Abstract
Background
To describe autofluorescence patterns of choroidal melanocytic lesions using the Heidelberg Retinal Angiograph 2 system (HRA2).
Methods
20 patients with choroidal melanocytic lesions in the ocular fundus underwent ophthalmologic examination, fundus photography, autofluorescence and optical coherence tomography (OCT). Pathologic examination was performed on one enucleated eye with a large choroidal melanoma.
Results
15 patients had choroidal nevi and 5 had malignant choroidal melanoma (1 small, 1 medium and 3 large tumours). Choroidal nevi did not show any characteristic autofluorescence pattern, although secondary retinal pigment epithelium (RPE) changes, such as drusen and pigment epithelium detachment, appeared faintly hyperautofluorescent in 2 patients. Only the small malignant choroidal melanomas had prominent orange pigmentation, although all melanomas had an intense confluent hyperautofluorescent signal over the lesions. Pathology of one large malignant melanoma revealed lipofuscin underlying RPE.
Conclusion
Most nevi did not have characteristic hyperautofluorescent features, but choroidal melanomas seemed to have a pattern of confluent hyperautofluorescence. Therefore, autofluorescence may be a useful non‐invasive tool to assess lipofuscin in pigmented choroidal lesions, which may contribute to the diagnosis of malignancy. This hypothesis, however, remains to be confirmed in large prospective studies.
Choroidal melanoma is the most frequent primary intraocular tumour and the second most frequent malignant melanoma of the body.1 Although it is a malignancy of the melanocytic cells of the choroid, it directly affects the retinal pigment epithelium (RPE). This secondary epitheliopathy appears as areas of atrophy, drusen, lipofuscin accumulation, and localised detachment over the RPE with an intense phagocytic activity, which is responsible for changes in fundus colouration.2
Lipofuscin accumulates in RPE cells and in macrophages of malignant choroidal tumours3 and is usually seen as orange pigment over the lesion. Colour may vary from orange to brown or red–brown depending on the nature of the tumour, whether markedly melanotic or amelanotic. Moreover, orange pigment has been suggested to be a sign of malignancy.4
Fundus autofluorescence has been used by several authors to investigate the amount of lipofuscin in the RPE.5 The recently developed Heidelberg Retina Angiograph 2 system (HRA2) (Heidelberg Engineering, Heidelberg, Germany) provides better assessment of autofluorescence, as well as identification of the spatial distribution of lipofuscin‐mediated fundus autofluorescence in vivo.6
In this study, autofluorescence patterns of choroidal melanocytic lesions were investigated using the HRA2 system. Histological studies were also performed on one enucleated eye with a large choroidal melanoma to investigate tumoural lipofuscin content.
Materials and methods
This retrospective observational study was conducted with a consecutive case series of 20 patients with melanocytic lesions of the ocular fundus at Paulista School of Medicine, Federal University of Sao Paulo (EPM‐UNIFESP). This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of EPM‐UNIFESP. Patients with pigmented choroidal lesions and no previous treatment were included.
Lesions were classified clinically as benign or malignant by a blinded investigator according to the Collaborative Ocular Melanoma Study (COMS).1 Benign (choroidal nevi) lesions were less than 1 mm in height and less than 5 mm in basal diameter according to ultrasound measurements and had no risk factors for growth, such as symptoms, orange pigmentation, subretinal fluid, juxtapapillary location and hot spots on angiography. Malignant lesions were classified as small, medium or large.1 All patients underwent ophthalmic examination, colour fundus photography, autofluorescence studies and optical coherence tomography (STRATUS OCT3).
Autofluorescence (AF) imaging was performed with the Heidelberg Retina Angiograph 2 system (HRA2, Heidelberg, Germany).7 AF images were recorded at 488 nm wavelength using a barrier filter for detection of emitted light above 500 nm. At least 15 single AF images of 512×512 pixels were acquired. Several images were aligned and a mean image was calculated after detection and correction of eye movements using image analysis software. Abnormal autofluorescence was defined as either increased or decreased fundus autofluorescence in comparison with background fundus autofluorescence, and classified as either hyperautofluorescence or hypoautofluorescence.8 AF image readers were blinded to clinical findings and diagnoses.
Enucleation was performed in one patient with a diagnosis of large malignant choroidal melanoma, and the eye was sent to routine pathology and histochemical examination with techniques for lipofuscin determination: periodic acid Schiff (PAS), long Ziehl‐Neelsen, Sudan black B and fluorescence microscopy.
