Abstract
Purpose
To describe a quick and simple “small‐bubble” technique to immediately determine the success of attaining complete Descemet's membrane (DM) separation from corneal stroma through Anwar's “big‐bubble” technique of deep anterior lamellar keratoplasty (DALK) for complete stromal removal.
Methods
A partial trephination was followed by a lamellar dissection of the anterior stroma. Deep stromal air injection was then attempted to achieve the big bubble to help separate the stroma from the DM. To confirm that a big bubble had been achieved, a small air bubble was injected into the anterior chamber (AC) through a limbal paracentesis. If the small bubble is then seen at the corneal periphery, it confirms that the big‐bubble separation of DM was successful because the convex nature of the bubble will cause it to protrude posteriorly, forcing the small AC bubble to the periphery. If the small AC bubble is not seen in the corneal periphery, this means that it is present in the centre, beneath the opaque corneal stroma, and therefore the big bubble has not been achieved.
Results
We used the small‐bubble technique to confirm the presence of the big bubble in three (one keratoconus, one interstitial keratitis and one dense corneal scar) out of 41 patients who underwent DALK. The small‐bubble technique confirmed that the big bubble was achieved in the eye of all three patients. Complete stromal removal with baring of the DM was achieved, and postoperatively all three eyes achieved best corrected vision of 6/6.
Conclusion
The small‐bubble technique can be a useful surgical tool for corneal surgeons attempting lamellar keratoplasty using the big‐bubble technique. It helps in confirming the separation of DM from the deep stroma, which is important in achieving total stromal replacement. It will help to make the transition to lamellar keratoplasty smoother, enhance corneal graft success and improve visual outcomes in patients.
Keywords: deep anterior lamellar keratoplasty, big bubble, small bubble, DALK
Anterior lamellar keratoplasty involves heterotopic lamellar replacement of the anterior layers of the cornea while retaining the posterior layers of the recipient's eye, including the posterior stroma, DM and the endothelial layer. A major disadvantage of lamellar keratoplasty as opposed to penetrating keratoplasty (PKP) for optical reasons is the irregularity of the corneal stromal bed, which occurs following manual lamellar dissection techniques, and this problem limits the final best visual acuity. Different approaches have been devised to overcome this problem, including the use of microkeratomes1,2 to achieve smoother lamellar dissections and transplanting full thickness stroma onto bare DM3. To completely obviate this problem, a variety of surgical techniques have been described for performing “maximum depth” DALK, in which complete stromal removal with baring of DM is attempted, at least in the central visual axis.3,4,5,6,7,8 These include techniques using viscoelastic9,10 and Anwar's big‐bubble technique using air to cleave the stroma from the DM.3 Anwar's big‐bubble technique for DALK has good visual outcomes for keratoconus.3 This could be because of the presence of a smooth donor‐to‐recipient interface between the DM and the donor stroma, which reduces interface irregularities.
Although Anwar's air dissection is an established and efficient technique for reproducibly detaching the DM from the corneal stroma, injecting air into the stroma, which precedes DM separation, invariably produces a dense white overlying opacity, and if is extensive, it often precludes visualization of the AC. The classic circular silvery sheen of the “big bubble” is often masked, and it often it is not possible to ascertain whether complete air separation of DM has taken place. To overcome this problem, endoscopic visualization has been used.11,12 We describe here a simple and elegant technique, which we name the “small‐bubble technique”, that reliably delineates the extent of the big bubble without the use of ancillary instruments to increase the success of the “big‐bubble” technique.
Methods
Patients
Out a total of 41 patients who underwent the DALK procedure by the Anwar method in our centre, three patients required the small‐bubble technique to help confirm the presence of the big bubble. The presence of the big bubble could not be ascertained in these cases because of either the presence of intrastromal air after injection (in patient number 1) or the primary pathology (in patients 2 and 3), which precluded a view of the big bubble.
Surgical technique
After performing partial‐thickness trephination with a Hanna trephine (Moria, France) set at 60–70% of the preoperative stromal pachymetry, manual dissection and excision of the anterior stroma is initiated with a Mini‐Crescent blade (1.25 mm, Sharpoint, UK) and completed by the crescent blade (2.25 mm, BD Visitec, UK). In keratoconus and chemical‐injury patients, we set the trephine to cut to a shallower depth. The diameter of the trephine is based on the size of the cone in the keratoconus. Anwar's “big‐bubble” technique is then initiated with the use of a 30‐gauge needle attached to a 3 ml syringe. The needle tip, with the bevel facing down, is carefully inserted tangentially into paracentral cornea to an approximate depth of 90% stromal thickness. Under direct visual control, the needle is carefully advanced and a firm injection of approximately 0.5 ml of air is performed.
