Abstract
Aim
With the rationale that amyloid beta (AB) is toxic to the retina, we here assessed the role of TRAIL, a mediator of AB toxicity and related signal transduction, in a rat model. We also attempted to demonstrate possible protective effects of σ1 receptor agonists in these processes.
Methods
AB and the σ1 receptor agonist Pre‐084 were injected intravitreally in the anaesthetised rat. In additional experiments, the σ1 receptor antagonist BD1047 was administered to assess specificity of the effects of Pre‐084. Western blot analysis was performed on retinas to evaluate the expression of TRAIL and TRAIL receptors in the retina, as well as of Bax and phosphorylated JNK following the different treatments. Lactic dehydrogenase (LDH) levels were measured as a cytotoxicity marker.
Results
All TRAIL receptors were expressed in rat retinas. Intravitreal injection of AB in rat eyes induced overexpression of TRAIL and the proapoptotic protein Bax, as well as phosphorylation of JNK. All these effects of AB were abrogated by pretreatment with the σ1 receptor agonist Pre‐084.
Conclusions
It is likely that TRAIL is a mediator of AB effects on the retina. In light of their specific inhibitory effects upon TRAIL expression, it is plausible to hypothesise that σ1 receptor agonists could represent potential pharmacological tools for restraining AB related retinal damage.
Keywords: cytokines, receptors, retinal protection
Amyloid beta (AB) is the pivotal molecule in Alzheimer's disease related neurodegenerative processes.1 Nevertheless, detrimental effects of AB have also been shown on the retina.2
We have previously shown that AB exerts part of its toxic effects on human neural cells via activation of the tumour necrosis factor related apoptosis inducing ligand (TRAIL) a proapoptotic cytokine belonging to the TNF family.3 TRAIL interacts with specific receptors, DR4, DR5, initiating the apoptotic cascade,4 which implies, among other things, phosphorylation of the stress kinase family, such as Jun‐N terminal kinase (JNK),5 and induction of proapoptotic gene expression, such as Bax.6 In addition, TRAIL also binds to two decoy receptors, designated as DcR1 and DcR2.7
The term “sigma” (σ) is used to refer to a unique class of non‐opioid, non‐phencyclidine binding sites heterogeneously distributed in the nervous system and in peripheral organs that may serve as receptors for any, as yet unidentified, endogenous ligand.8 σ Receptors appear involved in cellular functions9 as well as in neurotransmission.8 Interestingly, both σ1 and σ2 subtypes are expressed in various eye tissues,10 including retina.11 Interestingly, σ1 receptor agonists, which protect neurons from AB toxicity,12 exert similar effects upon retinal ganglion cells.13
This study was aimed to assess in vivo the involvement of TRAIL in the mechanisms related to the neurotoxicity induced by AB in the rat retina. After intravitreal injection of AB in rat eyes, the expression of the proteins TRAIL, JNK and Bax was evaluated. To test possible involvement of σ1 receptors, we treated rats with the neuroprotective specific σ1 receptor agonist Pre‐084.
Methods
Male Sprague‐Dawley rats weighing 250–300 g (six rats/group; Charles River, Calco, Italy) were used, following the guidelines of the animal care and use committee of the University of Catania, and conformed to the Association for Research in Vision and Ophthalmology (ARVO). Amyloid beta25‐35 (AB) was from Sigma‐Aldrich (Milan, Italy). Pre‐084 and BD1047 were from Tocris (Avonmouth, UK).
Animals were anaesthetised with ketamine hydrochloride (35 mg/kg) and xylazine hydrochloride (5 mg/kg) and unilaterally injected intravitreally, using a Hamilton syringe, with AB (5 nmol/2 μl) in phosphate buffered saline. A separate set of animals were treated intravitreally, immediately before AB injection, with the σ1 receptor agonist Pre‐084 (0.3 μg/2 μl) and/or σ1 receptor antagonist BD1047 (0.3 μg/2 μl). The contralateral eye of each animal was used as a control (injected with vehicle). After 48 hours the animals were sacrificed, the eyes enucleated and retinas collected for western blot analysis and lactic dehydrogenase (LDH) assay.