Results
Choroidal nevi
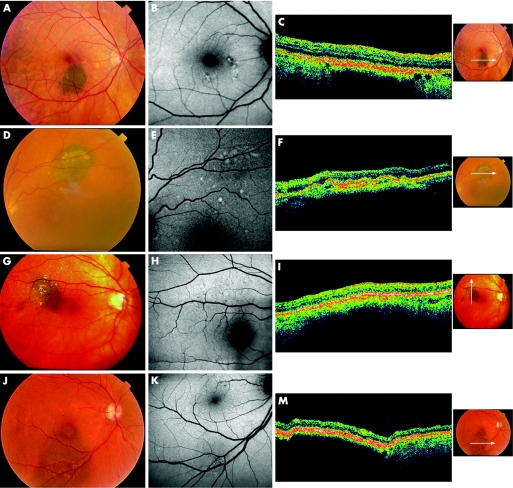
The 15 lesions classified as nevi had a brown to grey appearance, mainly in the central retina. Lesions ranged from homogeneous, small, flat and well delimited to more heterogeneous with secondary changes overlying the nevus, such as drusen and pigment mottling (fig 1). AF showed a normal pattern of background fundus fluorescence with no corresponding areas of hyperfluorescence or hypofluorescence over the nevi. Two patients, however, had faint localised hyperautofluorescent areas that corresponded to small drusen and drusenoid pigment epithelium detachment, which were also found elsewhere in the retina (fig 1). Ocular coherence tomography (OCT) of three patients showed RPE irregularities that corresponded to drusen and drusenoid pigment epithelium detachment (fig 1), which correlated with increased AF images.
Figure 1 Choroidal nevi. Ocular fundus photographs of choroidal nevi (A, D, G, J). Autofluorescence shows a normal pattern of background fundus fluorescence (B, E, H, K). Hyperautofluorescent areas correspond to drusen and drusenoid pigment epithelium detachment (D, H, F). OCT shows thickening of RPE‐choriocapillaris complex with posterior blockage of reflectivity (C, F, I, M) and drusen and drusenoid pigment epithelium detachment.
Choroidal melanoma
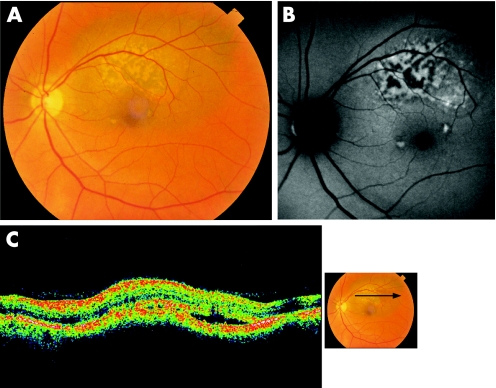
One patient was diagnosed with a small malignant choroidal melanoma, seen as a pigmented lesion, with orange pigment in a reticular and confluent pattern throughout its surface. We identified a confluent plaque‐like hyperautofluorescent area over the lesion, which corresponded to orange pigment (fig 2). OCT revealed thickening, elevation of the RPE‐choriocapillaris complex, irregularities over the RPE and subretinal fluid.
Figure 2 Small choroidal melanoma. A. Ocular fundus photograph shows orange pigment throughout the lesion surface. B. Autofluorescence shows a confluent plaque‐like hyperautofluorescent area. C. OCT shows subretinal fluid sparing the fovea and overlying the retina with mildly increased reflectivity.
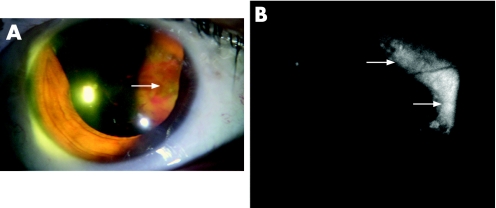
The medium choroidal melanoma was a large heterogeneous brown‐to‐grey elevated pigmented lesion with increased pigment at the apex. Superficial fibrosis and subretinal fluid were noted, and AF showed a plaque‐like hyperautofluorescent signal throughout the surface of the lesion, which corresponded to the areas of increased pigment and fibrous metaplasia. OCT revealed a serous detachment of the overlying neural retina with intraretinal cysts, thickening and loss of the internal retina architecture. The other patients with choroidal malignant melanomas were diagnosed with large lesions, and AF also revealed a confluent plaque‐like hyperautofluorescent area with central hypoautofluorescence (fig 3).
