Abstract
Background
Meta‐analyses of trials have shown greater benefits from angioplasty than thrombolysis after an acute myocardial infarction, but the time delay in initiating angioplasty needs to be considered.
Objective
To extend earlier meta‐analyses by considering 1‐ and 6‐month outcome data for both forms of reperfusion. To use Bayesian statistical methods to quantify the uncertainty associated with the estimated relationships.
Methods
A systematic review and meta‐analysis published in 2003 was updated. Data on key clinical outcomes and the difference between time‐to‐balloon and time‐to‐needle were independently extracted by two researchers. Bayesian statistical methods were used to synthesise evidence despite differences between reported follow‐up times and outcomes. Outcomes are presented as absolute probabilities of specific events and odds ratios (ORs; with 95% credible intervals (CrI)) as a function of the additional time delay associated with angioplasty.
Results
22 studies were included in the meta‐analysis, with 3760 and 3758 patients randomised to primary angioplasty and thrombolysis, respectively. The mean (SE) angioplasty‐related time delay (over and above time to thrombolysis) was 54.3 (2.2) minutes. For this delay, mean event probabilities were lower for primary angioplasty for all outcomes. Mortality within 1 month was 4.5% after angioplasty and 6.4% after thrombolysis (OR = 0.68 (95% CrI 0.46 to 1.01)). For non‐fatal reinfarction, OR = 0.32 (95% CrI 0.20 to 0.51); for non‐fatal stroke OR = 0.24 (95% CrI 0.11 to 0.50). For all outcomes, the benefit of angioplasty decreased with longer delay from initiation.
Conclusions
The benefit of primary angioplasty, over thrombolysis, depends on the former's additional time delay. For delays of 30–90 minutes, angioplasty is superior for 1‐month fatal and non‐fatal outcomes. For delays of around 90 minutes thrombolysis may be the preferred option as assessed by 6‐month mortality; there is considerable uncertainty for longer time delays.
Keywords: acute myocardial infarction, primary coronary angioplasty, thrombolytics, meta‐regression
In the UK, at least 87 000 people under the age of 75 years have an acute myocardial infarction (AMI) each year.1 The relationship between normal coronary artery blood flow and mortality after MI is well documented,2 so early restoration of normal myocardial blood flow is a prime therapeutic goal for the management of MI. Pharmacological treatment with thrombolytic therapy and primary angioplasty are two different modes of reperfusion treatment for ST elevation MI (STEMI).
Meta‐analyses of the various randomised trials comparing thrombolysis and primary angioplasty have shown substantial improvements in mortality, non‐fatal reinfarction and stroke from the use of angioplasty2,3,4,5; and they have also shown that angioplasty has lower recurrence rates and less residual stenosis.6,7 Despite the apparent clinical superiority of primary angioplasty, thrombolytic treatment is the default treatment option in many countries because of practical limitations on the use of percutaneous interventions, including a shortage of cardiac catheter facilities and appropriately skilled staff. The choice of appropriate management also needs to consider the possible time delay in initiating reperfusion with primary angioplasty compared with thrombolysis. The effect of this angioplasty‐related time delay in reducing the mortality benefit of angioplasty relative to thrombolysis has been demonstrated using meta‐regression methods.8,9
This work has been influential in clinical guidelines for the management of AMI.10,11 For example, European guidelines suggest that primary angioplasty is the “preferred treatment if performed by an experienced team less than 90 minutes after first medical contact”.11 However, there are some limitations in the analyses informing these guidelines. A key meta‐analysis only had abstracts available for some trials,2 and inaccuracy in data extraction has been observed.12 The quantification of the relationship between the benefit of angioplasty and time delay until its initiation did not measure the uncertainty around this relationship, and the analysis was restricted to a subset of major clinical events.8
This paper seeks to build on these previous analyses by extending their scope and statistical rigor. It assesses how the treatment effect of angioplasty on fatal and non‐fatal outcomes (reinfarctions and strokes) relates to the additional delay in initiating angioplasty. It also considers both the 1‐month and the 6‐month outcome data reported in randomised clinical trials. Furthermore, in using Bayesian statistical methods, the paper is able to quantify more fully the uncertainty associated with the estimated relationships.
Methods
Search strategy and data extraction
To identify trials comparing intravenous thrombolysis and primary angioplasty in patients with STEMI, the analysis used an earlier review2 as a starting point. To update this review, the following databases were searched: Cochrane Controlled Trials Register, UK National Research Register, Medline, Embase, Database of Abstracts of Reviews of Effects, UK National Health Service Economic Evaluation Databases, and the Health Technology Assessment Database. The searches were restricted to English language studies published between 2002 and 2004. The inclusion criteria were consistent with those used previously.2,5 Full details of the search strategy are available in a technical report (available at http://heart.bmj.com/supplemental).
Two researchers (YBV, CA) independently extracted the clinical data. Outcomes of interest were mortality, non‐fatal reinfarctions, fatal and non‐fatal strokes and haemorrhagic strokes, as well as any data about time delay to treatment initiation. Discrepancies were resolved by consensus, and a third researcher (SP) was consulted when necessary. Data were also extracted on the difference between time‐to‐balloon in angioplasty and time‐to‐needle in thrombolytic treatment. This definition emphasises the differences in times to initiation of treatment between the two reperfusion strategies, thus avoiding the problem of different timing definitions across studies. Mean times to treatment, together with their standard deviations, were preferred in the analysis, but medians and quartiles were used when these were not available. Where the earlier review2 had used preliminary data from conference abstracts, these were updated with final trial reports; the earlier data extraction was also checked and any inaccuracies were corrected.
Statistical methods
The comparison in the meta‐analysis was between primary angioplasty and thrombolysis (regardless of type of drug). Because only a limited number of trials reported 6‐month data on fatal or haemorrhagic strokes, these end points were excluded from the meta‐analysis. Thus three outcomes (death, non‐fatal strokes and non‐fatal reinfarctions), for which sufficient data were available, were analysed using an intention‐to‐treat principle.