For western blot analysis cells were lysed in lysis buffer (lysis buffer containing 50 mM TRIS, pH 7.6, 150 mM NaCl, 5 mM EDTA, 1 mM phenyl methyl sulphonyl fluoride, 0.5 mg/ml leupeptin, 5 mg/ml aprotinin, 1 mg/ml pepstatin). The samples were sonicated and centrifuged at 15 000 g for 30 minutes at 4°C. The resulting supernatants were isolated and protein content determined by a conventional method (BCA protein assay Kit, Pierce, Rockford, IL, USA).
Western blot analysis was performed as described elsewhere,14 using the following antibodies (all antibodies were from Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA unless otherwise specified): rabbit anti‐TRAIL polyclonal antibody, or goat anti‐DR5 polyclonal antibody; rabbit anti‐DR4 polyclonal antibody (Prosci Inc, Poway, CA, USA) or goat anti DcR1 polyclonal antibody; goat anti‐DcR2 polyclonal antibody; rabbit anti‐JNK1 polyclonal antibody; mouse anti‐p‐JNK monoclonal antibody; rabbit anti‐Bax polyclonal antibody or rabbit anti‐β‐tubulin polyclonal antibody. Horseradish peroxidase conjugated anti‐rabbit or anti‐mouse IgG or anti‐goat IgG secondary antibody were from Amersham Italia (Milan, Italy), and were detected by enhanced chemiluminescence (ECL; Amersham Italia, Milan, Italy). Densitometric analysis was performed using 1D Image Analysis Software; Scientific Imaging Kodak Company. β‐Tubulin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as an internal control to validate the right amount of protein loaded in the gels. Densitometric analysis is expressed as mean percentage increase integrated density vs control values, by previously normalising the sample value versus the corresponding β‐tubulin expression. All experiments were run twice and analysed in triplicate.
LDH release was measured on tissue lysate, following the protocol suggested for a LDH release toxicity kit (Sigma). Each sample was run in triplicate.
Statistical analysis of results was performed by means of the one way analysis of variance (ANOVA) test, followed by the Duncan's least significance test. Significance was accepted for a p value<0.05.
Results
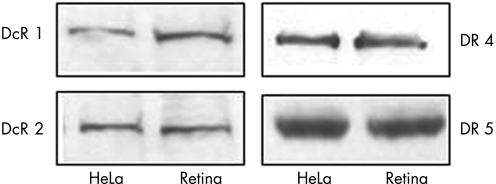
The expression of TRAIL receptors was first assessed in the normal rat retina by means of western blot analysis. Rat retina expressed the four receptors for TRAIL, showing levels of expression similar to that found in HeLa cells (fig 1).
Figure 1 Western blot analysis of the four TRAIL receptor proteins DcR1 (upper left), DcR2 (lower left), DR4 (upper right) and DR5 (lower right) in the retina of untreated, male adult Sprague‐Dawley rats (retina). Protein extracts from HeLa cells (HeLa) were used as a positive control.
Thus, to assess the mechanisms involved in the AB toxicity on the retina, tissues were analysed for LDH as well as by western blot for TRAIL expression.
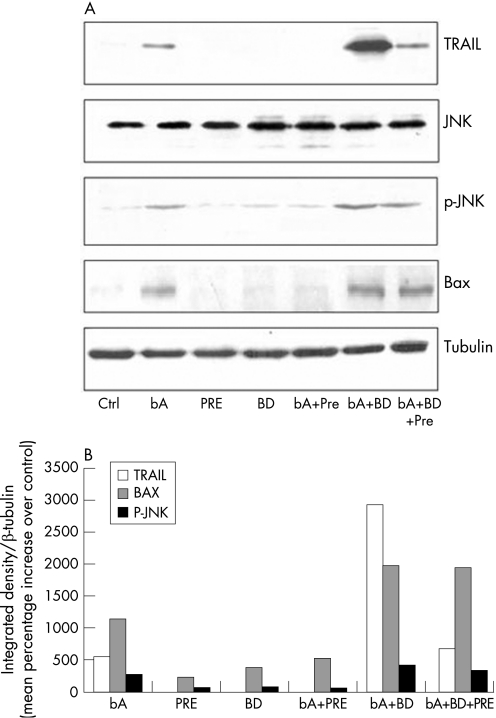
TRAIL expression increased significantly in retinas 48 hours after AB injection (fig 3, blot, lane 2). The expression of TRAIL was then analysed after pretreatment with the specific σ1 receptor agonist Pre‐084 and/or σ1 receptor antagonist BD1047. Pretreatment with the agonist Pre‐084 resulted in abrogation of AB dependent increase of TRAIL (fig 3, blot, lane 5). On the other hand, pretreatment with the antagonist BD1047 resulted in potentiation of the inducing effect of AB upon TRAIL (fig 3, blot, lane 6). Pretreatment with the combination of σ1 receptor agonist and antagonist resulted in significant decrease of TRAIL expression (fig 3, blot, lane 7) in AB treated retina. Either the agonist or the antagonist did not induce TRAIL when injected alone (fig 3, blot, lanes 3 and 4).
