Abstract
Purpose
A recently devised 3D computer‐automated threshold Amsler grid test was used to identify early and distinctive defects in people with suspected glaucoma. Further, the location, shape and depth of these field defects were characterised. Finally, the visual fields were compared with those obtained by standard automated perimetry.
Patients and methods
Glaucoma suspects were defined as those having elevated intraocular pressure (>21 mm Hg) or cup‐to‐disc ratio of >0.5. 33 patients and 66 eyes with risk factors for glaucoma were examined. 15 patients and 23 eyes with no risk factors were tested as controls. The recently developed 3D computer‐automated threshold Amsler grid test was used. The test exhibits a grid on a computer screen at a preselected greyscale and angular resolution, and allows patients to trace those areas on the grid that are missing in their visual field using a touch screen. The 5‐minute test required that the patients repeatedly outline scotomas on a touch screen with varied displays of contrast while maintaining their gaze on a central fixation marker. A 3D depiction of the visual field defects was then obtained that was further characterised by the location, shape and depth of the scotomas. The exam was repeated three times per eye. The results were compared to Humphrey visual field tests (ie, achromatic standard or SITA standard 30‐2 or 24‐2).
Results
In this pilot study 79% of the eyes tested in the glaucoma‐suspect group repeatedly demonstrated visual field loss with the 3D perimetry. The 3D depictions of visual field loss associated with these risk factors were all characteristic of or compatible with glaucoma. 71% of the eyes demonstrated arcuate defects or a nasal step. Constricted visual fields were shown in 29% of the eyes. No visual field changes were detected in the control group.
Conclusions
The 3D computer‐automated threshold Amsler grid test may demonstrate visual field abnormalities characteristic of glaucoma in glaucoma suspects with normal achromatic Humphrey visual field testing. This test may be used as a screening tool for the early detection of glaucoma.
Glaucoma is the second leading treatable cause of blindness in the USA, and one of the top three causes of blindness worldwide. An estimated 66.8 million people were projected to suffer from glaucoma in the world in the year 2000, with 6.7 million suffering from bilateral blindness.1 In the USA, an estimated 1.6 million people aged 40 years or older have primary open angle glaucoma (POAG). However, an estimated 50% of glaucoma in the USA is undiagnosed.2 Furthermore, it is estimated that up to 50% of axons can be lost before any visual field defect is apparent.3 Since standard glaucoma therapy is associated with a decrease in the incidence of visual field loss,4 earlier diagnosis and therapy may significantly preserve visual function and reduce blindness caused by this disease.
Traditional clinical tonometry has a low diagnostic specificity and sensitivity, rendering it alone inadequate for glaucoma screening.5 Therefore, a sensitive and widely available screening method that can reliably and quickly determine the extent and location of relative eccentric scotomas would be valuable. The Amsler grid, developed by Mark Amsler in the 1920s, was designed specifically to analyse visual field defects in the central 10 degrees.6,7 It utilises a suprathreshold target to test the central 10 degrees. This test is good for detecting metamorphopsia, but is not sensitive for the detection of relative scotomas.7
The detection of relative scotomas was advanced by the development of threshold Amsler grid testing by Wall and Sadun.7,8 The yield of scotoma detection in the central 10 degrees is significantly increased by using cross‐polarising filters to vary perceived luminance. Accordingly, it was found to be much more sensitive for shallow scotoma detection in patients with macular disease and optic neuropathies.7,8
Threshold Amsler grid testing of the central 25 degrees or more can now be easily and rapidly accomplished with the 3D computer‐automated threshold Amsler grid test (3D‐CTAG), recently introduced by Fink and Sadun,9,10,11,12,13,14 which uses an Amsler grid with varying contrast. Akin to conventional perimetry, this test also adds a third (Z) dimension in terms of contrast sensitivity to the X–Y coordinates of the visual field. However, it is unique in having a much higher spatial resolution of 1 degree or less, both horizontally and vertically. We believe this increases its sensitivity in characterising and distinguishing optic neuropathies and maculopathies.
