Abstract
Ciguatera fish poisoning and neurotoxic shellfish poisoning are distinct clinical entities characterized by gastrointestinal and neurological disturbances, following the consumption of certain reef fish and shellfish containing toxic polyether compounds sporadically present in certain toxic marine dinoflagellates. The biotransformation and bioaccumulation of gambierol and brevetoxin, and their congeners, are believed to be involved in the pathogenesis of these “food-chain diseases”, for which no effective treatments are available. Here, we describe for the first time the potent effect of gambierol and brevetoxin on TRPV1 channels, a key player in thermal and pain sensation. Our findings may lead to promising new therapeutic interventions.
Introduction
Characterized by gastrointestinal (nausea, vomiting, diarrhea and abdominal cramping) and neurological (paraesthesias, temperature reversal and cardiac arrhythmias) signs and symptoms, ciguatera fish poisoning (CFP) and neurotoxic shellfish poisoning (NSP) are acute intoxications resulting from the consumption of tropical reef fishes and shellfish [1]. Neurological disturbances typically include tingling of the lips, hands and feet, unusual temperature perception [2], burning mouth syndrome [3], numbness in the extremities and pain. Several polyether toxins, such as gambierol and brevetoxin, are believed to underlie these clinical findings but very little is known about their molecular targets and their mechanisms of action. Since TRPV1, a non-selective cation channel expressed in nociceptive neurons, is known to be involved in temperature and burning-pain sensation [4, 5], we examined whether TRPV1 is involved in these typical neurological disturbances.
Materials and methods
Compound synthesis and solution preparation
Gambierol was synthesized as described previously [6–8], dissolved in DMSO and diluted in ND96. Brevetoxin (PbTx-3 form Latoxan) is dissolved in ND96. Linoleoyl ethanolamide (NAE 18:2 from Cayman Chemical) was diluted in ND96 solution. The total DMSO concentration was maximum 0.5 %.
RNA preparation and electrophysiological recordings
cRNA transcripts were synthesized from XbaI-linearized VR1 cDNA templates using T7 RNA polymerase (Ambion). Oocytes, harvested from anaesthetized female Xenopus laevis frogs as previously described [9], were injected with 0.5–5 ng TRPV1 cRNA. Two to seven days after injection, two-electrode voltage-clamp recording was performed. Currents were measured in ND96 solution using a protocol of –90 mV during 400 s. Current-voltage (IV) curves were obtained using a series of 400 ms step pulses from –90 to +90 mV. The recording chamber was perfused at a rate of 2 mL min−1 with a ND-96 solution containing (in mM) 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES, pH 7.4. Temperature of the perfusate was kept at 22°C and controlled using a SC-20 dual in-line heater/cooler (Warner Instruments) and pH was kept at 7.4 unless otherwise described. As previously described [10], capsaicin (2 μM) was used as an agonist and capsazepine (10 μM) as an antagonist of TRPV1. Capsaicin and capsazepine were purchased from Sigma, and anandamide from Tocris. To obtain EC50 values, the toxin-induced total current was normalized to the capsaicin current (=100%), plotted against the concentration of toxin used and a fit with the Hill equation yielded the EC50.
Results
Administration of 10 μM gambierol (figure 1A) showed no visible effect on TRPV1 channels (figure 2A). In contrast, when gambierol (10 μM) was applied together with capsaicin, a clear allosteric effect with an EC50 of 613 nM (figure 2B and 2D) was found. IV curve is shown in figure 2C. No allosteric effect was visible when the same testing method was used with a ND96 solution of pH 5.4 as an activator of TRPV1 channels instead of capsaicin (data not shown). These results indicate that the acting site of gambierol is most likely related to the intracellular binding site of capsaicin, in contrast with the extracellular binding site of protons. Figure 2E shows the same allosteric effect when linoleoyl ethanolamide was used as endogenous activator instead of capsaicin.
Figure 1.
Structure of gambierol (A) and brevetoxin (B).
Figure 2.
