Abstract
Background
Hypoxia‐inducible factor (HIF) is a common transcription factor for many angiogenic proteins. Retinal pigment epithelial (RPE) cells are an important source of angiogenic factors in the retina. The expression of HIF, its regulation by proline hydroxylase (PHD) enzymes, and its downstream regulation of angiogenic factors like vascular endothelial growth factor (VEGF) and erythropoietin (EPO) was studied in RPE cells in order to determine some of the molecular mechanisms underlying ischaemic retinal disease.
Methods
ARPE‐19 cells were cultured for various times under hypoxic conditions. Cellular HIF and PHD isoforms were analysed and quantified using western blot and densitometry. VEGF and EPO secreted into the media were assayed using enzyme‐linked immunosorbent assay (ELISA). Messenger RNA (mRNA) was quantified using real‐time quantitative reverse transcriptase polymerase chain reaction (qPCR). RNA interference was achieved using siRNA techniques.
Results
HIF‐1α was readily produced by ARPE‐19 cells under hypoxia, but HIF‐2α and HIF‐3α could not be detected even after HIF‐1α silencing. HIF‐1α protein levels showed an increasing trend for the first 24 h while HIF‐1α mRNA levels fluctuated during this time. After 36 h HIF‐1α protein levels declined to baseline levels, a change that was coincident with a rise in both PHD2 and PHD3. Silencing HIF‐1α significantly decreased VEGF secretion. Significant production of EPO could not be detected at the protein or mRNA level.
Conclusions
HIF‐1α appears to be the main isoform of HIF functioning in ARPE‐19 cells. Under hypoxia, HIF‐1α levels are likely self‐regulated by a feedback loop that involves both transcriptional and post‐translational mechanisms. VEGF production by human RPE cells is regulated by HIF‐1α. EPO was not produced in significant amounts by RPE cells under hypoxic conditions, suggesting that other cells and/or transcription factors in the retina are responsible for its production.
Keywords: diabetic retinopathy, VEGF, erythropoietin, hypoxia‐inducible factor, proline hydroxylase
Diabetic retinopathy is a leading cause of irreversible vision loss in North America.1 Since the discovery that vascular endothelial growth factor (VEGF) is elevated in ocular fluids of patients with diabetic retinopathy,2 the important role of this growth factor in the pathogenesis of diabetic retinopathy has been well established.3,4,5 Recently, the pathogenic role of erythropoietin (EPO) in diabetic retinopathy has also been recognised.6,7 Both VEGF and EPO transcription are regulated by the hypoxia‐inducible factor (HIF).8
HIF is the primary hypoxic signalling protein in cells and is able to induce the transcription of more than 70 genes which mediate angiogenesis, cell proliferation/survival, and glucose/iron metabolism.9,10,11 HIF is a heterodimeric transcription factor composed of a labile α subunit, which is post‐translationally regulated by oxygen, and a stable β subunit which is constitutively expressed.12 Three different isoforms of the α subunit exist, with the HIF‐1α being the most widely studied.13 Oxygen plays a key role in stabilising HIF‐1α and its function by acting as a co‐factor for proline hydroxylase (PHD) enzymes. Under normoxic conditions, conserved proline residues on HIF‐1α are hydroxylated by PHDs, leading to proteasomal degradation of HIF‐1α.13 Under hypoxic conditions, proline residues are not hydroxylated and HIF‐1α escapes degradation, accumulates in the cytoplasm, and then moves to the nucleus to act as a transcription factor.14
Novel therapies for diabetic retinopathy have recently emerged based on an increased understanding of the biochemical factors underlying ischaemic retinal disease. These therapies, however, are all based on antagonism of VEGF.15,16,17 HIF‐1α has been proposed to represent a novel therapeutic target in ischaemia‐induced retinal diseases like diabetic retinopathy.13 The important role of HIF in ischemic retinal disease is becoming increasingly recognised. HIF‐1α expression is related to protection of retinal elements from ischaemic injury.18 In models of retinal degeneration, HIF‐1α and EPO induction by hypoxic preconditioning has been shown to be protective.19
Retinal pigment epithelial (RPE) cells are a functionally and metabolically diverse group of cells in the retina. Under hypoxic conditions, RPE cells have been demonstrated to produce multiple angiogenic factors including VEGF.20 The purpose of this study was to study the expression patterns of HIF and its downstream genes in RPE cells under hypoxic conditions in order to better understand some of the molecular mechanisms underlying ischaemic retinal diseases like diabetic retinopathy.