The 3D‐CTAG has already demonstrated promise as an effective tool in accurately evaluating, characterizing and monitoring visual field defects in patients with age‐related macular degeneration (AMD), with the potential as a screening tool for the early diagnosis of AMD.15
The objectives of this pilot study are (1) to test the potential of the 3D‐CTAG for a sensitive and accurate screening method for glaucoma‐suspect patients with demonstrated ocular hypertension or physiologic cupping and normal Humphrey visual field tests; (2) to establish the location, shape, pattern and extent of the visual field defects; and (3) to compare the results to those obtained by standard Humphrey visual field tests (ie, achromatic standard or SITA standard 30‐2 or 24‐2).
Patients and methods
Fifty‐five patients from the Doheny Eye Institute, Keck School of Medicine at the University of Southern California were enrolled as test subjects. Over 500 glaucoma‐suspect patients who were being followed at the Doheny Eye Institute were contacted by telephone to participate in the programme or directly enrolled from the glaucoma clinic. Institutional review board approval was obtained. Glaucoma suspects were defined as those with intraocular pressure >21 mm Hg or cup‐to‐disc ratio of >0.5, and a normal achromatic standard or SITA standard 30‐2 or 24‐2 Humphrey's visual field (HVF) test. Visual fields with scattered, non‐specific defects consistent with minor artefact were included as normal. However, 11 HVF tests were eliminated from the study due to defects suspicious for but not characteristic of glaucomatous loss. These included superior or inferior peripheral defects involving two to three test points of −4 to −8 dB, or an isolated nasal defect of −4 to −8 dB or greater. Patients who had additional eye diseases were excluded from the study. A total of 100 eyes were tested using the 3D computer‐automated visual field test. Seventy‐seven eyes with the defined risk factors for glaucoma were examined and 66 included. In addition, a total of 23 eyes of normal subjects with no risk factors for glaucoma were tested as controls. Participation was voluntary and informed consent was obtained for each patient.
We utilised an IBM‐compatible Pentium II PC running Windows 98 in conjunction with a 17″ touch‐sensitive computer monitor. The same cathode ray tube (CRT) monitor was used without any subsequent changes in the brightness and luminance settings, both throughout each examination and between examinations. The CRT monitor was turned on at least 30 minutes prior to the testing sessions to ensure brightness and luminance stability. Each patient was positioned in front of the computer monitor at a distance of 30 cm, maintainable by a chin headrest. This distance from the central fixation marker on the computer screen (0 degrees horizontally and 0 degrees vertically from fixation) determined the angle of the visual field tested. An eye‐cover was used to occlude the eye that was not being examined. When necessary, refractive correction was used with the patient's contact lenses or eyeglasses.
An Amsler grid at a pre‐selected greyscale (ie, contrast) level and angular resolution was displayed by the 3D‐CTAG software. All patients were tested with the same user‐defined contrast levels (see below) at an angular resolution of one degree, respectively. The patient was asked to focus on an automatically changing stimulus (random ASCII‐character set) of constant size and brightness at the centre of the grid to suppress the central Troxler effect and to maintain fixation and attention. The patient was subsequently asked to mark the areas on the grid that were missing from his field of vision by tracing the border of this region with his finger on the touch screen while maintaining fixation. The patient's response was recorded by the computer program. After completion of a particular greyscale level, the contrast of the Amsler grid with respect to the constant, black background of the computer screen was changed and the patient had to perform the same task as outlined above. This procedure was repeated for a total of five different contrast levels (20%, 40%, 60%, 80% and 100%), with one level being the standard Amsler grid, in our case a white grid on a black background, that is, 100% (absolute) contrast difference with respect to the background of the computer screen. Areas of 0% contrast sensitivity were then defined as the inability of a patient to recognise an Amsler grid displayed at 100% contrast difference in those areas. Areas of 100% contrast sensitivity were defined as the ability of a patient to recognise an Amsler grid displayed at the lowest preset contrast, that is, the darkest grid. Changing the Amsler grid contrast resulted in horizontal “cuts” through the hill‐of‐vision at various contrast sensitivity levels. The results of each tested level were recorded, and, in combination, resulted in an instantaneous 3D depiction of the central (25 degrees) hill‐of‐vision both as topographical contour rings and 3D wire/shaded diagrams. The results were subsequently used to establish the location, extent, slope, depth and shape of the scotomas in the glaucoma‐suspect patients. The data were compared to Humphrey visual field tests (ie, achromatic standard or SITA standard 30‐2 or 24‐2). The examination was repeated for each eye tested.