The achievement of a big bubble is usually confirmed by the rapid appearance of a silvery opaque circle representing the big bubble's edges, delineating the size of the bubble achieved. The size of big bubble can vary depending on the amount of air that has been injected. Further injection of air into the bubble can enlarge the bubble if required. It is adequate to bare the DM in the central 5 mm of the cornea for the patient to have a good visual result. The peripheral stroma then needs to be dissected to a depth of about 10% to allow a full thickness donor graft to be placed, after removal of the donor DM and endothelium. Unlike the appearance of a big bubble, intrastromal air appears white rather than silvery, with irregular, fibrillary edges. If this intrastromal air extends peripherally, it will obscure the big bubble, and it may be impossible to ascertain whether DM separation has truly occurred. It is in this situation that our technique would be helpful.
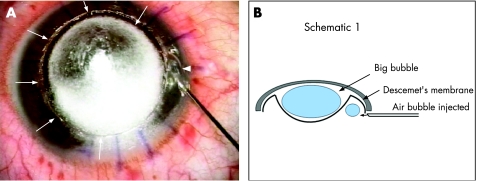
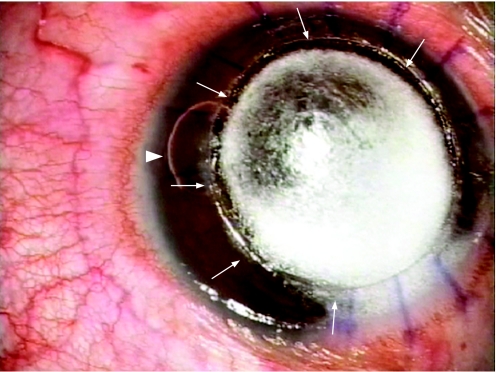
Our technique involves injecting a small amount of air into the AC at the limbus with a 27‐ or 30‐gauge needle (fig 1A). The small bubble cannot be injected before injecting the big bubble because the breach in the DM while making the paracentesis will prevent the big bubble from forming. Visualization of this is best achieved by selecting a portion of the limbus where intrastromal air does not obscure the peripheral chamber angle, and care needs to be taken when advancing the needle to visualize the needle tip when injecting air, so as to avoid inadvertent perforation of an unseen big bubble. The amount of air to be injected is small, the objective being to achieve an AC air bubble of approximately 2 mm in diameter. If the small bubble remains visible at the periphery, this suggests that the AC periphery is the highest point of the AC, and thus confirms that a central big bubble is present, with its posterior convexity bulging centrally (fig 1B). More clues to this may be the shape of the bubble, which may assume a “kidney‐bean” configuration and which can be made to travel completely around the corneal periphery by circular tilting of the globe, demonstrating the annular or “doughnut”‐shaped configuration of the AC periphery (fig 2). If, by contrast, the small bubble immediately disappears upon injection, this means it has moved centrally to the highest point of the AC, in this case, the central AC, and therefore no central big bubble has been achieved. In this situation, a repeat air injection should be performed, and, if a big bubble has now been achieved, the small bubble will migrate to the periphery and appear again. This technique is helpful if the big bubble is achieved after air injection. The success rate for achieving the big bubble has been quoted in previous publications to be 60–70%.13,14 In the event that even after multiple air injections the big bubble is not achieved, then a manual dissection of the remaining stroma is done. It should, however, be noted that this technique is less useful if there is any pathology, such as peripheral anterior synechiae, that interferes with the movement of the air bubble in the AC.
Figure 1 (A) Intraoperative photograph showing the injection of a small air bubble into the anterior chamber with a 27‐gauge needle (arrowhead); multiple arrows indicate the peripheral extent of the “big bubble”. (B) Schematic diagram in cross section showing the “big bubble” with its posterior convexity, which causes the “small bubble” to be present at the periphery of the anterior chamber.
Figure 2 By manipulating the globe, the “small bubble” (arrowhead) helps to delineate the extent of the “big bubble” (arrows).