The analysis was undertaken using Bayesian statistical methods.13,14,15 These methods were used because they are more suitable for synthesising evidence when there are differences between trials in, for example, follow‐up times and reported outcomes. An important feature of Bayesian methods is that they use external evidence (so called “prior distributions”), which represent beliefs about the evidence and its uncertainty external to the data extracted from the trials. This analysis has used “non‐informative” prior distributions so that the data are dominant in the results presented. A sensitivity analysis was undertaken to verify that changing the specification of the prior distribution did not alter the results substantially. Bayesian methods also enable direct probability statements to be made about quantities of clinical interest—for example, the probability that one intervention is better than another.13,14
Full details of the statistical methods are presented in the technical report (http://heart.bmj.com/supplemental). Briefly, the meta‐analysis models all outcomes of interest as probabilities on a log odds scale, and results are reported as the absolute probability of specific events and odds ratios (ORs; with 95% credible intervals (CrI)). It is assumed that baseline event rates (ie, clinical events in the thrombolysis arms) vary randomly between trials, where the degree of variation is estimated from the data (a “random effect” assumption). That is, although the patient populations in different trials are not identical, they are similar to each other. So the results of the analysis are only valid for patient populations similar to a hypothetical “average” trial population.
For each outcome measure, the relative treatment effect of primary angioplasty compared with thrombolytic treatment is modelled as a “random effect”; similar but not identical between trials. This relative treatment effect is estimated as a function of the time delay related to the initiation of angioplasty. This relationship is used to establish the extent to which any additional effectiveness of angioplasty is affected by the additional time it takes to deliver the intervention compared with thrombolysis, while taking into account the uncertainty surrounding the average delay in each trial. When interpreting the results of such a “meta‐regression”, caution is needed in extrapolating the relationship beyond the data on time delay observed in the trials. Also, it should be recognised that, at the extremes of the time‐delay data, uncertainty in the estimates relationship will be greater than around the midpoint.
A feature of the evidence base is that some trials report outcomes at 1 month's follow‐up, some at 6 months' follow‐up and some at both. In order that all these data can be used, outcomes at 1 month and 6 months are assumed to differ by a random effect.16 This reflects the fact that clinical events are more likely to occur within the first month after an AMI and, by allowing a relationship between outcomes at the two time points, more of the data can be used in the analysis. Thus, those studies which do not report at 6 months can “borrow strength” both from those that do and from their own results at 1 month.
Results
Summary of the trial evidence
A total of 24 studies met the inclusion criteria. Two of the studies were subsequently excluded from the meta‐analysis. One of these was excluded because it did not report times to treatment and thus could not provide data on the delay to primary angioplasty.17 The SHOCK study18 was also excluded because the primary comparison was between emergency revascularisation without differentiating results by type of intervention (angioplasty 64%, surgery 36%), and hence this treatment strategy was not directly comparable with primary angioplasty in the other trials.
Table 1 lists the remaining 22 studies included. In comparison with the earlier review,2 one additional trial19 was identified which had not been published at the time. In addition, full trial results were available for three studies that had previously been reported in abstract form only.20,21,22
Table 1 Overview of trials, key end points and time to treatment for primary angioplasty (A) and thrombolysis (T).
| Study | 1 Month (4–6 weeks) | 6 Months | Time (min) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Death | NF Reinf | NF Stroke | No | Death | NF Reinf | NF Stroke | Mean | Mean | |||||||||