Figure 3 Large choroidal melanoma. A. Colour photograph shows a very large heterogeneous anterior pigmented choroidal lesion with superficial haemorrhage. B. A confluent plaque‐like hyperautofluorescent area is also seen.
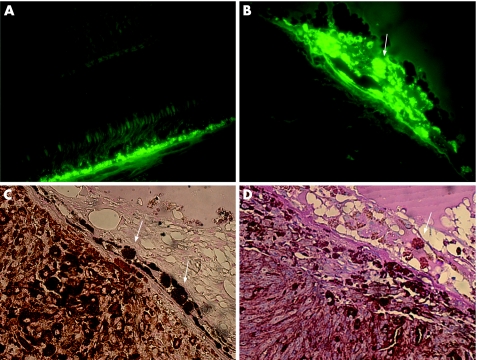
The histopathological study of one enucleated eye revealed cell proliferation in the choroidal lesion composed of spindle and epithelioid malignant melanocytic cells. Hyperautofluorescent deposits within the RPE and macrophages were overlying the choroidal melanoma, which stained positive for Sudan black B, PAS and long Ziehl‐Neelsen (fig 4). The final diagnosis confirmed the initial hypothesis of malignant choroidal melanoma and confirmed the presence of lipofuscin throughout the tumour.
Figure 4 Large choroidal melanoma: pathology studies. Fluorescence microscopy shows increased fluorescence throughout the RPE of adjacent normal retina (A; 400× magnification) and in situ hyperautofluorescent deposits under RPE cells over choroidal mass (B; 600× magnification). RPE cells and macrophages overlying the tumour stained for Sudan black B (C; 400× magnification) and PAS (D; 400× magnification).
Discussion
Large tumours are not difficult to characterise as malignant, but the diagnosis and management of small choroidal melanomas remains controversial. Documented growth of a recently diagnosed small choroidal melanocytic lesion is considered a hallmark for the diagnosis of choroidal melanomas. Quantitative (tumour size) and qualitative factors, such as symptoms, drusen, subretinal fluid, RPE changes, juxtapapillary location and orange pigmentation, may be predictive of tumour growth in patients with small choroidal melanocytic lesions.9
Secondary epitheliopathy and particularly orange pigmentation overlying the lesion have been described as major risk factors for progression to melanoma. These findings are, therefore, key features in serial evaluations.2,4,10,11 In 1974, Font and colleagues described the nature of the orange pigment using histochemistry and electron microscopy studies, and reported that both proliferated RPE cells and macrophages were seen in clusters within tumours containing lipofuscin.12 Singh and colleagues recently studied a large series of small choroidal melanocytic lesions and found that orange pigment significantly correlated with the risk of subsequent growth.9
We studied 15 patients with a diagnosis of choroidal nevi and epitheliopathy in a range of sizes and degrees, and no characteristic patterns of AF were found for most of them. Keilhauer and Delori also found no AF pattern for choroidal nevi, which usually appeared as normal background fundus on AF.13 It is still unclear whether transforming lesions can be identified by a newly diagnosed hyperautofluorescent area over these lesions.
One patient in our series had a large choroidal nevus with multiple small drusen and drusenoid pigment epithelium detachment, which appeared as faintly hyperautofluorescent. Karadimas and Bouzas (2005) demonstrated that, in cases of drusenoid pigment epithelium detachment, AF patterns may vary from hyperautofluorescence to hypoautofluorescence.14 Nevertheless, hyperautofluorescence in these cases may not be a sign of lipofuscin accumulation in the RPE, such as in choroidal melanomas; rather, it may be caused by different fluorophores inside the fluid of drusenoid detachments, or even by detached RPE cells within the fluid. Long‐term evaluations should be conducted to determine the predictive and prognostic value of these findings in malignant transformation.
AF areas in small, medium and large melanomas shared common characteristics. They showed a homogeneous increase in the intensity of fluorescence, with a confluent plaque‐like configuration. As small lesions start to damage the RPE and the underlying choriocapillaris, lipofuscin accumulates and hyperautofluorescence can be seen concurrently or even before the appearance of orange pigment on ocular fundus photography. However, this technique is designed for relatively flat retinas, and it is possible that highly elevated retinas caused by large melanomas may produce artefactual changes. Moreover, areas of increased pigmentation and fibrous metaplasia also showed increased AF in medium and large tumours.