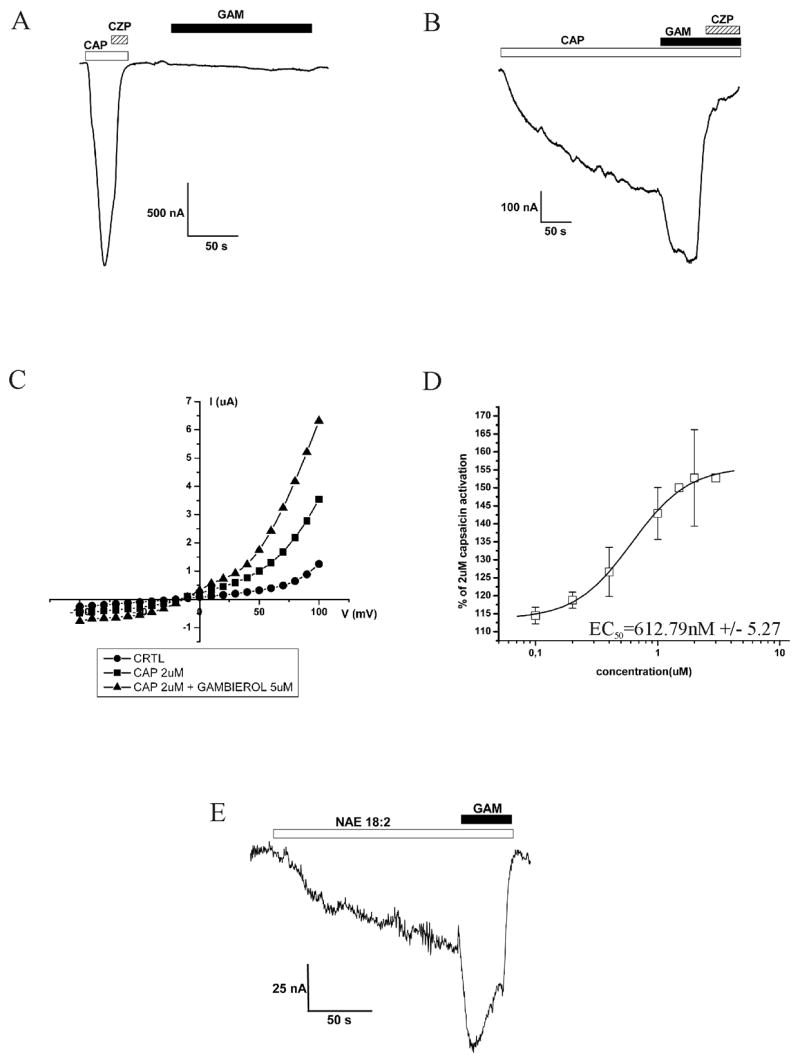
Electrophysiological tests of gambierol alone (A) and in the presence of capsaicin (B). IV curve and dose-response of the allosteric effect shown in respectively C and D. (E) Current showing the allosteric effect of gambierol with linoleoyl ethanolamide (NAE 18:2) as endogenous activator.
The polycyclic ether toxin involved in NPS is brevetoxin (figure 1B). In order to see whether the described effects are a hallmark for this group of toxins, brevetoxin (PbTx-3 from Latoxan) was also tested on TRPV1. Figure 3A shows there is no effect of brevetoxin (2 μM) alone on TRPV1, although an allosteric effect, with an EC50 of 352 nM, was visible when capsaicin (2 μM) was added together with brevetoxin (figure 3B and 3D). Figure 3C shows the IV. Using an acidic ND96 solution (pH 5,4) instead of capsaicin as an activator, did not give an allosteric effect (data not shown).
Figure 3.
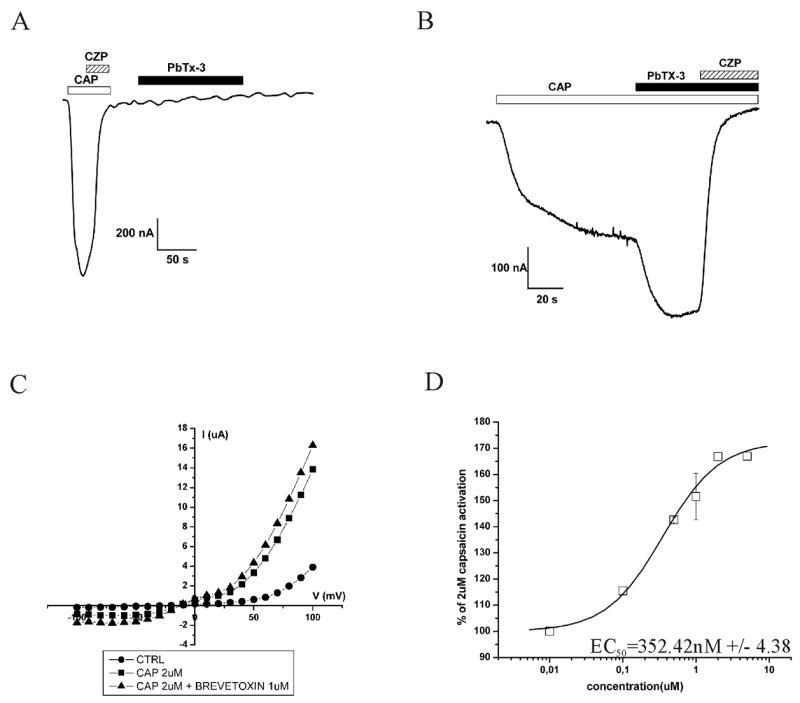
Electrophysiological tests of brevetoxin alone (A) and in the presence of capsaicin (B). IV curve and dose-response of the allosteric effect shown in respectively C and D.