| (A) | (T) | No (A)/(T) | OR (95% CrI) | No (A)/(T) | OR (95% CrI) | No (A)/(T) | OR (95% CrI) | (A) | (T) | No (A)/(T) | OR (95% CrI) | No (A)/(T) | OR (95% CrI) | No (A)/(T) | OR (95% CrI) | (A) | (T) | |
| Zijlstra et al 199323‡ | 70 | 72 | 0/4 | 0.1 (0 to 7.7) | 0/9 | 0.1 (0.0 to 4.8) | 0/2 | 0.2 (0.0 to 12.4) | – | – | – | – | – | – | – | – | 61 | 30 |
| Ribeiro et al 199324‡ | 50 | 50 | 3/1 | 3.1 (0.2 to 16) | 4/5 | 0.7 (0.2 to 3.5) | – | – | – | – | – | – | – | – | – | – | 238 | 179 |
| de Boer et al 199419‡ | 152 | 149 | 3/11 | 0.3 (0.2 to 2) | 2/15 | 0.1 (0.0 to 1.7) | 1/2 | 0.4 (0.0 to 8.1) | – | – | – | – | – | – | – | – | 195 | 176 |
| Berrocal et al 200320‡ | 54 | 58 | 5/6 | 0.9 (0.3 to 3.3) | 1/2 | 0.5 (0.0 to 8.6) | – | – | – | – | – | – | – | – | – | – | 82 | 15 |
| Zijlstra et al 199725‡ | 45 | 50 | 1/0 | 2.3 (0 to 43) | 0/8 | 0.0 (0.0 to 5.2) | 1/2 | 0.5 (0.0 to 8.7) | 45 | 50 | 1/0 | 2.2 (0.0 to 43.4) | 0/8 | 0.0 (0.0 to 5.2) | 1/2 | 0.5 (0.0 to 8.7) | 68 | 29 |
| Widimsky et al 200026‡ | 101 | 99 | 7/14 | 0.5 (0.3 to 1.8) | 1/10 | 0.0 (0.0 to 2.7) | 0/0 | 1 (0.0 to 50.4) | – | – | – | – | – | – | – | – | 96 | 90 |
| de Boer et al 200227‡ | 46 | 41 | 3/8 | 0.3 (0.1 to 2.4) | 1/6 | 0.1 (0.0 to 3.5) | 1/2 | 0.4 (0.0 to 7.9) | – | – | – | – | – | – | – | – | 59 | 31 |
| Widimsky et al 200321†‡ | 429 | 421 | 29/42 | 0.7 (0.5 to 1.4) | 6/13 | 0.4 (0.2 to 1.8) | 1/9 | 0.1 (0.0 to 3.0) | – | – | – | – | – | – | – | – | 97 | 12 |
| DeWood et al 199028 | 46 | 44 | 3/2 | 1.5 (0.2 to 7.4) | – | – | – | – | – | – | – | – | – | – | – | – | 126 | 84 |
| Grines et al 199329 | 195 | 200 | 5/13 | 0.4 (0.2 to 1.9) | 5/13 | 0.3 (0.2 to 1.8) | 0/3 | 0.1 (0.0 to 9.2) | 188 | 190 | 7/15 | 0.4 (0.2 to 1.7) | – | – | – | – | 60 | 32 |
| Gibbons et al 199330 | 47 | 56 | 2/2 | 1.2 (0.1 to 8) | – | – | – | – | 47 | 56 | 3/2 | 1.8 (0.2 to 8.1) | 0/2 | 0.2 (0.0 to 13.2) | – | – | 277 | 232 |
| Ribichini et al 199831 | 55 | 55 | 1/3 | 0.3 (0.1 to 6.1) | 1/2 | 0.4 (0.0 to 8.3) | 0/0 | 1 (0.0 to 51.31) | 55 | 55 | 1/4 | 0.2 (0.0 to 4.9) | 2/2 | 1 (0.1 to 7.3) | – | – | 53.2 | 36.5 |
| Garcia et al 199932 | 109 | 111 | 3/12 | 0.2 (0.1 to 1.9) | 4/6 | 0.6 (0.2 to 3.0) | 0/2 | 0.2 (0.0 to 12.3) | 99 | 91 | 5/13 | 0.3 (0.2 to 1.7) | 6/8 | 0.6 (0.2 to 2.5) | – | – | 197 | 150 |
| GUSTO IIb 199733 | 565 | 573 | 32/40 | 0.8 (0.6 to 1.5) | 25/37 | 0.6 (0.4 to 1.4) | 1/5 | 0.2 (0.0 to 4.2) | 565 | 573 | – | – | – | – | – | – | 228 | 180 |
| Le May et al 200134 | 62 | 61 | 3/2 | 1.5 (0.2 to 7.4) | 3/5 | 0.5 (0.1 to 3.4) | 1/1 | 1 (0.0 to 16.2) | 62 | 61 | 3/2 | 1.5 (0.1 to 7.3) | 4/10 | 0.3 (0.1 to 2.1) | 1/3 | 0.3 (0.0 to 6.0) | 77 | 15 |
| Bonnefoy et al 200235 | 421 | 419 | 20/16 | 1.3 (0.6 to 2.2) | 7/15 | 0.4 (0.2 to 1.7) | 0/4 | 0.1 (0.0 to 7.6) | – | – | – | – | – | – | – | – | 190 | 130 |
| Schomig et al 200036 | 71 | 69 | 3/5 | 0.6 (0.2 to 3.4) | 2/4 | 0.4 (0.1 to 4.0) | – | – | 71 | 69 | 3/9 | 0.2 (0.1 to 2.2) | – | – | – | – | 65 | 30 |
| Vermeer et al 199937† | 75 | 75 | 5/5 | 1 (0.3 to 036) | 1/7 | 0.1 (0.0 to 3.4) | 2/1 | 2 (0.1 to 15.3) | – | – | – | – | – | – | – | – | 85 | 10 |
| Kastrati et al 200238 | 81 | 81 | 2/5 | 0.4 (0.1 to 3.5) | 0/4 | 0.1 (0.0 to 7.6) | 1/1 | 1 (0.0 to 16.2) | 70 | 71 | 5/7 | 0.7 (0.2 to 2.8) | – | – | – | – | 75 | 35 |
| Aversano et al 200239 | 225 | 226 | 12/16 | 0.7 (0.4 to 1.9) | 11/20 | 0.5 (0.3 to 1.6) | 3/8 | 0.3 (0.1 to 2.4) | 225 | 226 | 14/16 | 0.8 ( 0.4 to 1.9) | 12/24 | 0.4 (0.3 to 1.4) | 5/9 | 0.5 (0.2 to 2.3) | 101.5 | 46 |
| Grines et al 200240 | 71 | 66 | 6/8 | 0.7 (0.3 to 2.6) | 1/0 | 1.8 (0 to 39.7) | 0/3 | 0.1 (0.0 to 8.9) | – | – | – | – | – | – | – | – | 174 | 63 |
| Andersen et al 2003: Referral22* | 567 | 562 | 37/48 | 0.7 (0.6 to 1.4) | 11/35 | 0.2 (0.2 to 1.1) | 9/11 | 0.8 (0.3 to 2.2) | – | – | – | – | – | – | – | – | 90 | 20 |
| Andersen et al 2003: Invasive22* | 223 | 220 | 15/13 | 1.1 (0.5 to 2.3) | 2/14 | 0.1 (0.0 to 1.8) | 0/5 | 0.0 (0.0 to 6.6) | – | – | – | – | – | – | – | – | 63 | 20 |
Reinf, reinfarction; CrI, credibility interval.
*This trial consisted of two subtrials, labelled “Referral” and “Invasive”, and these are analysed as if they are two separate studies.
†Includes a third group of patients who received thrombolytic treatment followed by transfer to angioplasty; these third comparators were excluded from the present analysis.
‡Trial used streptokinase as part the thrombolytic arm, all other trials used tissue plasminogen activator.