Figure 3 (A) Western blot analysis of the effects of σ1 receptor agonists upon the expression of TRAIL (upper blot), JNK (second blot from the top), phosphorylated JNK (p‐JNK, third blot from the top), Bax (fourth blot from the top) and tubulin (bottom blot). Rats were untreated (lane 1), or received amyloid beta (lane 2) or, respectively the σ1 receptor agonist Pre084 (lane 3) or the σ1 receptor antagonist BD1047 (lane 4) alone. Other rats treated with AB were also pretreated with Pre084 (lane 5), BD1047 (lane 6), or both compounds (lane 7). (B) Densitometric analysis related to treatments mentioned above expressed as mean percentage increase (from each of three single experiments) over respective controls. Vertical bars are means (SEM). *p<0.05 (one way ANOVA, followed by a least significance Duncan's test).
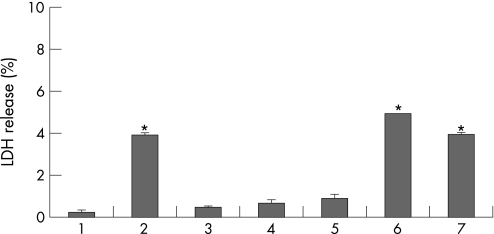
LDH release from retina was always parallel to TRAIL expression in all samples (fig 2).
Figure 2 Lactate dehydrogenase (LDH) release from the whole retina of rats treated with amyloid beta (AB) injected in the vitreal body. Rats were untreated (lane 1), or received AB (lane 2) or, respectively, the σ1 receptor agonist Pre‐084 (lane 3) or the σ1 receptor antagonist BD1047 (lane 4) alone. Other rats treated with AB were also pretreated with Pre084 (lane 5), BD1047 (lane 6) or both compounds (lane 7). Vertical bars are means (SEM). *p<0.05 (one way ANOVA, followed by a least significance Duncan's test).
To verify activation of the related signal transduction after induction of TRAIL expression by AB treatment, we evaluated the phosphorylation level of the TRAIL receptor downstream kinase JNK in the rat retina. Western blot analysis showed that the amount of the phosphorylated form of JNK was increased in rats treated with AB (fig 3, lane 2), an effect attenuated by Pre‐084 (fig 3, lane 5). Pretreatment with the antagonist BD1047 resulted in a further increase of JNK phosphorylation (fig 3, lane 6) after AB treatment. Combination of Pre‐084 and BD1047 resulted in a decreased AB induced JNK phosphorylation (fig 3, lane 7). Either the agonist or the antagonist alone did not have any effect (fig 3, lanes 3 and 4).
In addition, the proapoptotic protein Bax was increased in rats treated with AB (fig 3, lane 2); such effect was attenuated in the presence of Pre‐084 (fig 3, lane 5), whereas pretreatment with BD1047 resulted in a further increase of Bax after AB treatment (fig 3, lane 6). A combination of Pre‐084 and BD1047 decreased the expression of Bax induced by AB (fig 3, lane 7). Either the σ1 agonist or the σ1 antagonist alone were unable to modify Bax expression (fig 3, lanes 3 and 4).
Densitometric analysis performed on all western blots reported above confirmed statistically significant differences versus control values in all the experimental groups (fig 3, histogram). Control values vs tubulin were respectively 4.26 (0.05) for TRAIL; 8.81 (0.15) for BAX; 15.87 (0.43) for p‐JNK. The overall significance was assumed for p<0.05.
