to 0.94; p = 0.01) as the independent predictors of TLR.
Univariate predictors (p value <0.15) of cardiac events were PR (p = 0.007), diabetes mellitus (p = 0.04), plaque rupture (p = 0.12) and hypertension (p = 0.14). Multivariate logistic regression analysis yielded PR (χ2 6.59, OR 2.70; 95% CI 1.27 to 5.78; p = 0.01) and plaque rupture (χ2 4.17, OR 2.38; 95% CI 1.04 to 5.45; p = 0.04; table 5) as the independent predictors of cardiac events.
Table 5 Multivariate predictors of cardiac events (death, myocardial infarction, target lesion revascularisation (TLR) or non‐TLR).
| χ2 | p Value | OR (95% CI) | |
|---|---|---|---|
| Positive remodelling | 6.58 | 0.01 | 2.70 (1.27 to 5.78) |
| Plaque rupture | 4.17 | 0.04 | 2.38 (1.04 to 5.45) |
| Hypertension | 2.87 | 0.09 | 1.89 (0.91 to 3.96) |
| Diabetes mellitus | 2.82 | 0.09 | 1.91 (0.90 to 4.04) |
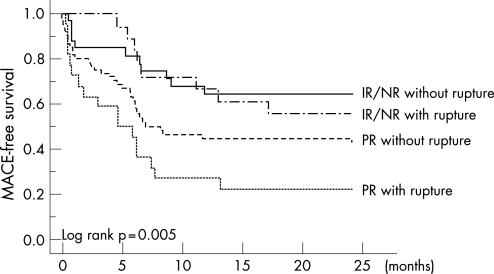
Accordingly, each group was further divided into two subgroups based on the presence or absence of the plaque rupture. Kaplan–Meier plot of cardiac event‐free survival curve demonstrated that PR with plaque rupture was the highest risk group among the four groups (log‐rank, p = 0.005; fig 2).
Figure 2 Kaplan–Meier curves comparing the four subgroups. Cardiac event‐free survival curve showed that patients with both positive remodelling (PR) and plaque rupture were associated with the worst clinical outcome. IR, intermediate remodelling; MACE, major adverse cardiac event; PR, positive remodelling.
Results from patients with AMI alone are also summarised in tables 1–4. In patients with AMI alone, the only independent predictor of the cardiac events (death, myocardial infarction, TLR and non‐TLR) was PR (χ2 3.95, OR 3.19; 95% CI 1.01 to 9.98; p = 0.047). Kaplan–Meier plot of cardiac event‐free survival rate was significantly lower in patients with PR than in those with IR/NR (log‐rank, p = 0.01).
IVUS results at follow‐up
Serial (post and 6 months follow‐up) IVUS imaging of the target lesion was available in 23 patients (16 patients with PR and 7 patients with IR/NR). Changes in in‐stent neointimal area were significantly larger in patients with PR than in those with IR/NR (+5.0 (2.2) vs +3.1 (1.7) mm2, p = 0.03). On the other hand, changes in peri‐stent P+M CSA did not differ between the groups (−0.0 (4.5) vs 1.0 (2.5) mm2, p = NS), suggesting that exaggerated in‐stent neointimal proliferation rather than a lack of peri‐stent remodelling may be related to the higher incidence of in‐stent restenosis.
IVUS imaging was performed in 19 of 44 (43%) lesions that required PCI during follow‐up. EEM, P+M and lumen CSAs of these non‐TLR lesions were 11.7 (5.2), 9.5 (5.1) and 2.2 (0.8) mm2, respectively. PR was present in 11 of 19 (57%) lesions and IR/NR in 8 of 19 (43%) lesions.
Discussion
This study shows that PR and the presence of plaque rupture are predictors of poor clinical outcome including both higher TLR and non‐TLR rates after bare metal stent implantation in patients with ACS.
Remodelling and TLR
Several IVUS studies have suggested that lesion characteristics may be related to clinical presentation—that is, culprit lesions for ACS have a higher incidence of positive arterial remodelling compared with those for stable angina pectoris.1,2,3 In addition, patterns of lesion remodelling before intervention have been shown to affect long‐term clinical outcome after non‐stent intervention4 as well as stenting5,6 in patients with angina pectoris. Our present result, showing that PR is related to unfavourable clinical outcome following PCI, is in concordance with previous studies on patients with angina pectoris.4,5,6,9 Although one study has shown the possible relationship between pre‐intervention remodelling and recurrence after thrombolysis in patients with AMI,10 our results further suggest that, even after stenting, patterns of remodelling have an impact on prognosis in patients with ACS.