Table 1 lists the data extracted from the 22 trials. In total, these trials included 3760 and 3758 patients randomised to primary angioplasty and thrombolysis, respectively. Eight of the 22 trials used streptokinase as the form of thrombolysis, and 14 used tissue plasminogen activator. For angioplasty, 13/22 trials used coronary stents, and eight studies used glycoprotein IIb/IIIa antagonists. The mean (SE) value of angioplasty‐related time delay (over and above time to thrombolysis) was 54.3 (2.2) minutes. All trials reported outcomes at between 30 days and 6 weeks (both are referred to as “1 month” in the meta‐analysis results) after the initial MI; 10/22 trials also reported outcomes at 6 months' follow‐up.
Meta‐analysis
Table 2 shows the estimated probability of each outcome occurring within 1 month or 6 months after initial treatment with primary angioplasty or thrombolytic drugs. These results are based on the average angioplasty‐related time delay of 54.3 minutes reported in the trials, estimated as a weighted average, the weights being the total number of patients in each trial. For all outcomes, the mean probability of an event occurring is lower for patients randomised to primary angioplasty. In particular, mortality within 1 month is estimated to be 4.5% after angioplasty and 6.4% after thrombolysis, with OR = 0.68 (95% CrI 0.46 to 1.01). For non‐fatal reinfarction, OR = 0.32 (95% CrI 0.20 to 0.51); and for non‐fatal stroke OR = 0.24 (95% CrI 0.11, 0.50). Table 2 also shows estimated results for the 6‐month end points, which are similar to those at 1 month, indicating that most events happen in the first month after randomisation.
Table 2 Estimated absolute probabilities of the occurrence of various end points 1 month or 6 months after angioplasty or thrombolytic treatment, together with the odds ratios comparing primary angioplasty and thrombolysis and probabilities that angioplasty is superior.
| End points | Probability (angioplasty)* Mean (95%CrI) | Probability (thrombolytics)* Mean (95%CrI) | Odds ratio (95%CrI) | Probability angioplasty superior |
|---|---|---|---|---|
| At 1 month | ||||
| Death | 4.5 (3.0 to 6.5) | 6.4 (4.5 to 9.0) | 0.68 (0.46 to 1.01) | 0.97 |
| Non‐fatal reinfarction | 2.0 (1.2 to 3.1) | 6.1 (4.1 to 8.5) | 0.32 (0.20 to 0.51) | 1.00 |
| Non‐fatal stroke | 0.5 (0.2 to 0.9) | 1.9 (1.0 to 3.2) | 0.24 (0.11 to 0.50) | 1.00 |
| At 6 months | ||||
| Death | 5.5 (3.4 to 8.8) | 7.7 (5.0 to 11.8) | 0.70 (0.42 to 1.18) | 0.93 |
| Non‐fatal reinfarction | 2.6 (1.4 to 4.8) | 6.9 (4.4 to 10.7) | 0.33 (0.20 to 0.67) | 0.99 |
| Non‐fatal stroke | 0.8 (0.2 to 1.0) | 2.8 (1.1 to 6.9) | 0.26 (0.08 to 0.72) | 0.99 |
*Results are given as percentages.
The results are for the average observed “angioplasty‐related time delay” (ie, 54.3 minutes).
As the additional time delay to initiation of primary angioplasty is modelled explicitly, it is possible to predict how particular angioplasty‐related time delays influence the clinical superiority of angioplasty. For angioplasty delays of 30, 60 or 90 minutes, the absolute probability differences and the odds ratios of angioplasty versus thrombolytic treatment are shown in table 3. If angioplasty could be initiated within 30 minutes of possible thrombolysis, the absolute probabilities of mortality, non‐fatal reinfarction and non‐fatal stroke at 6 months would be, respectively, 3.7%, 4.6% and 1.7% lower than those with thrombolysis. For any of these outcomes, the benefit of angioplasty decreases with longer delay until its initiation.
Table 3 Absolute probability differences (thrombolysis minus angioplasty), odds ratios for the 6‐month treatment effects of angioplasty compared with thrombolytic treatment (mean and 95% credibility interval (95% CrI)) and probability that angioplasty is superior at assumed “angioplasty‐related time delays” of 30, 60 and 90 minutes.
| End point | Primary angioplasty‐related time delay | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 Minutes | 60 Minutes | 90 Minutes | |||||||
| Probability difference* (95% CrI) | Odds ratio | Probability angioplasty is a better treatment | Probability difference* (95% CrI) | Odds ratio | Probability angioplasty is a better treatment | Probability difference* (95% CrI) | Odds ratio | Probability angioplasty is a better treatment | |
| Death | –3.7 (–7.2 to –0.5) | 0.54 (0.29 to 0.92) | 0.98 | –1.8 (–5.6 to +1.7) | 0.77 (0.44 to 1.29) | 0.87 | +0.7 (–4.6 to +8.1) | 1.15 (0.49 to 2.36) | 0.44 |
| Non‐fatal reinfarction | –4.6 (–8.2 to –2.2) | 0.30 (0.14 to 0.59) | 0.99 | –4.2 (–7.5 to –1.6) | 0.39 (0.21 to 0.72) | 0.97 | –3.1 (–7.1 to +1.6) | 0.55 (0.29 to 1.27) | 0.93 |
| Non‐fatal stroke | –1.7 (–5.8 to –0.5) | 0.47 (0.05 to 0.69) | 0.99 | –2.0 (–5.6 to –0.4) | 0.56 (0.09 to 0.75) | 0.98 | –1.6 (–5.3 to +0.8) | 0.79 (0.08 to 1.43) | 0.93 |
*Results are shown as percentages.