To calculate the 3D‐CTAG reproducibility, we assumed no difference between right and left eye testing. Out of a total of 77 eyes (39 right and 38 left eyes) considered, 15 had been examined once, 18 twice and 44 three times. Only the exams tested at least twice were used to evaluate the reproducibility of the novel 3D test (62). Since three exams of the same eye, consisting of one initial test and two retests, contribute more to the reproducibility than two exams of the same eye, consisting of one initial test and one retest, we considered a weighted mean and standard deviation for the reproducibility: A total of three exams (a, b and c) per eye allows for three comparisons: a–b, a–c and b–c. A total of two exams (a and b) per eye allows for one comparison: a–b. Therefore, the scoring was established as follows: all three out of three possible comparisons “similar” (short: 3/3) resulted in a score of 3, 2/3 in 1, 1/3 in 0, 2/2 in 1, and 1/2 in 0. It should be pointed out that this is a very conservative approach. The “similarity” of examination results was defined qualitatively and independently by three experts (two glaucoma specialists, one neuro‐ophthalmologist) with respect to presence or absence of defects, and scotoma location, size, shape and depth.
The visual field data obtained with the 3D‐CTAG were classified according to the Ocular Hypertensive Treatment Study (OHTS).16
Results
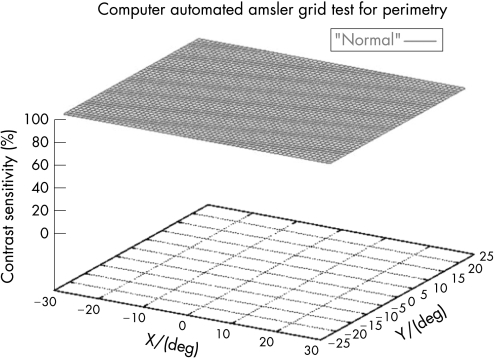
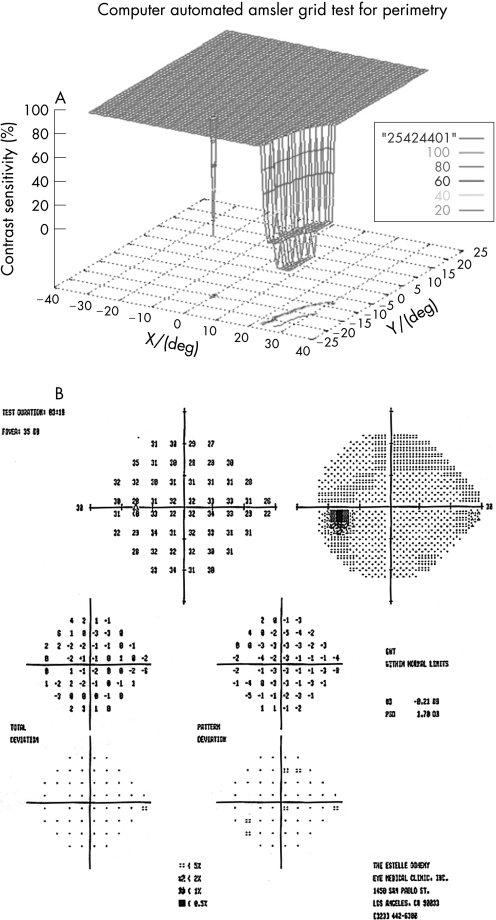
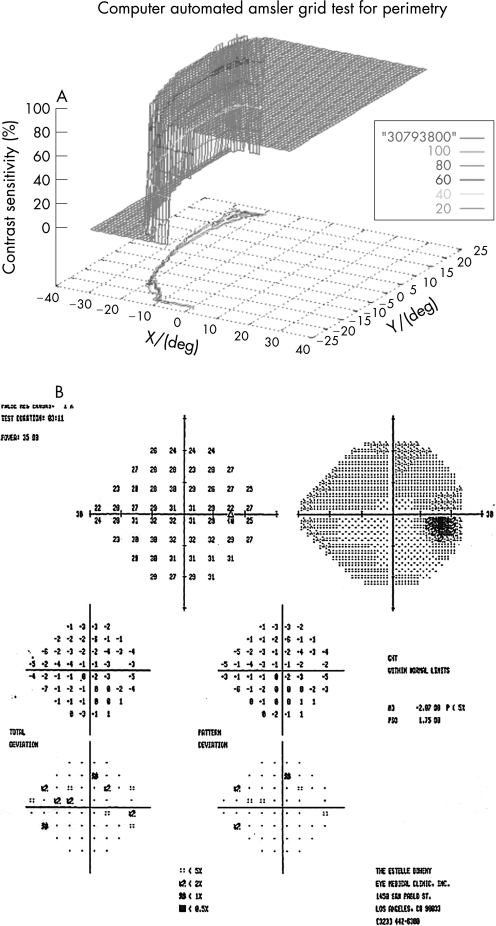
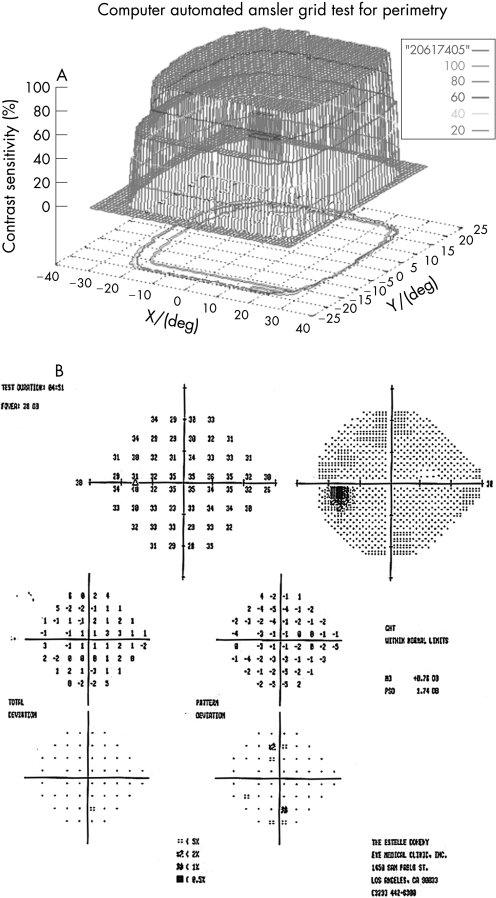
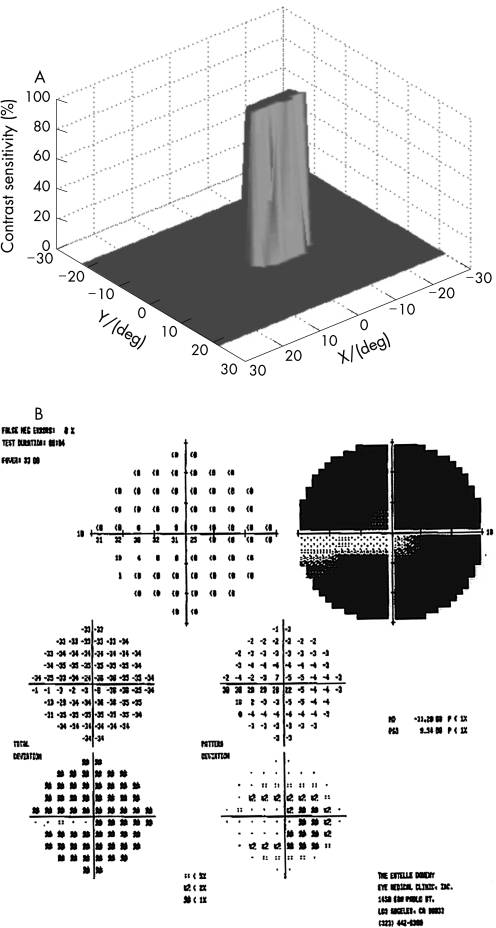