Discussion
The described effects seem to be general for the group of polycyclic ether toxins inducing CFP and NSP. Typical neurological symptoms, like burning mouth syndrome and unusual temperature sensation [2, 3], can be explained as follows: Endogenuous activators like anandamide [11] or unsaturated fatty acids (e.g. linoleoyl ethanolamide) [12], may give a constant small activation of TRPV1 channels. When contaminated fish is eaten, polycyclic ether toxins come into contact with the endogenuous activated TRPV1 channels and the described allosteric effect leads to an increased inward current of cations. This inward current is well known to be associated with a typical burning-pain sensation [10, 13] and to play a key role in temperature sensation [5].
An interesting mechanistic convergence is our finding of allosteric effects on TRPV1 by compounds isolated from cnidarian sources [14]. It is intriguing to consider this in the context of a case report of CFP associated with jellyfish (cnidaria) ingestion [15]. Further, recent and ongoing studies by us demonstrate the presence of a diverse suite of bioactive lipidic compounds amongst the active cocktail of substances in cnidaria venoms.
In conclusion, the elucidation that polycyclic ether toxins activate TRPV1 is novel and may lead to the development of effective therapies. Work to further elucidate the mechanism by which the toxins activate TRPV1 is ongoing.
Acknowledgments
We thank Dr. David Julius for providing the rat TRPV1 clone and Sarah Debaveye for support in molecular biology work. This was supported by the following grants: OT-05-64 (K.U. Leuven), G.0330.06 (F.W.O.-Vlaanderen) and P6/31 (Interuniversity attraction Poles Programme - Belgian State - Belgian Science Policy), GM 56677 (NIGMS, JDR). Dr. Yanagihara’s work was supported by 20061497, Hawaii Community Foundation, Honolulu Hawaii.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lewis RJ. Ciguatera: Australian perspectives on a global problem. Toxicon. 2006;48:799–809. doi: 10.1016/j.toxicon.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 2.Cameron J, Capra MF. The basis of the paradoxical disturbance of temperature perception in ciguatera poisoning. J Toxicol Clin Toxicol. 1993;31:571–579. doi: 10.3109/15563659309025762. [DOI] [PubMed] [Google Scholar]
- 3.Heir GM. Ciguatera neurotoxin poisoning mimicking burning mouth syndrome. Quintessence Int. 2005;36:547–550. [PubMed] [Google Scholar]
- 4.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 5.Patapoutian A. TRP Channels and Thermosensation. Chem Senses. 2005;30(Suppl 1):i193–i194. doi: 10.1093/chemse/bjh180. [DOI] [PubMed] [Google Scholar]
- 6.Johnson HW, Majumder U, Rainier JD. The total synthesis of gambierol. J Am Chem Soc. 2005;127:848–849. doi: 10.1021/ja043396d. [DOI] [PubMed] [Google Scholar]
- 7.Johnson HW, Majumder U, Rainier JD. Total synthesis of gambierol: subunit coupling and completion. Chemistry. 2006;12:1747–1753. doi: 10.1002/chem.200500994. [DOI] [PubMed] [Google Scholar]
- 8.Majumder U, Cox JM, Johnson HW, Rainier JD. Total synthesis of gambierol: the generation of the A-C and F-H subunits by using a C-glycoside centered strategy. Chemistry. 2006;12:1736–1746. doi: 10.1002/chem.200500993. [DOI] [PubMed] [Google Scholar]
- 9.Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- 10.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 11.Smart D, Jerman JC. Anandamide: an endogenous activator of the vanilloid receptor. Trends Pharmacol Sci. 2000;21:134. doi: 10.1016/s0165-6147(00)01459-0. [DOI] [PubMed] [Google Scholar]
- 12.Movahed P, Jonsson BA, Birnir B, Wingstrand JA, Jorgensen TD, Ermund A, Sterner O, Zygmunt PM, Hogestatt ED. Endogenous unsaturated C18 N-acylethanolamines are vanilloid receptor (TRPV1) agonists. J Biol Chem. 2005;280:38496–38504. doi: 10.1074/jbc.M507429200. [DOI] [PubMed] [Google Scholar]
- 13.Numazaki M, Tominaga M. Nociception and TRP Channels. Curr Drug Targets CNS Neurol Disord. 2004;3:479–485. doi: 10.2174/1568007043336789. [DOI] [PubMed] [Google Scholar]
- 14.Cuypers E, Yanagihara A, Karlsson E, Tytgat J. Jellyfish and other cnidarian envenomations cause pain by affecting TRPV1 channels. FEBS Lett. 2006;580:5728–5732. doi: 10.1016/j.febslet.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zlotnick BA, Hintz S, Park DL, Auerbach PS. Ciguatera poisoning after ingestion of imported jellyfish: diagnostic application of serum immunoassay. Wilderness Environ Med. 1995;6:288–294. doi: 10.1580/1080-6032(1995)006[0288:cpaioi]2.3.co;2. [DOI] [PubMed] [Google Scholar]