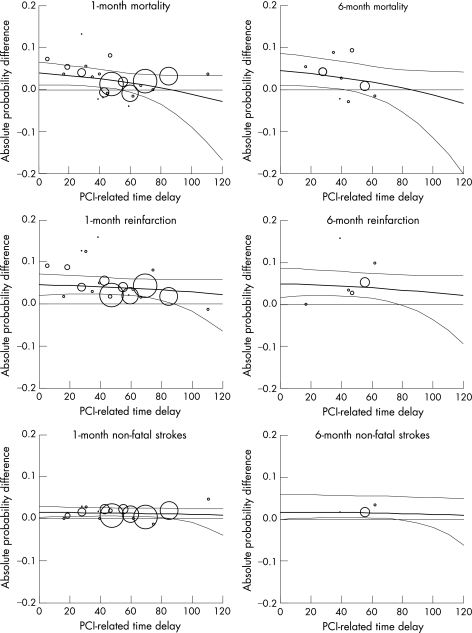
Figure 1 shows this effect in more detail. For mortality, angioplasty is better than thrombolysis, on average, at time delays up to 90 minutes. Moreover, for the 1‐month outcome of mortality, the probability that it is superior is 97%, for an additional delay of up to around 60 minutes. For the 6‐month outcome of mortality, there is more than 95% probability that angioplasty is superior for delays of up to around 45 minutes and 87% for delays up to around 60 minutes. However, this probability goes below 50% for delays at 90 minutes and beyond, where thrombolysis might therefore be the preferred option at least for the 6‐month mortality outcome. For non‐fatal reinfarction and non‐fatal stroke, primary angioplasty is superior, on average, even if it requires an additional time of up to 2 hours to achieve reperfusion with that method. For both non‐fatal outcomes at 1 month, there was over 95% probability that angioplasty is superior at additional delays of up to 90 minutes. For the corresponding 6‐month outcomes, there was over 95% probability that angioplasty was superior at delays up to 80 minutes.
Figure 1 Treatment effect of primary angioplasty relative to thrombolytic treatment, shown as the absolute probability differences for each key outcome (death, non‐fatal reinfarctions, non‐fatal strokes) and point of follow‐up (1 month, 6 months). The graphs show means and 95% credibility intervals plotted against the additional time delay to initiating primary angioplasty. Values above the 0.0 horizontal line indicate that angioplasty results in fewer clinical events. Each point represents a trial and their size is proportional to the trial sample size.
Discussion
The contribution of this review is twofold. First, it updates the most comprehensive recent meta‐analysis of randomised trials comparing primary angioplasty and thrombolysis in patients with STEMI.2 Second, it extends the evidence synthesis by evaluating the relationship between the treatment effects of angioplasty and time delay, expressed as the difference in times to initiation of treatment between the two reperfusion strategies. Furthermore, to our knowledge, this is the first study that explicitly models the measurement uncertainty associated with angioplasty‐related time delay.
Although Keeley et al2 do not deal directly with time delay, the main results in that study can be compared with those presented here for the average time delay of 54.3 minutes. For mortality at 1 month, Keeley et al found an odds ratio of 0.70 (95% CI 0.58 to 0.85), which is similar to that reported here, although our estimate does not reach statistical significance. This lack of significance is likely to be due to differences in the data extraction,12 the inclusion of additional evidence, and also because the measurement uncertainty in the time‐delay covariate is explicitly considered here. For the outcome of non‐fatal reinfarction, the results here are similar to those of Keeley et al, for both the magnitude and uncertainty of the odds ratio. The analysis of the stroke outcome is not comparable to that in Keeley et al, which included all strokes, compared with the non‐fatal strokes considered here. Although a separate analysis of the longer‐term follow‐up data was undertaken by Keeley et al, these results were not presented in sufficient detail to allow a reliable comparison between the two sets of analyses at 6 months. Another reason why there will be slight differences between the two meta‐analyses is that in ours the uncertainty in the between‐study variability of the effect is appropriately taken in to account, thus producing slightly wider CrIs than those obtained using classical meta‐analysis methods.14,15
Based on research undertaken during a similar period to our own, Boersma et al41 have also recently demonstrated, using individual patient data from 22 trials, that angioplasty is associated with significantly lower 30‐day mortality, reinfarction and stroke than thrombolysis, regardless of delay in presentation. The main results in that study for the overall angioplasty‐related delay of 55 minutes can be compared with those presented here for the average time delay of 54.3 minutes at 1 month (table 2), although there were minor differences in the trials included in the two studies. The absolute differences in the risks of non‐fatal MI and stroke between angioplasty and thrombolysis at 1 month were similar (4.3% vs 4.1% for non‐fatal MI and 1.7% vs 1.4% for non‐fatal stroke in Boersma et al and the current study, respectively). The estimated absolute reduction in mortality risk with angioplasty at 1 month was higher in the study of Boersma et al: 2.6% versus 2%. As seen in previous studies,8,42 the benefit of angioplasty for mortality decreases the longer the time delay to initiation of angioplasty. However, none of these studies (including Boersma et al41) quantify the uncertainty in this relationship fully. The comprehensive handling of uncertainty in the current analysis allows the precision associated with the relationship to be presented (fig 1). The Bayesian approach also facilitates the presentation of results as the probability of one intervention being the better treatment.
This is the first study to link explicitly short‐term (1 month) with longer‐term (6 months) outcomes using as much of the available clinical evidence as possible. Although none of the trial data indicate systematic differences between the relative treatment effect of primary angioplasty at 1 month and at 6 months, fewer data are generally available at 6 months, resulting in greater uncertainty. It is, therefore, not surprising that the point estimates of the relative treatment effect of angioplasty are similar at the two time points, but with greater uncertainty at 6‐month end points. Thus, a probability of the superiority of angioplasty measured by a 6‐month mortality end point of greater than 0.95 can be identified for delays of up to around 45 minutes only, while for delays at around 90 minutes thrombolysis appears to be superior. However, angioplasty appears to be better for achieving 6‐month non‐fatal outcomes, on average, for delays up to around 90 minutes. It should be noted, however, that the uncertainty in these relationships shown here is less than it would have been had only 6‐month follow‐up data been used in the analysis owing to the paucity of the data.
