In recent years, a new cardiac syndrome with transient left ventricular dysfunction has been described in Japanese patients. This new entity has been referred to as “tako‐tsubo cardiomyopathy” or “apical ballooning”, named for the particular shape of the end‐systolic left ventricle in ventriculography.1 To date, tako‐tsubo cardiomyopathy has also been reported to occur in the western population. The following clinical characteristics of this phenomenon must be met: (1) transient akinesis or dyskinesis of left ventricular wall motion abnormalities (ballooning) with chest pain; (2) new electrocardiographic changes (either ST elevation or T wave inversion); (3) no significant obstructive epicardial coronary artery disease; (4) absence of recent significant head trauma, intracardial bleeding, phaeochromocytoma, myocarditis, and hypertrophic cardiomyopathy.2
Emotional or physical stress usually precedes this cardiomyopathy. A unifying mechanistic explanation responsible for this acute but rapidly reversible contractile dysfunction is still lacking. Multivessel epicardial coronary artery vasospasm, coronary microvascular dysfunction or spasm, impaired fatty acid metabolism, transient obstruction of the left ventricular outflow, and catecholamine‐mediated myocardial dysfunction have been proposed as potential mechanisms.3,4,5,6 The optimal treatment of patients presenting with this syndrome depends primarily on the haemodynamic conditions and remains rather symptomatic in nature.
This article primarily addresses the clinical setting of tako‐tsubo cardiomyopathy and describes a broad spectrum of diagnostic tools. Moreover, currently proposed pathophysiological mechanisms are discussed in detail, providing more insight into this new cardiac entity.
Definition
Tako‐tsubo cardiomyopathy is characterised by acute onset of chest pain and a completely reversible regional contractile dysfunction. In left ventriculography typical wall motion abnormalities, such as apical and mid‐ventricular akinesia and a hypercontractile basis, can be documented. Coronary angiography reveals no relevant epicardial coronary artery disease (fig 1). Recently, several cases of transient ballooning involving the mid‐ventricular part, sparing the apical and basal segments, have also been documented.7 Tako‐tsubo cardiomyopathy mimics symptoms of acute myocardial infarction with acute chest pain, ECG changes, and a slight increase in specific cardiac biomarkers. In 1991 Sato and Dote first described this transient contractile dysfunction as tako‐tsubo cardiomyopathy, named after the bottle with a round bottom and a narrow neck used for trapping octopus.1 Other groups called the syndrome apical “ballooning”, “broken heart”, “scared to death”, “ampulla‐syndrome”, or “acute stress cardiomyopathy”.4 Owing to its clinical and imaging characteristics, this syndrome is frequently misdiagnosed as an acute coronary syndrome related to obstructive coronary artery disease (fig 2).
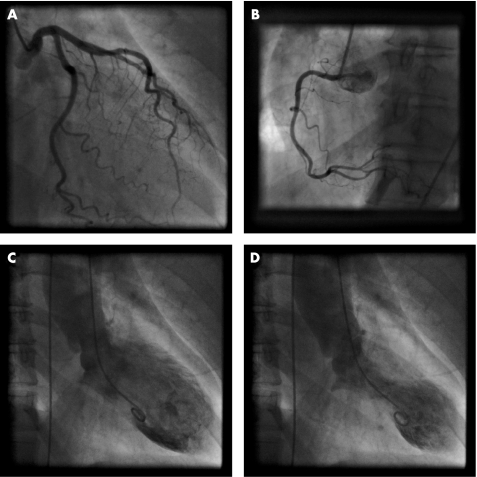
Figure 1 Selective coronary angiography. Left (A) and right (B) coronary arteries in a patient presenting with tako‐tsubo cardiomyopathy, excluding coronary artery disease. Left ventriculography during diastole (C) and systole (D) demonstrate the typical left ventricular apical ballooning and a hypercontractile base.
Figure 2 Stepwise approach to the diagnosis and management of patients with tako‐tsubo cardiomyopathy. AICD, automatic implantable cardioverter‐defibrillator; BNP, B‐type natriuretic peptide; CK, creatine kinase.