Methods
Cell culture
ARPE‐19 cells were purchased commercially (ATCC, Manassas, USA). Cells were cultured (37°C, 5% CO2) in 8.8 cm2 Nunclon™Δ culture dishes (Nalge Nunc International, Rochester, USA) with D‐MEM/F‐12 growth medium (3 ml, ATCC, Manassas, USA) supplemented with 10% heat‐inactivated fetal bovine serum (FBS) and penicillin (100 units/ml) and streptomycin (100 μg/ml). For hypoxia experiments, medium was changed at time = 0 and cells were placed in a hypoxic incubator (37°C, 3% O2, 5% CO2) for various amounts of time (12, 24 or 36 h). At the endpoint of each experiment, culture medium was removed and frozen at −80°C until further use while protein/RNA was extracted from cells immediately. All experiments were done using cells within the first five passages.
siRNA transfection
Cells were grown to 90% confluence for transfection. For siRNA experiments ON‐TARGETplus SMARTpool HIF‐1α siRNA was used, while fluorescent siGLO RISC‐free siRNA was used as a negative control (Dharmacon, Lafayette, USA). Prior to transfection, cells were washed with Opti‐MEM® I reduced‐serum medium (Invitrogen, Burlington, Canada). Following this, cells were incubated (37°C, 8 h) in reduced‐serum medium (2 ml) containing 100 pmol siRNA and 5 μl Lipofectamine™ 2000 (Invitrogen, Burlington, Canada). The transfection solution was then aspirated and the cells washed with culture medium. Cells were given 36 h to recover from transfection, and then experiments (time = 0) were begun. The amount of siRNA and transfection agent used was optimised, and under the conditions used we observed 100% transfection under fluorescence microscopy and little to no toxicity under light microscopy.
Protein extraction and western blotting
Cultured cells were washed with phosphate‐buffered saline (PBS), and treated with 200 μl of cell lysis buffer (Cell Signaling Technology, Beverly, USA). Cells were then scraped from the culture dish and the suspension centrifuged (14 000×g, 10 min). Supernatant containing protein extract was aliquoted and stored at −80°C until further use. For western blot, 10 μl of protein extract per lane was used. Electrophoresis was performed under reducing conditions using NuPAGE® 4–12% Bis‐Tris gels with NuPAGE® MOPS SDS running buffer (Invitrogen, Burlington, Canada). Proteins were transferred to a Hybond™‐C Extra nitrocellulose membrane (GE Healthcare Life Sciences, Piscataway, USA). The membrane was blocked in Tris‐buffered saline containing Tween 20 (TBST) with 5% non‐fat milk for 1 h at room temperature (RT), and then membranes were incubated with primary antibody in TBST/5% non‐fat milk overnight at 4°C. Following this, membranes were washed with TBST, probed with secondary antibody in TBST/5% non‐fat milk (1 h, RT) and then washed again. Protein bands were visualised using Western Lightning® ECL Chemiluminescence Reagent (Perkin Elmer, Montreal, Canada). Densitometry was performed with background subtraction and protein bands were normalised against the β‐tubulin band of the same sample. Each western blot was repeated three times (total of four blots), and the results averaged on densitometry.