Several areas of increased fluorescence were seen throughout the RPE, as well as in underlying macrophages within the tumour. Lohmann and colleagues studied the endogenous fluorescence of ocular malignant melanomas and found that lipofuscin granules were cleaved off and broken into small remnants in the melanoma.15 Our histochemical and fluorescent microscopy studies confirmed the presence of lipofuscin throughout a large melanoma and some areas of accumulated lipofuscin, similar to the findings by Lohmann. Therefore, we suggest that these deposits may be responsible for the large area of hyperautofluorescence identified clinically.
In summary, choroidal melanomas may have a pattern of confluent hyperautofluorescence over the tumour. Most nevi did not have hyperautofluorescent areas, although two patients had faint hyperfluorescent signals that were clearly correlated with small drusen and seen throughout the retina. Therefore, AF studies may be a useful non‐invasive tool to assess the presence of lipofuscin in pigmented choroidal lesions, especially nevi and small choroidal melanomas, which may contribute to the diagnosis of malignant lesions. However, prospective studies with a larger number of patients are needed and are currently being carried out to confirm our initial hypothesis.
Abbreviations
AF - autofluorescence
HRA2 - Heidelberg Retinal Angiograph 2 system
OCT - optical coherence tomography
PAS - periodic acid Schiff
RPE - retinal pigment epithelium
Footnotes
Funding: Vision Institute, UNIFESP‐EPM.
Competing interests: None declared.
References
- 1.Margo C E. The Collaborative Ocular Melanoma Study: an overview. Cancer Control 200411304–309. [DOI] [PubMed] [Google Scholar]
- 2.Damato B E, Foulds W S. Tumor‐associated retinal pigment epitheliopathy. Eye 19904382–387. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy C J, Rakoczy P E, Constable I J. Lipofuscin of the retinal pigment epithelium: a review. Eye 19959763–771. [DOI] [PubMed] [Google Scholar]
- 4.Smith L T, Irvine R. Diagnostic significance of orange pigment accumulation over choroidal tumors. Am J Ophthalmol 197376212–216. [DOI] [PubMed] [Google Scholar]
- 5.Lois N, Halfyard A S, Bunce C.et al Reproducibility of fundus autofluorescence measurements obtained using a confocal scanning laser ophthalmoscope. Br J Ophthalmol 199983276–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holz F G, Bellmann C, Margaritidis M.et al Patterns of increased in vivo fundus autofluorescence in the junctional zone of geographic atrophy of the retinal pigment epithelium associated with age‐related macular degeneration. Graefe's Arch Clin Exp Ophthalmol 199923745–152. [DOI] [PubMed] [Google Scholar]
- 7.Von Ruckmann A, Fitzke F W, Bird A C. Distribution of fundus autofluorescence with a scanning laser ophthalmoscope. Br J Ophthalmol 199579407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delori F C. Autofluorescence method to measure macular pigment optical densities fluorometry and autofluorescence imaging. Arch Biochem Biophys 2004430156–162. [DOI] [PubMed] [Google Scholar]
- 9.Singh A D, Mokashi A A, Bena J F.et al Small choroidal melanocytic lesions: features predictive of growth. Ophthalmology 20061131032–1039. [DOI] [PubMed] [Google Scholar]
- 10.Augsburger J J, Schroeder R P, Territo C.et al Clinical parameters predictive of enlargement of melanocytic choroidal lesions. Br J Ophthalmol 198973911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shields C L, Cater J, Shields J A.et al Combination of clinical factors predictive of growth of small choroidal melanocytic tumors. Arch Ophthalmol 2000118360–364. [DOI] [PubMed] [Google Scholar]
- 12.Font R L, Zimmerman L E, Armaly M F. The nature of the orange pigment over a choroidal melanoma. Arch Ophthalmol 197491359–362. [DOI] [PubMed] [Google Scholar]
- 13.Keilhauer C N, Delori F C. Near‐infrared autofluorescence imaging of the fundus: visualization of ocular melanin. Invest Ophthalmol Vis Sci 2006473556–3564. [DOI] [PubMed] [Google Scholar]
- 14.Karadimas P, Bouzas E A. Fundus autofluorescence imaging in serous and drusenoid pigment epithelial detachments associated with age‐related macular degeneration. Am J Ophthalmol 2005401163–1165. [DOI] [PubMed] [Google Scholar]
- 15.Lohmann W, Wiegand W, Stolwijk T R.et al Endogenous fluorescence of ocular malignant melanomas. Ophthalmologica 19952097–10. [DOI] [PubMed] [Google Scholar]