The analysis suggests, therefore, that angioplasty performs better than thrombolytic treatment, but this superiority is related to angioplasty‐related time delay. It should be emphasised, however, that no trials have been identified which show a statistically significant advantage for thrombolysis at very long angioplasty‐related time delays. Moreover, the PRAGUE‐2 trial indicates that angioplasty performs better than thrombolysis even when it involves a patient transfer of up to 3 hours.21 Without more evidence at long angioplasty‐related time delays, the linear regression model estimated here will inevitably indicate that the relative treatment effect of primary angioplasty becomes negative at an unspecified delay. This is not because of data showing this effect, but simply because a consistent relationship has been observed for a range of relatively short time delays. In reality, for delays approaching 2 hours, this study can neither confirm nor refute whether angioplasty is better than thrombolytic treatment.
This study has some limitations. First, the lack of individual patient data precludes an analysis of how the relative effect of angioplasty varies between patient subgroups, and although this analysis has taken account of the uncertainty in the average time delay, thus reducing the possibility of ecological fallacy,43 the presence of an ecological bias cannot be entirely eliminated. However, this is less of a problem when it is recognised that the aim of this study is to provide evidence to support population‐based decisions using cost‐effectiveness analysis as reported in the companion paper.44 However, an analysis of individual patient data would also enable a more appropriate estimate of the impact or otherwise of time delay on outcome to be obtained.46,47
Second, time‐to‐needle is a predictor of the success of thrombolytic treatment, but this effect could not be included in the analysis explicitly owing to inconsistent reporting of the data in the trials. Hence the results are based on the average time‐to‐needle in the studies considered, which, at 75 minutes, was shown to be similar to the median call to needle time (67 minutes) in the UK (personal communication, Dr John Birkhead, UK Myocardial Infarction National Audit Project). Further research would be desirable to identify all external evidence on the effect of time‐to‐needle on outcomes and incorporate this into our analysis by appropriate prior distributions taking account of relevance and quality.15
Third, given this review was an update of those published earlier, the effect of publication bias, study quality or the influence of individual studies on the overall meta‐analysis results was not formally assessed.
Fourth, further exploration of whether the potential relationship between time delay and effect (log odds ratio) is linear may be of merit.
The final limitation concerns the use of older streptokinase trials in the meta‐analysis. Keeley et al were criticised48 for including these trials in their meta‐analysis because by effectively averaging across the thrombolytic trials the additional benefit of angioplasty may have been overestimated. However, streptokinase is the most common form of thrombolytic treatment used in many countries and is used in about a third of patients in the UK (personal communication, Dr John Birkhead, UK Myocardial Infarction National Audit Project). In the present meta‐analysis, the differences between thrombolytic drugs were ignored with a focus on primary angioplasty or thrombolysis as two treatment groups. If only tissue plasminogen activator trials are analysed, the relative benefit of primary angioplasty is attenuated: 1‐month odds ratios for mortality are found to be 0.71 (95% CrI 0.44 to 1.16); for non‐fatal reinfarction, 0.41 (95% CrI 0.23 to 0.71); and for non‐fatal strokes, 0.23 (95% CrI 0.08 to 0.57). Full details of this sensitivity analysis are reported in the technical report (http://heart.bmj.com/supplemental).
The policy implications of this analysis should be seen in the context of the relevant healthcare system. For example, US guidelines currently recommend that primary angioplasty should be used only within an angioplasty‐related delay of less than 60 minutes.10 The guidelines, however, seem to be based largely on the work of Nallamothu and Bates,8 and may be premature because angioplasty seems to convey health benefits even when the delay is longer than 60 minutes. Even at delays longer than 1 hour, angioplasty is superior, on average, for all the 1‐month outcomes included in this study, although there is considerable uncertainty associated with these estimates.
What size of treatment effect would be necessary with primary angioplasty for it to be considered worthwhile, given the major changes in service organisation necessary for its implementation? This question is considered directly in the cost‐effectiveness analysis submitted as a companion paper, which examines whether the health benefits of primary angioplasty are sufficient to justify its additional cost.44 With respect to the absolute size of treatment effect with primary angioplasty, our analysis shows that the probability that primary angioplasty reaches at least a 1%, 2% and 3% improvement in survival at 1 month relative to thrombolysis is 0.82, 0.47 and 0.15, respectively, at the average angioplasty‐related time delay. In short, the benefit of timely treatment is the key: if primary angioplasty can be delivered in a appropriate fashion, current evidence supports its use; if not, the choice of treatment probably depends on time from onset of symptoms to presentation21,41 and the availability of pre‐hospital thombolysis.35
Decisions about appropriate methods of reperfusion should consider not only the effectiveness of each treatment option but also their cost effectiveness. With the quantification of both the expected treatment effects of angioplasty, with regard to several possible outcomes, and the uncertainties associated with these predictions, this meta‐analysis using Bayesian methods lays the foundations for a robust cost‐effectiveness analysis, in which other treatment strategies may be considered, and in which appropriate account is taken of statistical, clinical and methodological heterogeneity and all sources of uncertainty.48
Supplementary Material
Abbreviations
AMI - acute myocardial infarction
CrI - credibility interval
OR - odds ratio
STEMI - ST elevation myocardial infarction
Footnotes
Funding: This study was funded by an unrestricted educational grant from Cordis Ltd, which played no part in the design, execution or dissemination of the research. Mark Sculpher also receives funding via a Career Award in Public Health funded by the NHS Research and Development Programme.
Competing interests: Mark Sculpher and Mark de Belder have received research funding and consultancy fees from various manufacturers of medical devices such as coronary stents.