Aetiology
The cause of tako‐tsubo cardiomyopathy is unknown. However, all available evidence is consistent with the concept that this disease results from extreme emotional (33–45%) and/or physical stress (17–22%). There is a strong predominance of postmenopausal women. The seemingly increased susceptibility of women to stress‐related left ventricular dysfunction and potential gender‐related differences in response to catecholamines is not well understood.6 However, sex hormones exert important influences on the sympathetic neurohumoral axis as well as on coronary vasoreactivity.
In one patient, Kushiro et al demonstrated a type I CD36 deficiency, which is involved with many cardiovascular diseases and metabolic abnormalities. Further correlations between this cardiomyopathy and specific genetic profiles are unknown so far.
Epidemiology
Tako‐tsubo cardiomyopathy is a still rarely diagnosed cardiac syndrome. Over the last few years, however, the number of published reports of patients presenting with this syndrome has steadily increased. Several investigations assessed the prevalence of tako‐tsubo cardiomyopathy. Serial case studies coming from Japan revealed a prevalence of 1.2–2.0% among patients with acute coronary syndrome.8,9 In a recent US study, tako‐tsubo cardiomyopathy was diagnosed in about 2.2% of patients referred to hospital with suspected acute coronary syndrome. A German series reported an incidence of 0.1–2.3%, and a study from France investigating this syndrome in a large urban conurbation showed a prevalence of 0.9%. The incidence of tako‐tsubo cardiomyopathy in an Italian investigation was 2%.
Clinical findings
Tako‐tsubo cardiomyopathy is characterised by acute onset of chest pain, dyspnoea and sometimes syncope. In the acute stage respiratory insufficiency due to pulmonary oedema, cardiogenic shock and fatal arrhythmias can be observed.2,10 Moreover, in one case lethal rupture of the left ventricular wall was associated with tako‐tsubo cardiomyopathy 3 days after presenting with the syndrome, and in two patients left ventricular mural thrombus formation was reported.
Diagnostic findings
Electrocardiography
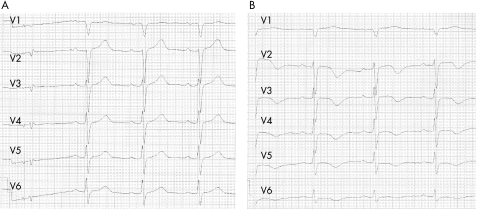
ST elevation (<2 mm) or T wave inversion in the anterior leads (V1–V6) have been the most commonly recorded findings (fig 3). In comparison to patients with anterior infarct, these ST elevations are less prominent. However, electrocardiography does not have a predictive value allowing reliable emergency differentiation of these entities. ECG changes may be present for several hours followed by normalisation and development of T wave inversion. Furthermore, in several cases transient prolongation of the QT interval was observed with a consecutive normalisation within some weeks.3 Even though QT interval prolongation is present in tako‐tsubo cardiomyopathy, rate adaptation of ventricular repolarisation is not significantly altered in comparison to acute ST elevation myocardial infarction, suggesting a different effect of autonomic nervous activity on the ventricular myocardium.11
Figure 3 ECGs of a patient presenting with tako‐tsubo cardiomyopathy showing ST elevations in the anterior leads in the acute phase (A) and changes in T waves and QT interval after 5 days (B).
Biochemistry
Serum values of myocardial creatine kinase (CK), CK‐MB, and troponin are often normal or only slightly elevated. Akashi et al first documented increased concentrations of B‐type natriuretic peptide (BNP) in patients presenting with tako‐tsubo cardiomyopathy.12 Recently, serum concentrations of the N‐terminal fragment of BNP (NT‐proBNP) were shown to be a valuable marker for assessment of myocardial deterioration and recovery. Moreover, low NT‐proBNP values on admission were shown to be a reliable indicator of a rather favourable prognosis for patients presenting with tako‐tsubo cardiomyopathy.