The mechanism of higher incidence of clinical restenosis observed in patients with PR may be explained by increased neointimal hyperplasia. This exaggerated neointimal response seen in the PR group may be explained by the fact that lesions with PR may be biologically active, and therefore may have increased response to mechanical stimuli caused by metallic stents. Recent volumetric IVUS studies have shown that adaptive vessel dilatation of the stented segment following stenting (ie, peri‐stent remodelling) may be related to lesser degree of neointimal response.11 Lack of this adaptive vessel remodelling, as was shown in lesions with PR after plain old balloon angioplasty,12 may also play a role in an increased amount of intrastent intimal hyperplasia in lesions with PR.
Recently, a drug eluting stent technology was developed and has clinically become available. Clinical trials have suggested that drug‐eluting stent may almost eliminate in‐stent neointimal hyperplasia.13,14 Although drug‐eluting stent may be promising, there may still be some impact of pre‐intervention lesion remodelling on in‐stent neointimal hyperplasia after drug eluting stent implantation.15 Whether use of drug‐eluting stent eliminates the impact on remodelling needs further investigations.
Remodelling and non‐TLR
A higher incidence of non‐target lesion revascularisation in the culprit lesion in patients with PR was documented in our present study. This is partly in concordance with a previous report on stable angina pectoris.5 As expected, the major difference between our study and the previous study was the incidence of PR (62% in our study vs 29% in the previous study) as a result of difference in clinical presentation (ACS vs stable angina). In addition, our study extended the clinical follow‐up period to 2 years (vs 7.7 months in the previous study). Despite the higher‐risk population and extended clinical follow‐up period, incidence of death and myocardial infarction were quite similar to those in the previous study. The relatively higher incidence of target lesion restenosis among patients with IR (21.8%) or NR (23.4%) in the previous study as compared with the present study may be partly associated with the infrequent use of stent (100% in our present study and 54.1% in the previous study). Conversely, incidence of non‐TLR in the present study was higher than that in the previous report. This may be attributed to the higher‐risk population (ACS vs stable angina) and the longer follow‐up period in our present study. Indeed, longer‐term (3–5 years) follow‐up results after stenting showed the comparable incidence (21%) of non‐TLR.16 Recently, an analysis of the National Heart, Lung, and Blood Institute's Dynamic Registry has demonstrated that 5.8% of patients with PCI required non‐TLR during 1 year.17 Another analysis of large numbers of patients from the second‐generation stent clinical trials with 5 years clinical follow‐up has shown that, during the first year after bare metal stent implantation in a low‐risk clinical trial population, the hazard rate was higher (18.3%) for target lesion events than for events unrelated to the target lesion (12.4%).18 However, after the first year, the average annual hazard rate of non‐target lesion events was significantly higher (6.3%) than that of target lesion events (1.7%). Both of these studies have shown that diabetes was the independent clinical predictor for non‐TLR.17,18 Diabetes was one of the univariate predictors for cardiac events in our present study, which is quite in concordance with these larger registries. However, diabetes was found to be a borderline predictor for cardiac events by multivariate analysis. This may be a result of the smaller sample size of our study population.
Interestingly, IVUS analysis of the culprit lesions for non‐TLR demonstrated the similar incidence of PR (57% of the non‐TLR lesion vs 62% of the original target lesion) in the original target lesion. This may suggest that PR is a pan‐coronary arterial response rather than lesion‐specific response.
Plaque rupture and clinical outcome
Recent IVUS studies have shown multiple plaque rupture to be the consequence of vulnerability of the pan‐coronary artery inflammation (vulnerable patients).19,20,21 In addition, presence of multiple plaque rupture has been reported as a poor prognostic indicator.20 Although IVUS was not performed in all three vessels at the time of the initial presentation, vulnerable or biologically active plaque might have developed throughout the entire coronary trees in patients with ACS. Recently, a relationship between systemic inflammation and coronary atherosclerosis progression has been reported.22 In addition, several trials have addressed the possible role of high‐sensitivity C reactive protein and low‐density lipoprotein‐cholesterol as independent markers of atherosclerosis progression.23,24 Although, high‐sensitivity C reactive protein did not differ between the PR and IR/NR groups in our present study, systemic and/or local inflammatory process may play a role in the disease progression.