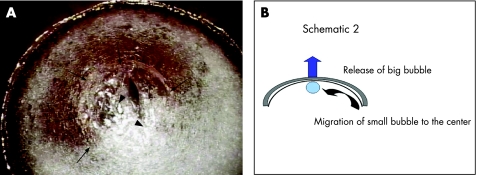
Once the big bubble has been confirmed, it is released by carefully incising the posterior stroma centrally to gain direct excess to the DM. Rapid collapse of the big bubble now causes migration of the small bubble towards the centre (fig 3A), and it will be seen to immediately appear beneath the posterior stroma when the big bubble's air is lost (fig 3B). Careful removal of the posterior stroma up to the margins of the lamellar trephination is now relatively easily achieved using blunt‐tipped corneal scissors because of the complete separation of the DM. Because there is complete removal of the recipient stroma with this technique, there is no need to perform stromal lamellar dissection of the donor. Donor tissue is trephined to the same diameter as the recipient bed, and DM membrane is usually easily stripped away with Macpherson's forceps or a dry Wexel sponge. This is then positioned onto the bare DM of the recipient bed for suturing.
Figure 3 (A) Schematic diagram in cross section showing the migration of the small bubble to the centre (curved arrow) after releasing the “big bubble” (block arrow), which is the highest point in the anterior chamber. (B) The “small bubble” migrates to the centre (arrows), after the incision of the posterior stroma to release the “big bubble” (arrowheads).
Case reports
Patient number 1
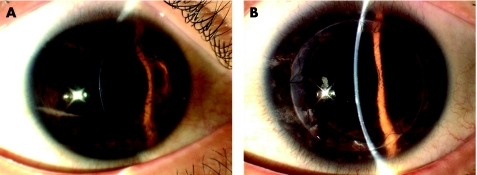
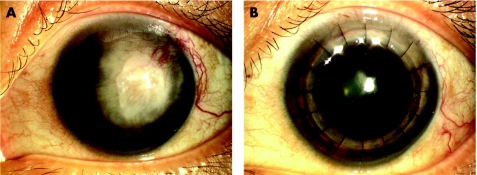
A 24‐year‐old Indian man with a clinical diagnosis of keratoconus was referred to us because of his intolerance of rigid gas‐permeable contact lenses. There was no history of previous surgery. His best corrected visual acuities were 6/18 in his right eye and 6/24 in his left eye. Slit‐lamp examination revealed clinical findings of advanced keratoconus (fig 4A). Keratometric values were 57 D in the right eye and 63 D in the left eye. In view of the clinically advanced keratoconus, a DALK was performed on the patient in his left eye.
Figure 4 (A) Preoperative slit‐lamps photograph of patient number 1 showing the advanced keratoconus in his left eye. (B) Postoperative photograph of the same patient one year later showing a clear DALK with a BCVA of 6/6.
Intraoperatively, the stromal‐air injection resulted in intrastromal air characterised by dense white opacity with fibrillary edges instead of the the explosive appearance of a white semi‐opaque disk for a big bubble.3 The small bubble helped confirm the presence of the big bubble, and dissection up to the DM was done. Donor cornea was then sutured on to the recipient bed. His postoperative course was uneventful. On the 6‐month follow up visit, his BCVA was 6/6 (fig 4B).
Patient number 2
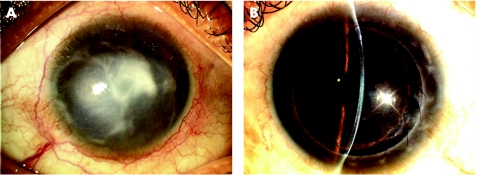
The second patient was a 42‐year‐old Chinese woman with a 9‐year of recurrent herpes simplex virus (HSV) stromal keratitis, following which she developed into a dense leucoma in her left eye (fig 5A). She presented with visual acuity of hand movements and was keen on visual rehabilitation. Her eye had been quiescent for the past year without any recurrence of HSV keratitis. She underwent DALK for her left eye. Intraoperatively the vascularized leucoma prevented visualisation of the big bubble. The small‐bubble technique helped to completely remove the pathological stroma up to the DM after confirmation of the prescence of the big bubble. Postoperatively she was maintained on oral Acyclovir 400 mg twice a day for 1 year. On her last follow‐up visit one year later, she had a BCVA of 6/6 with a clear lamellar graft (fig 5B).
Figure 5 (A) Slit‐lamp photographs of the second patient showing the dense leucoma caused by HSV stromal keratitis. (B) Postoperative photograph of the patient with a clear donor graft.