Echocardiography
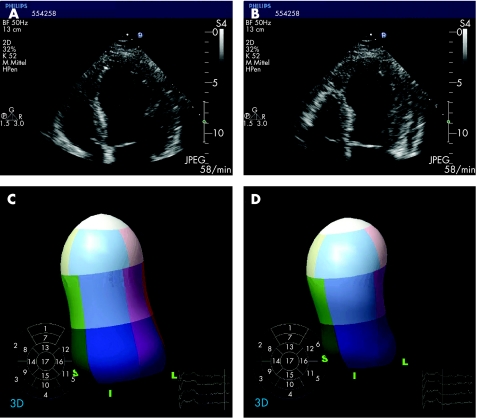
In the apical four‐chamber view, a typical akinesia of the left ventricular apex and/or the mid‐portions of the left ventricle as well as a hypercontractile base are consistently found (fig 4). Interestingly, the wall motion abnormalities exceed the area assigned to one coronary vessel. In a few cases, left ventricular outflow tract (LVOT) obstruction with an end‐systolic pressure gradient up to 60 mm Hg was observed.4 After normalisation of myocardial function the pressure gradient disappeared. These findings of a mid‐cavity dynamic obstruction in the acute phase of tako‐tsubo cardiomyopathy were correlated with a localised mid‐ventricular septal thickening when cardiac function returned to normal.
Figure 4 Transthoracic echocardiogram showing four‐chamber views during diastole (A) and systole (B) in a patient presenting with tako‐tsubo cardiomyopathy. Real time three‐dimensional echocardiography shows the typical contractile pattern of tako‐tsubo cardiomyopathy with akinesia of apical segments and hypercontractility of the basal segments (diastole, C; systole, D).
Coronary angiography and ventriculography
In all reported cases coronary angiography could exclude relevant coronary artery obstruction in patients presenting with tako‐tsubo cardiomyopathy (fig 1A, B). In a small case series involving five patients investigated with intravascular ultrasound, the finding of a single disrupted atherosclerotic plaque in the middle portion of the left anterior descending coronary artery was described. Ventriculography usually displays typical apical ballooning and a hypercontraction of the basal segments (fig 1C, D). In some cases, mid‐ventricular ballooning sparing the basal and apical segments can be present.7
Cardiovascular magnetic resonance imaging
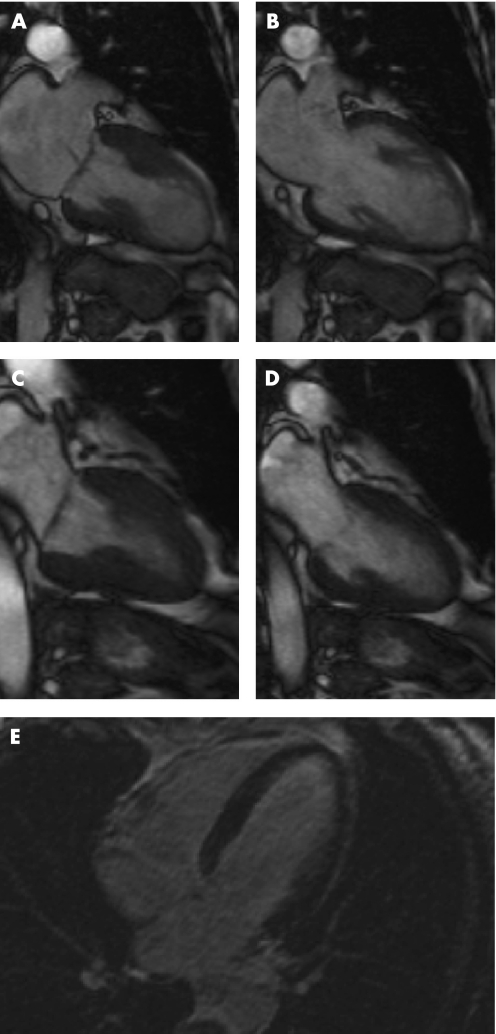
Cardiovascular magnetic resonance (CMR) imaging provides morphologic and precise functional information of left ventricular function (fig 5). More recently published data also documented regional wall motion abnormalities of the right ventricle in the acute phase of this syndrome.13 Sporadically, focal signal increase in different segments was detectable in the T2‐weighted turbo‐spin echo sequences, indicating myocardial oedema. First‐pass perfusion imaging does not show any evidence of focal perfusion abnormalities, corresponding to a specific vascular territory. Most commonly, in late enhancement imaging no pathological signal activity can be documented excluding myocardial infarction or inflammatory processes. So far only three cases of delayed hyperenhancement have been reported. In all cases the observed endocardial delayed hyperenhancement was small in comparison to the extent of the wall motion abnormalities. In view of the fact that in myocarditis areas of hyperenhancement originate from the epicardium, late enhancement sequences can assist in the differential diagnosis of tako‐tsubo cardiomyopathy.