Antibodies
Rabbit polyclonal to human HIF‐1α (Abcam, Cambridge, USA) was used at a dilution of 1:500. Rabbit polyclonal to human HIF‐2α (Abcam, Cambridge, USA) was used at a dilution of 1:100. Rabbit polyclonal to human HIF‐3α (Novus Biologicals, Littleton, USA) was used at a dilution of 1:1000. Rabbit polyclonal to human PHD1, PHD2, and PHD3 (Abcam, Cambridge, USA) were all used at dilutions of 1:500. As a loading control, rabbit polyclonal to human β‐tubulin (Abcam, Cambridge, USA) was used at a dilution of 1:1000. Peroxidase‐conjugated mouse anti‐rabbit IgG (Jackson ImmunoResearch, West Grove, USA) was used as the secondary antibody at a dilution of 1:20 000.
Enzyme‐linked immunosorbent assay (ELISA)
Culture medium was assayed for VEGF and EPO using the Quantikine® human VEGF immunoassay (R&D Systems, Minneapolis, USA) according to the manufacturer's instructions. Assays were done in duplicate. For the EPO ELISA, conditioned medium was concentrated 10 times using Amicon® Centricon® centrifugal filter devices (Millipore, Billerica, USA) according to manufacturer's instructions.
Real‐time reverse quantitative polymerase chain reaction (qPCR)
RNA was extracted from cells using TRIzol® Reagent (Invitrogen, Burlington, Canada), and cDNA was synthesised using SuperScript™ II RNAase H‐ Reverse Transcriptase (Invitrogen, Burlington, Canada). Real‐time quantitative PCR was performed using TaqMan® Gene Expression Assays (Applied Biosystems, Foster City, USA) at 50 cycles with 100 ng of starting cDNA. Amplification reactions were performed as specified by the manufacturer using TaqMan® Universal Master Mix without AmpErase® UNG and a 7900 Real Time PCR System (Applied Biosystems, Foster City, USA). RNA was quantified using the ΔΔCt method of relative quantification using SDS software version 2.2.1 (Applied Biosystems, Foster City, USA). Each reaction was performed as five replicates. RNA levels were normalised to β‐actin as an endogenous control, and calibrated against Stratagene® QPCR Human Reference Total RNA (Stratagene, La Jolla, USA) using respective primers.
Statistics
Densitometry, ELISA and qPCR data were analysed using one‐way ANOVA with the Newman–Keuls post‐hoc test. Post‐hoc analysis was only performed if the groups were significantly different. Statistical significance was set at 5%.
Results
HIF‐1α protein expression in hypoxic RPE cells
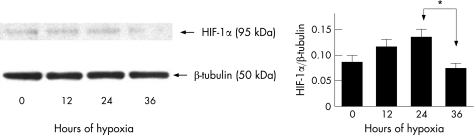
We did not detect any statistically significant changes in HIF‐1α protein levels during the first 24 h (fig 1). However, there appeared to be an increasing trend in HIF‐1α levels during the first 24 h. At 36 h of hypoxia HIF‐1α protein levels decreased significantly compared to the 24 h time point (p < 0.05). HIF‐1α protein levels at 36 h were not significantly different than at baseline (time = 0). Observation of the cells under light microscopy demonstrated that all cells appeared morphologically viable and no cell death was seen (data not shown).
Figure 1 HIF‐1α Expression by human RPE cells under hypoxia. ARPE‐19 cells were grown under hypoxia (3% O2) and HIF‐1α levels assayed using western blot. Densitometry was performed on four separate blots and the results averaged. HIF‐1α levels showed an increasing trend during the first 24 h, and at 36 h were significantly decreased from the 24 h time point (*p < 0.05). A representative blot is shown above. Error bars indicate SEM.