References
- 1.British Heart Foundation Incidence of myocardial infarction. http://www.heartstats.org/datapage.asp?id = 1085 (accessed 3 July 2007)
- 2.Keeley E C, Boura J A, Grines C L. Primary angioplasty versus intravenous thromblytic therapy for acute myocardial infarction: a quantitative review of 23 randomized trials. Lancet 200336113–20. [DOI] [PubMed] [Google Scholar]
- 3.Dalby M, Bouzamondo A, Lechat P.et al Transfer for primary angioplasty versus immediate thrombolysis in acute myocardial infarction: a meta‐analysis.[see comment]. Circulation 20031081809–1814. [DOI] [PubMed] [Google Scholar]
- 4.Grines C, Patel A, Zijlstra F, et al; PCAT collaborators Primary coronary angioplasty compared with intravenous thrombolytic therapy for acute myocardial infarction: six‐month follow up and analysis of individual patient data from randomized trials. Am Heart J 200314547–57. [DOI] [PubMed] [Google Scholar]
- 5.Cucherat M, Bonnefoy E, Tremeau G. Primary angioplasty versus intravenous thrombolysis for acute myocardial infarction. Cochrane Database Syst Rev 2003(3)CD001560. [DOI] [PMC free article] [PubMed]
- 6.Zijlstra F. Acute myocardial infarction: primary angioplasty. Heart 200185705–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith D, Channer K S. Primary angioplasty should be first line treatment for acute myocardial infarction. For and against. BMJ 20043281254–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nallamothu B K, Bates E R. Percutaneous coronary intervention versus fibrinolytic therapy in acute myocardial infarction: is timing (almost) everything? Am J Cardiol 200392824–826. [DOI] [PubMed] [Google Scholar]
- 9.Kent D M, Lau J, Selker H P. Balancing the benefits of primary angioplasty against the benefits of thrombolytic therapy for acute myocardial infarction: the importance of timing. Eff Clin Pract 20014214–220. [PubMed] [Google Scholar]
- 10.Antman E M, Anbe D T, Armstrong P W.et al ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction ‐ executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2004110588–636. [DOI] [PubMed] [Google Scholar]
- 11.Silber S, Albertsson P, Avilés F F.et al Guidelines for percutaneous coronary interventions: the Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology. Eur Heart J 200526804–847. [DOI] [PubMed] [Google Scholar]
- 12.Nallamothu B K, Antman E M, Bates E R. Primary percutaneous coronary intervention versus fibrinolytic therapy in acute myocardial infarction: does the choice of fibrinolytic agent impact on the importance of time‐to‐treatment? Am J Cardiol 200494772–774. [DOI] [PubMed] [Google Scholar]
- 13.Spiegelhalter D J, Myles J P, Jones D R.et al An introduction to Bayesian methods in health technology assessment. BMJ 1999319508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutton A J, Abrams K R. Bayesian methods in meta‐analysis and evidence synthesis. Stat Methods Med Res 200110277–303. [DOI] [PubMed] [Google Scholar]
- 15.Spiegelhalter D J, Abrams K R, Myles J P.Bayesian approaches to clinical trials and health‐care evaluation. Chichester: Wiley, 2004
- 16.Larose D T, Dey D K. Grouped random effects models for Bayesian meta‐analysis. Stat Med 1997161817–1829. [DOI] [PubMed] [Google Scholar]
- 17.Akhras F, Abu Ousa A, Swann G.et al Primary coronary angioplasty or intravenous thrombolysis for patients with acute myocardial infarction? Acute and late follow‐up results in a new cardiac unit. J Am Coll Cardiol 199729(suppl)A235–A236. [Google Scholar]
- 18.Hochman J S, Sleeper L A, Webb J G.et al Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med 1999341625–634. [DOI] [PubMed] [Google Scholar]
- 19.de Boer M, Hoorntje J C A, Ottervanger J P.et al Immediate coronary angioplasty versus intravenous streptokinase in acute myocardial infarction: left ventricular ejection fraction, hospital mortality and reinfarction. J Am Coll Cardiol 1994231004–1008. [DOI] [PubMed] [Google Scholar]
- 20.Berrocal D H, Cohen M G, Spinetta A D.et al Early reperfusion and late clinical outcomes in patients presenting with acute myocardial infarction randomly assigned to primary percutaneous coronary intervention or streptokinase. Am Heart J 2003146E22. [DOI] [PubMed] [Google Scholar]
- 21.Widimsky P, Budesinsky T, Vorac D.et al Long distance transport for primary angioplasty vs immediate thrombolysis in acute myocardial infarction. Final results of the randomized national multicentre trial—PRAGUE‐2. Eur Heart J 20032494–104. [DOI] [PubMed] [Google Scholar]
- 22.Andersen H R, Nielsen T T, Rasmussen K.et al A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med 2003349733–742. [DOI] [PubMed] [Google Scholar]
- 23.Zijlstra F, de Boer M J, Hoorntje J C.et al A comparison of immediate coronary angioplasty with intravenous streptokinase in acute myocardial infarction. N Engl J Med 1993328680–684. [DOI] [PubMed] [Google Scholar]
- 24.Ribeiro E E, Silva L A, Carneiro R.et al Randomized trial of direct coronary angioplasty versus intravenous streptokinase in acute myocardial infarction. J Am Coll Cardiol 199322376–380. [DOI] [PubMed] [Google Scholar]
- 25.Zijlstra F, Beukema W P, van't Hof A W J.et al Randomized comparison of primary coronary angioplasty with thrombolytic therapy in low risk patients with acute myocardial infarction. J Am Coll Cardiol 199729908–912. [DOI] [PubMed] [Google Scholar]
- 26.Widimsky P, Groch L, Zelizko M.et al Multicentre randomized trial comparing transport to primary angioplasty vs immediate thrombolysis vs. combined strategy for patients with acute myocardial infarction presenting to a community hospital withouth a catheterization laboratory. The PRAGUE study. Eur Heart J 200021823–831. [DOI] [PubMed] [Google Scholar]