Patient number 3
Similarly, our third patient had a dense corneal scar (fig 6A), which obscured the view of the big bubble intraoperatively. The small‐bubble technique was again useful for establishing the existence of the big bubble. The dissection of the posterior stroma was completed, and it was replaced with a clear donor lamellar graft (fig 6B).
Figure 6 (A, B) Preoperative and postoperative photographs of patient number 3 showing a dense corneal scar, and, after DALK, a clear lamellar graft.
Complete stromal removal was accomplished in all three eyes, and the visual outcomes in these patients are given in Table 1.
Table 1 Presenting and final best corrected visual acuity (BCVA) of the patients who underwent deep anterior lamellar keratoplasty using the “big bubble” with intraoperative use of the “small‐bubble technique”.
| Patient | Age/sex | Diagnosis | Presenting visual acuity | Postoperative BCVA | Refraction |
|---|---|---|---|---|---|
| 1 | 24/M | Keratoconus | 6/24 | 6/6 | −6.50/−2.50×150 |
| 2 | 42/F | HSV stromal keratitis | HM | 6/6 | −2.75/−2.25×165 |
| 3 | 36/F | Corneal scar (contact lens keratitis) | HM | 6/6 | +3.25/−3.0×35 |
Discussion
The advantages of retaining DM and endothelium in an anterior lamellar keratoplasty (less risk of allograft rejection and graft failure owing to endothelial cell loss compared with PK15,16) are often balanced against the poorer visual outcomes of conventional or manual lamellar surgery. However, recent reports indicate visual outcomes for DALK comparable to those for PKP, especially for keratoconus, stromal dystrophies and corneal scars.17,18,19,20,21 Hence, total stromal removal in DALK surgery, with its potential for better vision, is becoming a more popular option for the management of conditions without endothelial dysfunction. Additional advantages of lamellar keratoplasty are the extra‐ocular nature of this procedure and the greater wound strength. Endothelial cell counts of the donor are no longer critical; this is especially important in developing countries where there is a shortage of donor tissue.
The Anwar “big‐bubble” technique is perhaps the most dramatic, elegant and rapid form of achieving complete separation of DM from posterior stroma in DALK surgery. We prefer the big‐bubble technique to using viscoelastic for DM separation because the latter technique is more likely to cause significant enlargement of a small perforation. However, in advanced keratoconus and a thin recipient stroma, the big‐bubble technique might not be ideal for performing DALK owing to its higher chance of perforation.22
Teichmann and Anwar describe several signs that help the surgeon ascertain the presence of a “big bubble”. Firstly, blanching of the corneal stroma spreads in a wave‐like circular fashion with a true bubble. Secondly, a completed bubble frequently exhibits a feathery white band at its circular periphery. Thirdly, the anterior surface of the cornea “rises” as the bubble takes up space in the central cornea.3,4 If the big bubble has been achieved, the globe is firm on palpation. The above signs help to confirm the presence of the big bubble and are the only indications of it in the event that the peripheral stroma is opaque following the air injection and therefore it is not possible to visualise the small bubble.
Confirmation of achievement of the big bubble is important in DALK surgery because it determines the next step for the surgeon, that is, to go ahead with stromal removal or consider reinjecting air into the stroma. Our “small‐bubble” technique of injecting air into the AC to observe its behaviour is an elegant and simple method to confirm success of the big‐bubble injection.
Conclusion
The big‐bubble technique of baring the DM helps in the complete removal of the pathological stroma and its full thickness replacement with healthy donor stroma. It will be a useful surgical tool in the armament of corneal surgeons attempting to achieve the difficult transition from penetrating to lamellar surgery to enhance corneal graft success.
Footnotes
The authors do not have a proprietary or financial interest in the products used in the patients in this case series.
Competing interests: None declared.