Figure 5 Cine sequences of cardiac magnetic resonance imaging during systole (A) and diastole (B) in the acute phase. Normal function could be documented after 3 weeks (systole, C; diastole, D). Late enhancement does not show any increased signal intensity (E).
Myocardial single photon emission computed tomography (SPECT)
Several reports describe thallium‐201 (201Th) perfusion patterns in patients with tako‐tsubo cardiomyopathy. In many cases a perfusion defect in the apical region has been detected in the acute phase despite normal coronary arteries. These defects decrease with the clinical course of recovery. Some cases reported a diminished accumulation of iodine‐123 (123I) metaiodobenzylguanidine (MIBG) in the hypokinetic region. Kurisu et al found impaired myocardial fatty acid metabolism rather than a disturbed myocardial perfusion during the early phase.14 Ito et al performed technetium‐99 m (99mTc)‐tetrofosmin myocardial SPECT and showed that myocardial perfusion in the apical region was impaired immediately after hospitalisation but mostly recovered after 3–5 days.9
Myocardial biopsies
To date, several groups have investigated endomyocardial biopsies from both the right and left ventricle, revealing myocyte injury and a slight increase in connective tissue. From a systematic analysis it is known that tako‐tsubo cardiomyopathy is accompanied by severe morphological alterations, with many vacuoles of different size contributing to cellular deteriorations. Moreover, the specific arrangement of contractile (actin, myosin) and cytoskeletal (α‐actinin, titin, dystrophin) proteins is dissolved. The content of the contractile material is reduced and detected in the border area of the cells. Contraction bands are sporadically present. Clusters of mitochondria with abnormalities in size and shape and areas of non‐specified cytoplasm can be observed. The nuclei typically appear rounded or oval either in the middle or in the border area of the cells. Cell swelling associated with damage to the basal lamina or damaged mitochondria with flocculent densities are typical signs of oncotic cell death and are absent. Additionally, apoptotic and autophagic cell death can be excluded by electron microscopy and immunohistochemistry. Moreover, the interstitial space is widened and contains fibrotic material, including collagen fibrils, formations of cell debris, macrophages, and an increased number of fibroblasts. Most noteworthy, these alterations are transient and almost completely reversible after functional recovery.15
Pathophysiological mechanism of tako‐tsubo cardiomyopathy
Several pathophysiological concepts have been proposed for tako‐tsubo cardiomyopathy. However, the underlying mechanism remains unclear.
Concept of epimyocardial vasospasm
In all documented patients presenting with tako‐tsubo cardiomyopathy, relevant coronary artery obstruction could be excluded. Thus, epimyocardial spasms were proposed to be responsible for inducing ischaemia. Arguing against this hypothesis is the fact that the region of wall motion abnormalities does not correspond to the perfusion territory of a single coronary artery. Many studies have evaluated the presence of either spontaneous or provoked multivessel epicardial spasm during angiography. In a systematic review Gianni et al found that only a few patients experienced spontaneous multivessel epicardial spasm (1.4%). Using provocative tests (infusion of ergonovine or acetylcholine) 28% experienced multivessel spasm. However, results varied widely in different series. Taken together, epicardial vasospasm seems to be unlikely mechanism.10
Concept of microcirculation disturbance
Sadamatsu et al were the first to report that patients with tako‐tsubo cardiomyopathy have an impaired coronary microcirculation.16 They could document a diminished coronary flow reserve (CFR) using a Doppler guide wire. These results were confirmed by other groups suggesting that microvascular dysfunction contributes substantially to the development of this syndrome. Recently, a reduced coronary flow velocity in the absence of relevant coronary artery stenosis was also observed as measured by the TIMI frame count method immediately after onset of tako‐tsubo cardiomyopathy. Additionally, myocardial contrast echocardiographic studies revealed perfusion defects in the apex returning to a homogenous signal after a follow up of 4 weeks, suggesting that microvascular dysfunction might be responsible for the reversible contractile impairment.5 However, it is unclear whether coronary microvascular dysfunction is the primary mechanism involved in the pathogenesis of the syndrome or whether it is simply an associated secondary phenomenon. Furthermore, the underlying cause of the potential microvascular dysfunction is unknown.