- 27.de Boer M J, Ottervanger J P, van't Hof A W.et al Reperfusion therapy in elderly patients with acute myocardial infarction: a randomized comparison of primary angioplasty and thrombolytic therapy [see comments.]. J Am Coll Cardiol 2002391723–1728. [DOI] [PubMed] [Google Scholar]
- 28.DeWood D.et al Direct PTCA vs intravenous t‐PA in acute myocardial infarction: results from a prospective radomized trial. Thrombolysis and Interventional Therapy in Acute Myocardial Infarction Symposium VI. Washington: George Washington University, 1990
- 29.Grines C L, Browne K F, Marco J.et al A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. N Engl J Med 1993328685–691. [DOI] [PubMed] [Google Scholar]
- 30.Gibbons R J, Homes D R, Reeder G S.et al Immediate angioplasty compared wtih the administration of a thrombolytic agent followed by conservative treatment for myocardial infarction. N Engl J Med 1993328685–691. [DOI] [PubMed] [Google Scholar]
- 31.Ribichini F, Steffenino G, Dellavalle A.et al Comparison of thrombolytic therapy and primary coronary angioplasty with liberal stenting for inferior myocardial infarction with precordial ST‐segment depression: immediate and long‐term results of a randomized study. J Am Coll Cardiol 1998321687–1694. [DOI] [PubMed] [Google Scholar]
- 32.Garcia E, Elizaga J, Soriano J.et al Primary angioplasty versus thrombolysis with t‐PA in anterior myocardial infarction: results from a single trial. J Am Coll Cardiol 199933605–611. [DOI] [PubMed] [Google Scholar]
- 33.GUSTO investigators A clinical trial comparing primary coronary angioplasty with tissue plasminogen activator for acute myocardial infarction. The Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes (GUSTO IIb) Angioplasty Substudy Investigators [published erratum appears in N Engl J Med 1997;337: 287], N Engl J Med 19973361621–1628. [DOI] [PubMed] [Google Scholar]
- 34.Le May M R, Labinaz M, Davies R F.et al Stenting versus thrombolysis in acute myocardial infarction trial (STAT). J Am Coll Cardiol 200137985–991. [DOI] [PubMed] [Google Scholar]
- 35.Bonnefoy E, Lapostolle F, Leizorovicz A.et al Primary angioplasty versus prehospital fibrinolysis in acute myocardial infarction: a randomised study. Lancet 2002360825–829. [DOI] [PubMed] [Google Scholar]
- 36.Schomig A, Kastrati A, Dirschinger J.et al Coronary stenting plus platelet glycoprotein IIb/IIIa blockade compared with tissue plasminogen activator in acute myocardial infarction. N Engl J Med 2000343385–391. [DOI] [PubMed] [Google Scholar]
- 37.Vermeer F, Oude Ophuis A J, van der Berg E J.et al Prospective randomised comparison between thrombolysis, rescue PTCA and primary PTCA in patients with extensive myocardial infarction admitted to a hospital without PTCA facilities: a safety and feasibility study. Heart 199982426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kastrati A, Mehilli J, Dirschinger J.et al Myocardial salvage after coronary stenting plus abciximab versus fibrinolysis plus abciximab in patients with acute myocardial infarction: a randomised trial. Lancet 2002359920–925. [DOI] [PubMed] [Google Scholar]
- 39.Aversano T, Aversano L T, Passamani E.et al Thrombolytic therapy vs primary percutaneous coronary intervention for myocardial infarction in patients presenting to hospitals without on‐site cardiac surgery: a randomized controlled trial [see comments]. JAMA 20022871943–1951. [DOI] [PubMed] [Google Scholar]
- 40.Grines C L, Westerhausen D R, Jr, Grines L L.et al A randomized trial of transfer for primary angioplasty versus on‐site thrombolysis in patients with high‐risk myocardial infarction: the Air Primary Angioplasty in Myocardial Infarction study [see comments]. J Am Coll Cardiol 2002391713–1719. [DOI] [PubMed] [Google Scholar]
- 41. Boersma E, PCAT‐2 Trialists' Collaborative Group. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in‐hospital fibrinolysis in acute myocardial infarction patients. Eur Heart J 200627779–788. [DOI] [PubMed] [Google Scholar]
- 42.Kent D M, Schmid C H, Lau J.et al Is primary angioplasty for some as good as primary angioplasty for all? Modeling across trials and individual patients. J Gen Intern Med 200217887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz S. The fallacy of the ecological fallacy: the potential misuse of a concept and the consequences. Am J Public Health 199484819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bravo Vergel Y, Palmer S, Asseburg C.et al Is primary angioplasty cost effective in the UK? Results of a comprehensive decision analysis. Heart 2007931238–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Lambert P C, Sutton A J, Abrams K R.et al A comparison of summary patient‐level covariates in meta‐regression with individual patient data meta‐analysis. J Clin Epidemiol 20025586–94. [DOI] [PubMed] [Google Scholar]
- 46.Berlin J A, Santanna J, Schmid C H.et al Individual patient‐ versus group level data meta‐regressions for the investigation of treatment effect modifiers: ecological bias rears its ugly head. Stat Med 200221371–387. [DOI] [PubMed] [Google Scholar]
- 47.Fresco C. Primary coronary angioplasty versus thrombolysis for acute myocardial infarction [letter]. Lancet 20033611303. [DOI] [PubMed] [Google Scholar]
- 48.Cooper N J, Sutton A J, Abrams K R.et al Comprehensive decision analytical modelling in economic evaluation: a Bayesian approach. Health Econ 200413203–226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.