One hundred eyes from 55 patients were examined with the 3D‐CTAG in 3–5 minutes per eye. Seventy‐seven eyes from 40 patients with ocular hypertension or enlarged cup‐to‐disc ratios were initially included in the glaucoma‐suspect group. Seven patients and 11 eyes were excluded from the study as they had demonstrated visual field loss suspicious for glaucoma in the Humphrey visual field test. Sixty‐six eyes from 33 patients were finally included in the glaucoma‐suspect group. The average age of the participants in the glaucoma‐suspect group was 52.3 years (range 8–82). Twenty‐three eyes of normal subjects with no risk factors for glaucoma were tested as controls with the 3D‐CTAG, and none showed any visual field defects (fig 1). The average age in this group was 47.2 years (range 27–74). All of the visual field defects in the glaucoma‐suspect group were characteristic or compatible with glaucomatous visual field loss. In this group, 52/66 (79%) eyes demonstrated visual field defects detected with the 3D‐CTAG. Arcuate defects or a nasal step were seen in 37/52 (71%) of the eyes with defects. Twelve out of 52 (23%) showed arcuate defects: 7/12 (58%) demonstrated superior arcuate defects; 2/12 (17%) showed inferior arcuate defects; and superior and inferior arcuate defects together were seen in 3/12 (25%). Twenty‐five out of 52 (48%) eyes demonstrated nasal defects: inferior nasal scotomas were demonstrated in 9/25 (36%) eyes and 6/25 (24%) eyes showed superior nasal scotomas. Figure 2 shows a 3D depiction of an inferior nasal step as demonstrated by the 