Concept of catecholamine‐triggered myocyte injury
The most widely proposed hypothesis relates to the role of stress in patients with tako‐tsubo cardiomyopathy. In the majority of cases, triggering conditions that preceded onset were said to involve exposure to endogenous (emotional) or exogenous stresses (trauma, surgical procedure, exacerbation of a pre‐existing condition). This suggests that increased sympathetic activity plays a major role in the origin of this syndrome. Akashi et al first described notably elevated norepinephrine (noradrenaline) concentrations in patients with tako‐tsubo cardiomyopathy.17 This was confirmed by others showing significantly increased catecholamine concentrations in comparison to patients with Killip class III myocardial infarctions. Increased serum concentrations of catecholamines have been shown to generate direct myocyte injury. Oxidation of catecholamines results in the formation of highly toxic substances and free radicals causing intracellular calcium overload and myocardial cell damage. The typical histological signs of catecholamine toxicity, which are described as focal, mononuclear, inflammatory areas of fibrotic response and characteristic contraction bands, are also reported to be present in tako‐tsubo cardiomyopathy.6,15 Contraction bands have been reported in several clinical settings of extensive catecholamine production such as phaeochromocytoma or subarachnoid haemorrhage, showing that catecholamines may be an important link between emotional stress and cardiac injury.6
The distinctive contractile pattern may be explained by an enhanced responsiveness of apical myocardium to sympathetic stimulation. Alternatively, a base‐to‐apex gradient could result in regional differences in myocardial blood flow in the setting of catecholamine‐mediated epicardial or microvascular vasoconstriction.6
Interestingly, the wall motion abnormalities observed in tako‐tsubo cardiomyopathy are not the same as those found with subarachnoid haemorrhage or intracranial haemorrhage, in which only the basal segments of the left ventricle are affected.18
Concept of obstruction of left ventricular outflow tract
Villareal et al observed LVOT obstruction in three patients with tako‐tsubo cardiomyopathy.19 Other groups could confirm these pathologic findings, especially in women in the presence of abnormal myocardial functional architecture, such as localised mid‐ventricular septal thickening, suggesting an important factor in the development of this syndrome.4 They hypothesised that in the presence of increased concentrations of catecholamines caused by emotional stress, this mid‐ventricular septal thickening could lead to development of a severe transient left ventricular mid‐cavity obstruction, resulting in subendocardial ischaemia unrelated to a specific coronary artery territory. However, it remains unclear whether the observed intraventricular gradient is a consequence rather than a cause of tako‐tsubo cardiomyopathy.
Therapeutic options
There is no established treatment for patients with tako‐tsubo cardiomyopathy. However, these patients should be evaluated and treated initially in a manner similar to patients with acute myocardial infarction—that is, immediate invasive diagnostics. Complications, such as cardiogenic shock, pulmonary oedema or malignant arrhythmias, should be treated according to the usual management strategies. However, the overall prognosis of patients presenting with this syndrome is favourable; the reported in‐hospital mortality rates range from 0–8%.2
For those patients presenting in an unstable clinical condition, catecholamine administration is required (fig 2). Nevertheless, vasoactive agents should be used very carefully since they may further worsen the delicate situation. In cases of severe circulatory dysfunction, intra‐aortic balloon counterpulsation should be considered.