Interestingly, incidence of plaque rupture defined by IVUS varies among reports from 15.8% to 66%.8,25,26 Our result (24%) was quite within an acceptable range compared with these previous reports. As discussed by Kotani et al,8 incidence of the plaque rupture may be influenced by the time interval between the onset of symptom and IVUS imaging. In our current study, IVUS imaging was performed within 12 h from the onset of symptom. Coverage of the ruptured cavity by overlying thrombi may obscure the presence of plaque rupture, and might have contributed to the relatively lower incidence compared with some of the previous IVUS studies. Pathological studies have shown that plaque rupture was reported in 60–70% of the culprit lesion in ACS.27,28
Limitations
There are several possible limitations in this study. First, this is a single‐centre, retrospective analysis with relatively small numbers of patients. Therefore, these results need to be confirmed by a study of larger numbers of patients. Second, lesions without pre‐intervention IVUS imaging or heavily calcified lesions were excluded from this analysis. This exclusion might have affected the results. Third, several different definitions have been used and reported to classify patterns of arterial remodelling. Difference in the definition of remodelling might have affected the results.29 However, if we used, for example, another classification of remodelling that define PR as target lesion EEM CSA/proximal reference EEM CSA >1, and NR otherwise, the results would have been quite similar to those of our original analysis—that is, cardiac event‐free survival in the PR group would have been significantly lower than that in the NR group (log rank, p = 0.03, figure not shown). Fourth, in our series, only 25% of the patients were treated with statins, which is a relatively lower percentage than that in our current clinical practice (>50% of the patients with ACS are usually treated with statins). Therefore, this may have affected the clinical results. Fifth, both TLR and non‐TLR were performed frequently around 6 months after the index procedure. This may indicate the fact that routine angiographical as well as scintigraphical follow‐up were performed in our institution. However, intervention was based on symptom and/or myocardial ischaemia detected by Tl scintigraphy when angiographical restenosis was documented.
Finally, the impact of pre‐intervention remodelling on long‐term clinical follow‐up after drug‐eluting stent implantation needs to be investigated further.
Clinical implications
Our results suggest that patients with PR may require intensive risk factor modification to reduce target lesion recurrence and also non‐target lesion progression.
Abbreviations
ACS - acute coronary syndrome
AMI - acute myocardial infarction
CSA - cross‐sectional area
%DS - percent diameter stenosis
EEM - external elastic membrane
IR - intermediate remodelling
IVUS - intravascular ultrasound
MLD - minimal lumen diameter
MSA - minimal stent area
NR - negative remodelling
P+M - plaque plus media
PCI - percutaneous coronary intervention
PR - positive remodelling
TLR - target lesion revascularisation
Footnotes
Competing interests: None declared.
References
- 1.Kaji S, Akasaka T, Hozumi T.et al Compensatory enlargement of the coronary artery in acute myocardial infarction. Am J Cardiol 2000851139–1141. [DOI] [PubMed] [Google Scholar]
- 2.Schoenhagen P, Ziada K M, Kapadia S R.et al Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: an intravascular ultrasound study. Circulation 2000101598–603. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura M, Nishikawa H, Mukai S.et al Impact of coronary artery remodeling on clinical presentation of coronary artery disease: an intravascular ultrasound study. J Am Coll Cardiol 20013763–69. [DOI] [PubMed] [Google Scholar]
- 4.Dangas G, Mintz G S, Mehran R.et al Preintervention arterial remodeling as an independent predictor of target‐lesion revascularization after nonstent coronary intervention: an analysis of 777 lesions with intravascular ultrasound imaging. Circulation 1999993149–3154. [DOI] [PubMed] [Google Scholar]
- 5.Wexberg P, Gyongyosi M, Sperker W.et al Pre‐existing arterial remodeling is associated with in‐hospital and late adverse cardiac events after coronary interventions in patients with stable angina pectoris. J Am Coll Cardiol 2000361860–1869. [DOI] [PubMed] [Google Scholar]
- 6.Okura H, Morino Y, Oshima A.et al Preintervention arterial remodeling affects clinical outcome following stenting: an intravascular ultrasound study. J Am Coll Cardiol 2001371031–1035. [DOI] [PubMed] [Google Scholar]
- 7.Mintz G S, Nissen S E, Anderson W D.et al American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2001371478–1492. [DOI] [PubMed] [Google Scholar]