Protein expression of proline hydroxylase isoforms in hypoxic RPE cells
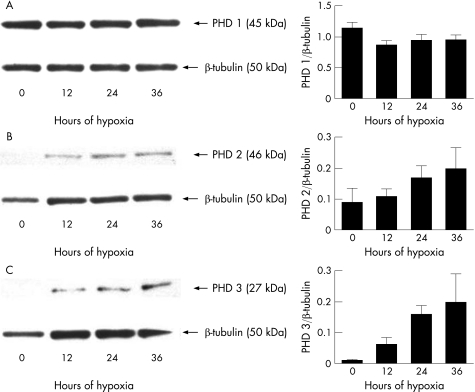
We did not detect any statistically significant changes in the protein levels of proline hydroxylase isoforms over the course of the experiment (fig 2). However, both proline hydroxylase 2 (PHD2) and proline hydroxylase 3 (PHD3) showed an increasing trend over the 36 h. Protein levels of proline hydroxylase 1 (PHD1), however, did not show any trend and remained essentially unchanged.
Figure 2 Expression of proline hydroxylase (PHD) isoforms by human RPE cells under hypoxia. While PHD1 (A) levels remained essentially unchanged, both PHD2 (B) and PHD3 (C) levels showed an increasing trend over time. Densitometry was performed on four separate blots and the results averaged. Representative blots are shown above. Error bars indicate SEM.
Protein expression of alternate HIF isoforms in hypoxic RPE cells
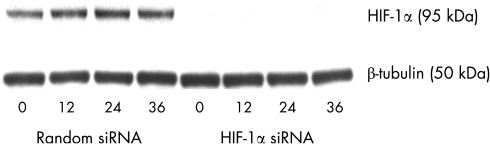
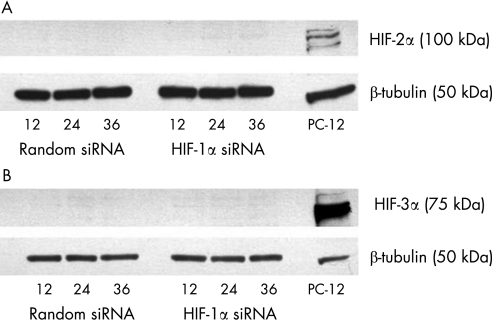
Using siRNA against HIF‐1α, HIF‐1α protein expression was effectively reduced to barely detectable levels over the time course of the experiment (fig 3). Alternate isoforms of HIF (HIF‐2α and HIF‐3α) could not be detected at any time point, even after HIF‐1α was silenced (fig 4).
Figure 3 Effective silencing of HIF‐1α expression by human RPE cells under hypoxia. When cells were transfected with random siRNA (negative control), HIF‐1α expression patterns were unchanged. After transfection with siRNA against HIF‐1α, protein levels were barely detectable using western blot techniques.
Figure 4 Expression of alternate isoforms of HIF by human RPE cells under hypoxia. When cells were transfected with random siRNA (negative control) or siRNA against HIF‐1α, neither HIF‐2α (A) or HIF‐3α (B) could be detected at the protein level. A representative blot is shown above. As a positive control, 20 μg of nuclear lysate from hypoxia‐treated PC‐12 rat adrenal pheochromocytoma cell line (GeneTex, San Antonio, USA) was used.
Analysis of mRNA expression patterns in hypoxic RPE cells
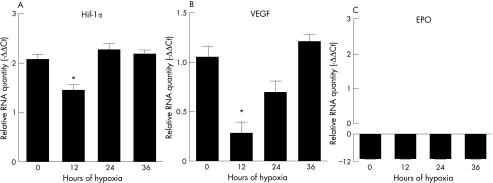
HIF‐1α mRNA levels declined at 12 h of hypoxia (fig 5, p < 0.05), but rose to baseline levels afterwards. Coincident with this decrease in HIF‐1α expression at 12 h was a decrease in levels of VEGF mRNA (fig 5, p < 0.05), demonstrating that the transcription of VEGF was indeed under the regulation of HIF‐1α. EPO mRNA was barely detectable, and its levels were several‐fold less than those of HIF‐1α and VEGF (fig 5).