Tako‐tsubo cardiomyopathy: key points
Tako‐tsubo cardiomyopathy is characterised by transient regional contractile dysfunction with hypokinesia or akinesia of the left ventricular apical segments and hypercontractile basal segments; a mid‐ventricular ballooning has also been observed
Absence of obstructive coronary artery disease
Electrocardiographic changes mimicking acute myocardial infarction (ST segment elevation)
Mild elevation of cardiac biomarkers can be observed
Tako‐tsubo cardiomyopathy occurs more frequently in elderly women after psychologically or physically stressful events
Complications of tako‐tsubo cardiomyopathy, such as cardiogenic shock, pulmonary oedema or malignant arrhythmias, should be treated according to current management strategies
After functional recovery, β‐blocker treatment might be appropriate
In a stable clinical setting, administration of anxiolytic agents is preferred. Data from an animal model of tako‐tsubo cardiomyopathy suggest that its development seems to be diminished after α‐ and β‐blockade.20 Thus, β‐blockers should be given in the acute and chronic phases and may possibly help to prevent recurrences, which have been described as occurring in 2.7–8% of patients.10 In order to prevent acute left ventricular thrombus formation, which has been observed in patients presenting with tako‐tsubo cardiomyopathy, the administration of low molecular weight heparin is warranted. After restitution of contractile function, further anticoagulation with warfarin is not required.
In the event of life threatening arrhythmias (torsade de pointes tachycardia, ventricular fibrillation), the implantation of a cardioverter‐defibrillator has to be considered.
Conclusion
Tako‐tsubo cardiomyopathy is a newly described heart syndrome characterised by transient left ventricular dysfunction. Although data suggest that catecholamine overload plays a central role in developing stress‐mediated myocardial stunning, the main mechanisms underlying its pathogenesis awaits further research and elucidation.
Additional references appear on the Heart website— http://heart.bmj.com/supplemental
INTERACTIVE MULTIPLE CHOICE QUESTIONS
This Education in Heart article has an accompanying series of six EBAC accredited multiple choice questions (MCQs).
To access the questions, click on BMJ Learning: Take this module on BMJ Learning from the content box at the top right and bottom left of the online article. For more information please go to: http://heart.bmj.com/misc/education.dtl Please note: The MCQs are hosted on BMJ Learning—the best available learning website for medical professionals from the BMJ Group.
If prompted, subscribers must sign into Heart with their journal's username and password. All users must also complete a one‐time registration on BMJ Learning and subsequently log in (with a BMJ Learning username and password) on every visit.
Additional references appear on the Heart website—http://heart.bmj.com/supplemental
Supplementary Material
Footnotes
In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article. All authors declare that the answer to the questions on your competing interest form are all “No” and therefore have nothing to declare
Additional references appear on the Heart website—http://heart.bmj.com/supplemental
References
- 1.Dote K, Sato H, Tateishi H.et al Myocardial stunning due to simultaneous multivessel coronary spasm: a review of 5 cases. J Cardiol 199121203–214.First report of a new cardiac syndrome called “tako‐tsubo” cardiomyopathy. [PubMed] [Google Scholar]
- 2.Bybee K A, Kara T, Prasad A.et al Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST‐segment elevation myocardial infarction. Ann Intern Med 2004141858–865. [DOI] [PubMed] [Google Scholar]
- 3.Tsuchihashi K, Ueshima K, Uchida T.et al Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina pectoris‐myocardial infarction investigations in Japan. J Am Coll Cardiol 20013811–18.Early and fundamental investigation in a large study population regarding tako‐tsubo cardiomyopathy. [DOI] [PubMed] [Google Scholar]