3D‐CTAG, with the corresponding normal Humphrey visual field test. 10/25 (40%) eyes had superior and inferior nasal step together. The 3D depiction of a superior and inferior nasal step together is seen in fig 3. Constricted visual field defects were demonstrated in 15/52 (29%) eyes. A constricted visual field in a patient with ocular hypertension and otherwise normal Humphrey visual field is seen in fig 4. None of the eyes demonstrated superior altitudinal or inferior altitudinal defects. Furthermore, there were no central or paracentral scotomas. Central or temporal islands were also not demonstrated. There were no visual field defects in the control group. Figure 5 demonstrates a visual field defect in a patient with advanced glaucoma, with good correlation between the 3D‐CTAG and standard automated perimetry.
Figure 1 3D visual field of a normal eye with no defects. The X‐axis denotes the horizontal visual field in degrees, the Y‐axis denotes the vertical visual field in degrees and the Z‐axis depicts the contrast sensitivity (see Patients and methods) as a function of location (X, Y).
Figure 2 A. 3D visual field test showing inferior nasal step in left eye of patient with ocular hypertension. B. Normal Humphrey visual field.
Figure 3 A. Superior and inferior nasal step together in right eye of glaucoma‐suspect patient. B. Normal Humphrey visual field.
Figure 4 A. Constricted visual field in left eye in patient with ocular hypertension. B. Normal Humphrey visual field.
Figure 5 A. Visual field in patient with advanced glaucoma. B. Corresponding Humphrey visual field.