Discussion
We investigated the involvement of TRAIL and its related signal transduction in the mechanism of neurotoxicity induced by AB on retinal cells after intravitreal injection and the effect of σ1 receptor agonists upon AB toxicity.
Indeed, Jen et al2 demonstrated that AB is toxic for rat retinal cells. In fact, when the retinas were injected with AB fragments, a distinct band of TUNEL positive photoreceptors cells was evident in the retina's outer nuclear layer. Moreover, it has been clearly demonstrated that AB is involved in pathological processes underlying age related macular degeneration.15
Here, we show that all TRAIL receptors are expressed in the rat retina and that intravitreal injection of the AB fragment 25–35 was followed by increased levels of TRAIL, paralleled by increased LDH release. In addition, AB treatment resulted in increased JNK kinase phosphorylation16 and Bax expression,12 events associated with TRAIL related signal transduction.5,6 Recent data show that TRAIL mediates AB toxicity in human neurons in vitro, resulting in excessive apoptosis and that AB is toxic to retinal cells in vitro and in vivo.2 The presence of AB in human pathological deposits associated with ageing and age related macular degeneration (AMD)17 and the caspase activation and amyloid precursor protein cleavage in rat ocular hypertension18 definitely suggest an involvement of AB in retina pathologies.
Taken together, all data support the hypothesis that AB toxicity actively involves TRAIL, which can thus be regarded as a candidate contributor to retinal damage along with other recognised factors such as, for example, superoxides19 and excitotoxicity.20
Intravitreal pretreatment with the σ1 receptor agonist Pre‐084 resulted in abrogation of AB effects on TRAIL, Bax and JNK. To corroborate the neuroprotective role of σ1 receptors,12,21 it is interesting to note that inactivation of σ1 receptors by specific antagonists rescues the apoptotic programme in tumour and self reliant cells.22 We showed that the effects of Pre‐084 are specifically mediated by activation of σ1 receptors. The σ1 antagonist BD1047 prevented the protective effect of Pre‐084, rescuing the effects induced by AB.
Our results show that the effects of AB on TRAIL expression in the retina, as well as the abrogating effect of pretreatment with the σ1 receptor agonist Pre‐084, suggest that the activation of σ1 receptors eventually interferes with the proapoptotic signalling initiated by AB and TRAIL. In support of this hypothesis, the σ1 receptor agonist Pre‐084 is able to reduce the expression level of Bax in AB treated neurons in vitro.12
In conclusion, we have shown that TRAIL could be regarded as one among candidate mediators of AB toxicity in the rat retina in vivo. σ1 Agonists might be considered potential agents for prevention of TRAIL dependent toxicity set into motion by AB in the retina.
Acknowledgements
This work was partly funded by a PRIN grant from MIUR and by ordinary funds for Research (FAR) from University of Catania to RB. The authors thank Bausch & Lomb for support.
Abbreviations
AB - amyloid beta
ECL - enhanced chemiluminescence
JNK - Jun‐N terminal kinase
LDH - lactic dehydrogenase
TRAIL - tumour necrosis factor related apoptosis inducing ligand
References
- 1.Selkoe D J. Alzheimer's disease: a central role for amyloid. J Neuropathol Expl Neurol 199453438–447. [DOI] [PubMed] [Google Scholar]
- 2.Jen L S, Hart A J, Jen A.et al Alzheimer's peptide kills cells of retina in vivo. Nature 1998392140–141. [DOI] [PubMed] [Google Scholar]
- 3.Cantarella G, Uberti D, Carsana T.et al Neutralization of TRAIL death pathway protects human neuronal cell line from beta‐amyloid toxicity. Cell Death Diff 200310134–141. [DOI] [PubMed] [Google Scholar]
- 4.Lee H O, Herndon J M, Barreiro R.et al TRAIL: a mechanism of tumor surveillance in an immune privileged site. J Immunol 20021694739–4744. [DOI] [PubMed] [Google Scholar]