Figure 5 mRNA Expression by human RPE cells under hypoxia. HIF‐1α mRNA (A) levels declined at 12 h and then rose to baseline levels. A similar pattern was seen with VEGF mRNA (B) expression. An asterix indicates a statistically significant difference (p < 0.05) between levels at 12 h and all other time points. Levels of EPO mRNA (C) were barely detectable. Errors bars indicate SEM.
Analysis of VEGF and erythropoietin secretion by hypoxic RPE cells
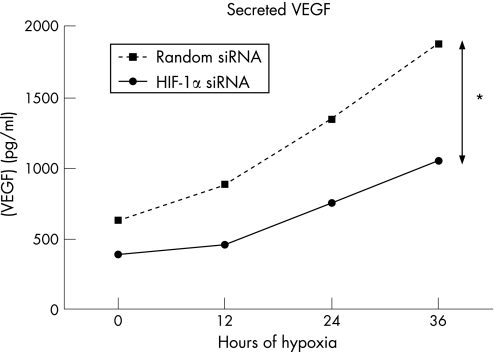
VEGF secreted into the media rose steadily over time and was significantly higher than baseline after 36 h in both groups (fig 6, p < 0.001). The HIF‐1α siRNA treated group produced significantly less VEGF (p < 0.001) at all time points. We could not detect any EPO in the conditioned medium, even following concentration of the medium to 10 times its original concentration (data not shown).
Figure 6 VEGF expression by human RPE cells under hypoxia. VEGF levels were significantly reduced at all time points after transfection with siRNA against HIF‐1α compared to random siRNA (*p < 0.001). Errors bars indicate SEM, but are too close together to be seen on this graph.
Discussion
We observed that during the first 24 h of hypoxia HIF‐1α protein expression in ARPE‐19 cells showed an increasing trend, and returned to baseline levels afterwards. HIF‐1α mRNA levels, on the other hand, showed significant fluctuation during the first 24 h. These observations suggest that both transcriptional and translational mechanisms may be responsible for regulating HIF‐1α levels in ARPE‐19 cells, and that these mechanisms are preferentially acting at different times.
PHD1 protein levels remained essentially unchanged during our experiments. However, PHD2 and PHD3 proteins steadily rose over the time course of our experiments. PHD2 and PHD3 levels peaked when HIF‐1α protein returned to baseline levels. It has been shown that under hypoxic conditions HIF‐1α regulates its own degradation through a feedback loop that involves either upregulation of PHD2 and PHD3 enzymes21,22 or transcription‐dependent (PHD independent) depletion.23 Our results support the hypothesis that both of these factors are regulating HIF‐1α protein levels in ARPE‐19 cells under hypoxic conditions. These two mechanisms are likely acting together as a homeostatic negative feedback loop to keep HIF‐1α protein levels in check.