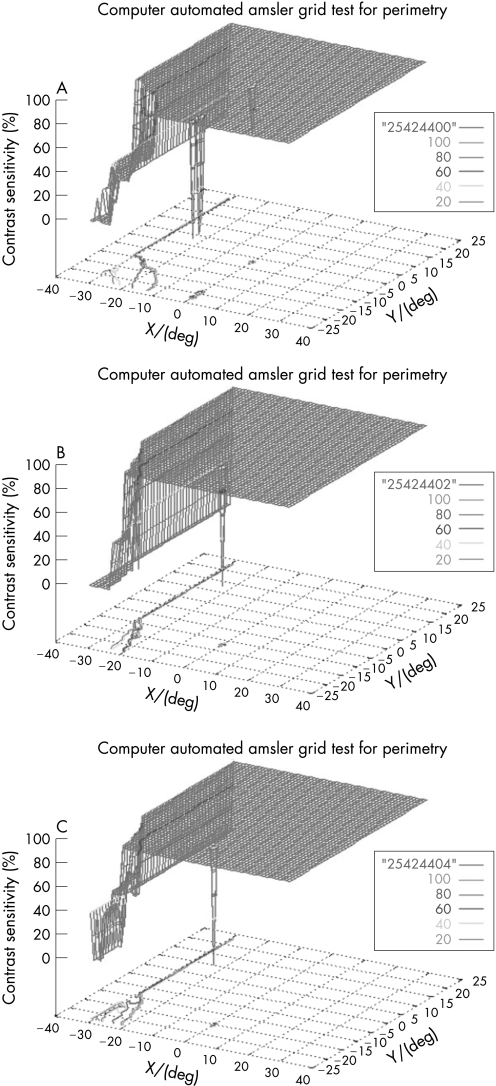
To determine the reliability of the results, each test was repeated. A similar pattern of visual field loss was attained with respect to the location, size and depth of the scotomas. Evaluation of reproducibility of the 3D‐CTAG demonstrated a reproducibility of 91±28%. Three repeated measurements of the same eye with an inferior nasal step are shown in fig 6, demonstrating the obtained reproducibility of the 3D‐CTAG.
Figure 6 A–C. Three repeated measurements of the same eye in a patient with inferior nasal step demonstrating the reproducibility of the 3D visual field test.
In contrast to standard automated perimetry, the physiological blind spot is not reported since there is no cortical representation of that area and thus the blind spot is “filled in” by the visual cortex when presented with a screenwide constant Amsler grid.
Discussion
We employed the 3D‐CTAG technology to determine the presence of visual field defects in glaucoma‐suspect patients. Of the eyes tested, 79% had visual field defects that were not detected by the Humphrey visual field test; 71% were characteristic and 29% were compatible with glaucoma. We believe that the majority of the visual field defects were not identified by standard automated perimetry because of its inherent lower resolution and sensitivity.
All of the visual field defects were characteristic or compatible with glaucoma in the glaucoma‐suspect group. These findings, in addition to the consistent location, shape and pattern of the scotomas in this group make it unlikely that they were due to other factors. Of course, there remains a possibility that the visual field defects demonstrated in this pilot study could also be caused by other than glaucomatous conditions, not known to us at the time of the examination. Since this is a pilot study rather than a definitive trial to introduce a new visual field test, additional and thorough longitudinal follow‐up studies are necessary to establish the relative value and indications for the 3D‐CTAG. Quantitative values for visual field characteristics, optic disc characteristics, and other clinical and demographics information were not obtained in this study. Follow‐up studies should determine the above as well as risk assessment values for the glaucoma suspects to clinically validate the 3D‐CTAG. So far, it appears that the location and shape of the scotomas in glaucoma‐suspect patients are unique for this disease. As expected, the scotomas were located in the periphery, and most included an arcuate defect or nasal step most often inferior. The results presented in this study certainly show a high percentage of abnormal findings, and some may represent false‐positive testing. We are currently completing a pilot study of glaucoma patients with known Humphrey visual field defects and their correlation to 3D‐CTAG field defects. Preliminary results of that study show agreement between Humphrey and the 3D‐CTAG.12 Future projects may include the comparison of the 3D‐CTAG with other early detection modalities such as short wave length automated perimetry, frequency doubling perimetry and structural measures such as nerve fibre layer analysis. Additionally, the relationship between psychophysical parameters and the anatomical substrate has been examined.17,18,19,20 A pathophysiological interpretation of our results is beyond the scope of this pilot study. Future definitive studies may elucidate this issue.