References
- 1.Tan D T, Ang L P. Modified automated lamellar therapeutic keratoplasty for keratoconus: a new technique. Cornea 2006251217–1219. [DOI] [PubMed] [Google Scholar]
- 2.Tan D, Ang L P K. Automated lamellar therapeutic keratoplasty for post‐PRK corneal scarring and thinning. Am J Ophthal 20041381067–1069. [DOI] [PubMed] [Google Scholar]
- 3.Anwar M, Teichmann K D. Deep lamellar keratoplasty: surgical techniques for anterior lamellar keratoplasty with and without baring of Descemet's membrane. Cornea 200221374–438. [DOI] [PubMed] [Google Scholar]
- 4.Anwar M, Teichmann K D. Big‐bubble technique to bare Descemet's membrane in anterior lamellar keratoplasty. J Cataract Refract Surg 200228398–403. [DOI] [PubMed] [Google Scholar]
- 5.Archila E A. Deep lamellar keratoplasty dissection of host tissue with intrastromal air injection. Cornea. 1984–85 3217–218. [PubMed] [Google Scholar]
- 6.Sujita J, Kondo J. Deep lamellar keratoplasty with complete removal of pathologic stroma for vision improvement. Br J Ophthalmol 199791184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melles G R J, Lander F, Rietveld F J R.et al A new surgical technique for deep stromal, anterior lamellar keratoplasty. Br J Ophthalmol 199983327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsubota K, Kaido M, Monden Y.et al A new surgical technique for deep lamellar keratoplasty with single running suture adjustment. Am J Ophthalmol 19981261–8. [DOI] [PubMed] [Google Scholar]
- 9.Melles G R J, Remeijer L, Geerards A J M.et al A quick surgical technique for deep, anterior lamellar keratoplasty using visco‐dissection. Cornea 200019427–432. [DOI] [PubMed] [Google Scholar]
- 10.Shimmura S, Shimazaki J, Omoto M.et al Deep lamellar keratoplasty (DLKP) in keratoconus patients using viscoadaptive viscoelastics. Cornea 200524178–181. [DOI] [PubMed] [Google Scholar]
- 11.Teichmann K D, Anwar M. Endoscopic visualization to aid deep anterior lamellar keratoplasty. Eye 2005191233–1234. [DOI] [PubMed] [Google Scholar]
- 12.Moore J E, Herath G, Sharma A. Endoscopic visualization to aid deep anterior lamellar keratoplasty. Eye 200418188–191. [DOI] [PubMed] [Google Scholar]
- 13.Fogla R, Padmanabhan P. Results of deep lamellar keratoplasty using the big‐bubble technique in patients with keratoconus. Am J Ophthalmol 2006141254–259. [DOI] [PubMed] [Google Scholar]
- 14.Fontana L, Parente G, Tassinari G. Clinical outcomes after deep anterior lamellar keratoplasty using the big‐bubble technique in patients with keratoconus. Am J Ophthalmol 2007143117–124. [DOI] [PubMed] [Google Scholar]
- 15.Thompson R W, Jr, Price M O, Bowers P J.et al Long‐term graft survival after penetrating keratoplasty. Ophthalmology 20031101396–1402. [DOI] [PubMed] [Google Scholar]
- 16.Williams K A, Muehlberg S M, Lewis R F.et al Long‐term outcome in corneal allotransplantation. The Australian Corneal Graft Registry. Transplant Proc 199729983. [DOI] [PubMed] [Google Scholar]
- 17.Funnell C L, Ball J, Noble B A. Comparative cohort study of the outcomes of deep lamellar keratoplasty and penetrating keratoplasty for keratoconus. Eye 200620527–532. [DOI] [PubMed] [Google Scholar]
- 18.Kawashima M, Kawakita T, Den S.et al Comparison of deep lamellar keratoplasty and penetrating keratoplasty for lattice and macular corneal dystrophies. Am J Ophthalmol 2006142304–309. [DOI] [PubMed] [Google Scholar]
- 19.Watson S L, Ramsay A, Dart J K.et al Comparison of deep lamellar keratoplasty and penetrating keratoplasty in patients with keratoconus. Ophthalmology 20041111676–1682. [DOI] [PubMed] [Google Scholar]
- 20.Shimazaki J, Shimmura S, Ishioka M.et al Randomized clinical trial of deep lamellar keratoplasty vs penetrating keratoplasty. Am J Ophthalmol 2002134159–165. [DOI] [PubMed] [Google Scholar]
- 21.Panda A, Bageshwar L M, Ray M.et al Deep lamellar keratoplasty versus penetrating keratoplasty for corneal lesions. Cornea 199918172–175. [DOI] [PubMed] [Google Scholar]
- 22.Michieletto P, Balestrazzi A, Balestrazzi A.et al Factors predicting unsuccessful big bubble deep lamellar anterior keratoplasty. Ophthalmologica 2006220379–382. [DOI] [PubMed] [Google Scholar]