- 4.Merli E, Sutcliffe S, Gori M.et al Tako‐tsubo cardiomyopathy: new insights into the possible underlying pathophysiology. Eur J Echocardiogr 2006753–61. [DOI] [PubMed] [Google Scholar]
- 5.Ako J, Takenaka K, Uno K.et al Reversible left ventricular systolic dysfunction—reversibility of coronary microvascular abnormality. Jpn Heart J 200142355–363. [DOI] [PubMed] [Google Scholar]
- 6.Wittstein I S, Thiemannv D R, Lima J A.et al Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005352539–548.Excellent study investigating catecholamine serum values in patients presenting with tako‐tsubo cardiomyopathy. [DOI] [PubMed] [Google Scholar]
- 7.Hurst R T, Askew J W, Reuss C S.et al Transient midventricular ballooning syndrome: a new variant. J Am Coll Cardiol 200648579–583. [DOI] [PubMed] [Google Scholar]
- 8.Stollberger C, Finsterer J, Schneider B. Tako‐tsubo‐like left ventricular dysfunction: clinical presentation, instrumental findings, additional cardiac and non‐cardiac diseases and potential pathomechanisms. Minerva Cardioangiol 200553139–145. [PubMed] [Google Scholar]
- 9.Ito K, Sugihara H, Kawasaki T.et al Assessment of ampulla (takotsubo) cardiomyopathy with coronary angiography, two‐dimensional echocardiography and 99mTc‐tetrofosmin myocardial single photon emission computed tomography. Ann Nucl Med 200115351–355. [DOI] [PubMed] [Google Scholar]
- 10.Gianni M, Dentali F, Grandi A M.et al Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J 2006272907–2908.Largest systematic review of tako‐tsubo cardiomyopathy including 286 patients. [DOI] [PubMed] [Google Scholar]
- 11.Bonnemeier H, Ortak J, Bode F.et al Modulation of ventricular repolarization in patients with transient left ventricular apical ballooning: a case control study. J Cardiovasc Electrophysiol 2006171340–1347. [DOI] [PubMed] [Google Scholar]
- 12.Akashi Y J, Nakazawa K, Sakakibara M.et al 123I‐MIBG myocardial scintigraphy in patients with “takotsubo” cardiomyopathy. J Nucl Med 2004451121–1127. [PubMed] [Google Scholar]
- 13.Haghi D, Athanasiadis A, Papavassiliu T.et al Right ventricular involvement in takotsubo cardiomyopathy. Eur Heart J 2006272433–2439.Large cardiac magnetic resonance investigation of tako‐tsubo cardiomyopathy with right ventricular involvement. [DOI] [PubMed] [Google Scholar]
- 14.Kurisu S, Inoue I, Kawagoe T.et al Myocardial perfusion and fatty acid metabolism in patients with tako‐tsubo‐like left ventricular dysfunction. J Am Coll Cardiol 200341743–748.Essential investigation with respect to myocardial perfusion in tako‐tsubo cardiomyopathy [DOI] [PubMed] [Google Scholar]
- 15.Nef H M, Möllmann H, Kostin S.et al Tako‐tsubo cardiomyopathy: intraindividual structural analysis in the acute phase and after functional recovery. Eur Heart J. (in press) First systematic structural analysis of endomyocardial biopsies from patients presenting with tako‐tsubo cardiomyopathy [DOI] [PubMed]
- 16.Sadamatsu K, Tashiro H, Maehira N.et al Coronary microvascular abnormality in the reversible systolic dysfunction observed after noncardiac disease. Jpn Circ J 200064789–792. [DOI] [PubMed] [Google Scholar]
- 17.Akashi Y J, Nakazawa K, Sakakibara M.et al The clinical features of takotsubo cardiomyopathy. QJM 200396563–573.Important serial case study of patients with tako‐tsubo cardiomyopathy in the clinical setting. [DOI] [PubMed] [Google Scholar]
- 18.Zaroff J G, Rordorf G A, Ogilvy C S.et al Regional patterns of left ventricular systolic dysfunction after subarachnoid hemorrhage: evidence for neurally mediated cardiac injury. J Am Soc Echocardiogr 200013774–779. [DOI] [PubMed] [Google Scholar]
- 19.Villareal R P, Achari A, Wilansky S.et al Anteroapical stunning and left ventricular outflow tract obstruction. Mayo Clin Proc 20017679–83. [DOI] [PubMed] [Google Scholar]
- 20.Ueyama T, Kasamatsu K, Hano T.et al Emotional stress induces transient left ventricular hypocontraction in the rat via activation of cardiac adrenoceptors: a possible animal model of ‘tako‐tsubo' cardiomyopathy. Circ J 200266712–713.First description of a potential animal model of tako‐tsubo cardiomyopathy [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.