Psychophysical testing has already been demonstrated to predict the onset of glaucomatous visual field loss in patients with ocular hypertension.21 The effects of glaucoma on contrast sensitivity have been examined.22,23,24,25 Improvements in resolution and reliability have made computer‐based testing increasingly common as a useful diagnostic tool for detecting glaucoma. For instance, scanning laser entoptic perimetry has recently been shown to be an inexpensive and effective screening test for retinal disease screening in visually asymptomatic patients and patients with glaucoma.26,27
The 3D‐CTAG provides several advantages over conventional perimetry, including superior angular resolution, contrast sensitivity as third dimension, potential interactive accessibility and distribution over the internet, shorter examination time and additional information through 3D depiction of scotomas. In contrast to several other retinal diseases, visual field loss from glaucoma first begins as shallow scotomas that then may progress and deepen over time and cause absolute visual field defects. Hence, used as a screening intervention, our results indicate that the 3D‐CTAG shows promise for the early detection of glaucoma in patients with ocular hypertension or cup enlargement. It may be used in large community‐based screening programmes and in the primary care setting for widespread screening of glaucoma‐suspect patients. Since most patients are evaluated in primary care centres, a potentially larger number of glaucoma‐suspect patients can be screened and then referred for treatment. Further, the demonstrated advantages of this test may enable visual field testing in locations where access to health care centres is not readily available.
Abbreviations
3D‐CTAG - 3D computer‐automated threshold Amsler grid test
CRT - cathode ray tube
HVF - Humphrey's visual field
POAG - primary open angle glaucoma
Footnotes
Competing interests: WF and AAS may have proprietary interest as patents on the test technology used in this study are issued. PPN, BF and DM have no proprietary interest.
References
- 1.Quigley H A. Number of people with glaucoma worldwide. Br J Ophthalmol 199680389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tielsch J M, Sommer A, Katz J.et al Racial variations in the prevalence of primary open‐angle glaucoma: the Baltimore Eye Survey. JAMA 1991266369–374. [PubMed] [Google Scholar]
- 3.Quigley H A, Addicks E M, Green R. Optic nerve damage in human glaucoma. III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. Arch Ophthalmol 1982146135–146. [DOI] [PubMed] [Google Scholar]
- 4.Kwon Y H, Kim C S, Zimmerman M B.et al Rate of visual field loss and long‐term visual outcome in primary open‐angle glaucoma. Am J Ophthalmol 200113247–56. [DOI] [PubMed] [Google Scholar]
- 5.Sponsel W E. Tonometry in question: can visual screening tests play a more decisive role in glaucoma diagnosis and management? Surv Ophthalmol 198933291–300. [PubMed] [Google Scholar]
- 6.Amsler M. Earliest symptoms of disease of the macula. Br J Ophthalmol 195337521–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wall M, Sadun A A. Threshold Amsler grid testing: cross‐polarizing lenses enhance yield. Arch Ophthalmol 1986104520–523. [DOI] [PubMed] [Google Scholar]
- 8.Wall M, May D R. Threshold Amsler grid testing in maculopathies. Ophthalmol 1987941126–1133. [DOI] [PubMed] [Google Scholar]
- 9.Fink W, Sadun A A. Novel 3D computerized threshold Amsler grid test, perimetry update 2002/2003, pp 207–212. In: Wall M, Henson D, eds. Proceedings of the XVth International Perimetric Society Meeting; June 2002; Stratford upon Avon, UK. Amsterdam/New York: Kugler Publications.