- 5.Gupta S, Barrett T, Whitmarsh A J.et al Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J 1996152760–2770. [PMC free article] [PubMed] [Google Scholar]
- 6.Le Blanc H, Lawrence D, Varfolomeev E.et al Tumour‐cell resistance to death receptor‐induced apoptosis through mutational inactivation of the proapoptotic Bcl‐2 homolog Bax. Nat Med 20028274–281. [DOI] [PubMed] [Google Scholar]
- 7.Sandra F, Hendarmin L, Nakamura S. Osteoprotegerin (OPG) binds with tumour necrosis factor‐related apoptosis‐inducing ligand (TRAIL): suppression of TRAIL‐induced apoptosis in ameloblastomas. Oral Oncol 200642415–420. [DOI] [PubMed] [Google Scholar]
- 8.Quirion R, Bowen W D, Itzhak Y.et al A proposal for the classification of sigma binding sites. Trends Pharmacol Sci 19921385–86. [DOI] [PubMed] [Google Scholar]
- 9.Roy S, Loh H. Effects of opioids on the immune system. Neurochem Res 1996211375–1386. [DOI] [PubMed] [Google Scholar]
- 10.Bucolo C, Campana G, Di Toro R.et al Sigma1 recognition sites in rabbit iris‐ciliary body: topical sigma1‐site agonists lower intraocular pressure. J Pharmacol Exp Ther 19992891362–1369. [PubMed] [Google Scholar]
- 11.Senda T, Matsuno K, Mita S. The presence of sigma receptor subtypes in bovine retinal membranes. Exp Eye Res 199764857–860. [DOI] [PubMed] [Google Scholar]
- 12.Marrazzo A, Caraci F, Salinaro E T.et al Neuroprotective effects of sigma‐1 receptor agonists against beta‐amyloid‐induced toxicity. Neuroreport 2005161223–1226. [DOI] [PubMed] [Google Scholar]
- 13.Martin P M, Ola M S, Agarwal N.et al The sigma receptor ligand (+)‐pentazocine prevents apoptotic retinal ganglion cell death induced in vitro by homocysteine and glutamate. Brain Res Mol Brain Res 200412366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sambrook J, Fritsch E F, Maniatis T.Molecular cloning: a laboratory manual. NY: Cold Spring Harbor Laboratory Press, 1989318.47–18.75. [Google Scholar]
- 15.Yoshida T, Ohno‐Matsui K, Ichinose S.et al The potential role of amyloid beta in the pathogenesis of age‐related macular degeneration. J Clin Invest 20051152793–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minogue A M, Schmid A W, Fogarty M P.et al Activation of the c‐Jun N‐terminal kinase signalling cascade mediates the effect of amyloid‐beta on long term potentiation and cell death in hippocampus: a role for interleukin‐1beta? J Biol Chem 200327827971–27978. [DOI] [PubMed] [Google Scholar]
- 17.Johnson L V, Leinter W P, Rivest A J.et al The Alzheimer's Aβ‐peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age‐related macular degeneration. Proc Natl Acad Sci USA 20029911830–11835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKinnon S J, Lehman D M, Kerrigan‐Baumrind L A.et al Caspase activation and amyloid precursor protein cleavage in rat ocular hypertension. Invest Ophtalmol Vis Sci 2002431077–1087. [PubMed] [Google Scholar]
- 19.Moreno M C, Campanelli J, Sande P.et al Retinal oxidative stress induced by high intraocular pressure. Free Radic Biol Med 200437803–812. [DOI] [PubMed] [Google Scholar]
- 20.Louzada P R, Lima A C, Mendonca‐Silva D L.et al Taurine prevents the neurotoxicity of beta‐amyloid and glutamate receptor agonists: activation of GABA receptors and possible implications for Alzheimer's disease and other neurological disorders. FASEB J 200418511–518. [DOI] [PubMed] [Google Scholar]
- 21.Bucolo C, Drago F, Lin L R.et al Sigma receptor ligands protect human retinal cells against oxidative stress. Neuroreport 200617287–291. [DOI] [PubMed] [Google Scholar]
- 22.Spruce B A, Campbell L A, McTavish N.et al Small molecule antagonists of the σ‐1 receptor cause selective release of the death program in tumour and self‐reliant cells and inhibit tumour growth in vitro and in vivo. Cancer Res 2004644875–4886. [DOI] [PubMed] [Google Scholar]