- 8.Kotani J, Mintz G S, Castagna M T.et al Intravascular ultrasound analysis of infarct‐related and non‐infarct‐related arteries in patients who presented with an acute myocardial infarction. Circulation 20031072889–2893. [DOI] [PubMed] [Google Scholar]
- 9.Gyongyosi M, Yang P, Hassan A.et al Intravascular ultrasound predictors of major adverse cardiac events in patients with unstable angina. Clin Cardiol 200023507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gyongyosi M, Wexberg P, Kiss K.et al Adaptive remodeling of the infarct‐related artery is associated with recurrent ischemic events after thrombolysis in acute myocardial infarction. Coron Artery Dis 200112167–172. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura M, Yock P G, Bonneau H N.et al Impact of peri‐stent remodeling on restenosis: a volumetric intravascular ultrasound study. Circulation 20011032130–2132. [DOI] [PubMed] [Google Scholar]
- 12.Okura H, Hayase M, Shimodozono S.et al Impact of pre‐interventional arterial remodeling on subsequent vessel behavior after balloon angioplasty: a serial intravascular ultrasound study. J Am Coll Cardiol 2001382001–2005. [DOI] [PubMed] [Google Scholar]
- 13.Morice M C, Serruys P W, Sousa J E.et al A randomized comparison of a sirolimus‐eluting stent with a standard stent for coronary revascularization. N Engl J Med 20023461773–1780. [DOI] [PubMed] [Google Scholar]
- 14.Moses J W, Leon M B, Popma J J.et al Sirolimus‐eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 20033491315–1323. [DOI] [PubMed] [Google Scholar]
- 15.Mintz G S, Tinana A, Hong M K.et al Impact of preinterventional arterial remodeling on neointimal hyperplasia after implantation of (non‐polymer‐encapsulated) paclitaxel‐coated stents: a serial volumetric intravascular ultrasound analysis from the ASian Paclitaxel‐Eluting Stent Clinical Trial (ASPECT). Circulation 20031081295–1298. [DOI] [PubMed] [Google Scholar]
- 16.Sonmez K, Turan F, Gencbay M.et al Long‐term (3–5 years) clinical and angiographic follow‐up results of coronary stenting in elderly patients. Circ J 2002661029–1033. [DOI] [PubMed] [Google Scholar]
- 17.Glaser R, Selzer F, Faxon D P.et al Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation 2005111143–149. [DOI] [PubMed] [Google Scholar]
- 18.Cutlip D E, Chhabra A G, Baim D S.et al Beyond restenosis: five‐year clinical outcomes from second‐generation coronary stent trials. Circulation 20041101226–1230. [DOI] [PubMed] [Google Scholar]
- 19.Rioufol G, Finet G, Ginon I.et al Multiple atherosclerotic plaque rupture in acute coronary syndrome: a three‐vessel intravascular ultrasound study. Circulation 2002106804–808. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka A, Shimada K, Sano T.et al Multiple plaque rupture and C‐reactive protein in acute myocardial infarction. J Am Coll Cardiol 2005451594–1599. [DOI] [PubMed] [Google Scholar]
- 21.Okura H, Kobayashi Y, Sumitsuji S.et al Baseline and follow‐up three vessel intravascular ultrasound evaluation of plaque rupture in acute coronary syndrome. Circulation 2005112II769 [Google Scholar]
- 22.Zouridakis E, Avanzas P, Arroyo‐Espliguero R.et al Markers of inflammation and rapid coronary artery disease progression in patients with stable angina pectoris. Circulation 20041101747–1753. [DOI] [PubMed] [Google Scholar]
- 23.Nissen S E, Tuzcu E M, Schoenhagen P.et al Statin therapy, LDL cholesterol, C‐reactive protein, and coronary artery disease. N Engl J Med 200535229–38. [DOI] [PubMed] [Google Scholar]
- 24.Ridker P M, Cannon C P, Morrow D.et al C‐reactive protein levels and outcomes after statin therapy. N Engl J Med 200535220–28. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka A, Kawarabayashi T, Nishibori Y.et al No‐reflow phenomenon and lesion morphology in patients with acute myocardial infarction. Circulation 20021052148–2152. [DOI] [PubMed] [Google Scholar]
- 26.Hong M K, Mintz G S, Lee C W.et al Comparison of coronary plaque rupture between stable angina and acute myocardial infarction: a three‐vessel intravascular ultrasound study in 235 patients. Circulation 2004110928–933. [DOI] [PubMed] [Google Scholar]
- 27.van der Wal A C, Becker A E, van der Loos C M.et al Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 19948936–44. [DOI] [PubMed] [Google Scholar]
- 28.Virmani R, Burke A P, Farb A.et al Pathology of the unstable plaque. Prog Cardiovasc Dis 200244349–356. [DOI] [PubMed] [Google Scholar]
- 29.Hibi K, Ward M R, Honda Y.et al Impact of different definitions on the interpretation of coronary remodeling determined by intravascular ultrasound. Catheter Cardiovasc Interv 200565233–239. [DOI] [PubMed] [Google Scholar]