- 10.Fink W, Sadun A A. 3D computer‐automated threshold Amsler grid test. J Biomed Opt 20049149–153. [DOI] [PubMed] [Google Scholar]
- 11.Fink W, Hsieh A K, Sadun A A. Computer‐automated 3D visual field testing in distinguishing paracentral scotomas of optic neuritis vs AION. [ARVO Abstract]. Invest Ophthalmol Vis Sci. 2000;41:S311 Abstract nr 1643
- 12.Fahimi A, Sadun A A, Fink W. Computer automated 3D visual field testing of scotomas in glaucoma. [ARVO Abstract]. Invest Ophthalmol Vis Sci. 2001;42:S149 Abstract nr 796
- 13.Nazemi P P, Fink W, Lim J I.et al Paracentral scotomas of age‐related macular degeneration detected by means of a novel computer‐automated 3D visual field test. [ARVO Abstract]. Invest Ophthalmol Vis Sci. 2001;42:S705 Abstract nr 3799
- 14.Nazemi P P, Fink W, Sadun A A.et al Early detection of glaucoma by means of a novel computer‐automated 3D visual field test. Poster and Abstract, American Academy of Ophthalmology Meeting in New Orleans 2001, Scientific Poster 33, p159.
- 15.Nazemi P P, Fink W, Lim J I.et al Scotomas of age‐related macular degeneration detected and characterized by means of a novel computer‐automated 3D visual field test. Retina 200525446–453. [DOI] [PubMed] [Google Scholar]
- 16.Keltner J L, Johnson C A, Cello K E.et al Classification of visual field abnormalities in the Ocular Hypertension Treatment Study. Arch Ophthalmol 2003121643–650. [DOI] [PubMed] [Google Scholar]
- 17.Quigley H A, Sanchez R M, Dunkelberger G R.et al Chronic glaucoma selectively damages large optic nerve fibers. Invest Ophthalmol Vis Sci 198728913–920. [PubMed] [Google Scholar]
- 18.Quigley H A, Dunkelberger G R, Green W R. Chronic human glaucoma causes selectively greater loss of large optic nerve fibers. Ophthalmology 198895357–363. [DOI] [PubMed] [Google Scholar]
- 19.Kielczewski J L, Pease M E, Quigley H A. The effect of experimental glaucoma and optic nerve transection on amacrine cells in the rat retina. Invest Ophthalmol Vis Sci 2005463188–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glovinsky Y, Quigley H A, Dunkelberger G R. Retinal ganglion cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci 199132484–491. [PubMed] [Google Scholar]
- 21.Johnson C A, Adams A J, Casson E J.et al Blue‐on‐yellow perimetry can predict the development of glaucomatous visual field loss. Arch Ophthalmol 199311645–650. [DOI] [PubMed] [Google Scholar]
- 22.Stamper R L. Psychophysical changes in glaucoma. Surv Ophthalmol 198933309–318. [PubMed] [Google Scholar]
- 23.Horn F, Martus P, Korth M. Comparison of temporal and spatiotemporal contrast‐sensitivity tests in normal subjects and glaucoma patients. Ger J Ophthalmol 1993231146–150. [PubMed] [Google Scholar]
- 24.Sample P A, Juang P S C, Weinreb R N. Isolating the effects of primary open‐angle glaucoma on the contrast‐sensitivity function. Am J Ophthalmol 1991112308–316. [DOI] [PubMed] [Google Scholar]
- 25.Wood M W, Livie‐Kitchin J E. Evaluation of the efficacy of contrast sensitivity measures for the detection of early primary open‐angle glaucoma. Optom Vis Sci 199263175–181. [DOI] [PubMed] [Google Scholar]
- 26.Plummer D J, Azen S P, Freeman W R. Scanning laser entoptic perimetry for the screening of macular and peripheral retinal disease. Arch Ophthalmol 20001181205–1210. [DOI] [PubMed] [Google Scholar]
- 27.Plummer D J, Lopez A, Azen S P.et al Correlation between static automated and scanning laser entoptic perimetry in normal subjects and glaucoma patients. Ophthalmology 20001071693–1701. [DOI] [PubMed] [Google Scholar]