Based on our observations, it would appear that during acute hypoxia (24 h) HIF‐1α protein levels are regulated mainly by transcription‐dependent mechanisms. HIF‐1α protein is more stable under hypoxia and accumulates under these conditions.14 The decreased HIF‐1α mRNA that we observed in ARPE‐19 cells after 12 h of hypoxia may represent a mechanism for keeping HIF‐1α protein levels in check and preventing excessive production and accumulation. Transcription‐mediated downregulation of HIF‐1α has been shown to occur during the first 24 h of hypoxia.23 Under chronic conditions (more than 24 h) these mechanisms likely no longer function, and PHD2 and PHD3 enzymes are upregulated to bring HIF‐1α down to baseline levels. In this case, PHD2 and PHD3 act as a ‘thermostat' mechanism to turn down HIF‐1α levels under chronic hypoxia. Upregulation of PHD2 and PHD3 as a self‐regulatory mechanism for reducing HIF‐1α protein levels in chronic hypoxia has been described in other systems.22 Furthermore, it has been shown that PHD2 and PHD3 are under transcriptional control of HIF‐1α, suggesting a possible feedback loop between these proteins.24,25
RNA interference using siRNA was effective at significantly reducing HIF‐1α protein levels during 36 h of hypoxia (72 h after transfection). In parallel to this, secreted VEGF protein levels were also significantly reduced over 36 h of hypoxia. Although secreted VEGF levels were reduced in the cells treated with siRNA against HIF‐1α, they were not completely depleted. Furthermore, secreted VEGF levels continued to rise despite a significant decrease in VEGF mRNA levels at 12 h. Increased stability of VEGF mRNA under hypoxic conditions26 and improved efficiency VEGF mRNA translation under hypoxia27,28 could explain these observations. We did not detect significant amounts of the HIF‐2α and HIF‐3α isoforms, even after HIF‐1α silencing, suggesting that the upregulation of alternate isoforms of HIF‐α is not responsible for VEGF production by ARPE‐19 cells. Expression of HIF‐1α and its relation to VEGF expression in ARPE‐19 cells has previously been reported.29 Our data suggest that the 1α isoform is the main isoform of HIF‐α in human RPE cells, and that loss of HIF‐1α function is not accompanied by upregulation of other isoforms. While HIF‐1α/β is the most widely studied isoform of HIF and considered the primary mediator of hypoxia‐induced gene expression, less is understood about HIF‐2α/β and HIF‐3α/β.13,30 Animal models and in vitro studies have shown that both HIF‐2α and HIF‐3α are upregulated in hypoxia, suggesting a role that is complementary rather than redundant with HIF‐1α.31,32 Whatever the function of HIF‐2α and HIF‐3α may be in other systems, it appears that these isoforms play little (if any) role in the hypoxic response of ARPE‐19 cells.
We were unable to detect EPO production from RPE cells at the protein level. Furthermore, EPO mRNA levels were barely detectable in RPE cells under hypoxia. It has previously been demonstrated that retina explants produce EPO under hypoxic conditions.6,33 A recent study34 has shown that in the murine liver, which expresses both HIF‐1α and HIF‐2α, EPO production in preferentially regulated by HIF‐2α and not HIF‐1α. We did not detect any significant HIF‐2α production in ARPE‐19 cells, even after HIF‐1α silencing, indicating that HIF‐1α is the major isoform in these cells. The lack of significant HIF‐2α and EPO expression in these cells suggests that retinal EPO production may also be regulated by HIF‐2α. The cellular elements responsible for EPO production in the retina remains elusive, and deserves further investigation.
In this report we have described HIF expression in human RPE cells. Similar to other systems, HIF levels appear to be self‐regulated in RPE cells by feedback mechanisms which operate at the transcriptional and post‐translational level. Furthermore, HIF‐1α appears to be the major isoform of HIF in human RPE cells and HIF‐1α regulates VEGF expression in these cells. EPO is not produced by human RPE cells under hypoxia, an effect which may be secondary to the fact that these cells do not express significant HIF‐2α. Although RPE cells are an important source of angiogenic factors in the retina, our results indicate that other retinal elements are likely also involved in the production of angiogenic factors like EPO. Our study was thus limited by its in vitro nature, and the fact that only RPE cells were studied. Examination of the role of these retinal elements as well as alternate isoforms of HIF in regulating the expression of angiogenic factors in vivo will likely unveil more clues to the molecular mechanisms underlying retinal ischaemic disease.
Acknowledgements
This work was funded by a grant from the Canadian Institutes of Health Research (CIHR grant # 11535). The authors declare no financial interests in any aspect of this report.
Footnotes
Competing interest: None declared.
References
- 1.Ferris F L, 3rd, Davis M D, Aiello L M. Treatment of diabetic retinopathy. N Engl J Med 1999341667–678. [DOI] [PubMed] [Google Scholar]
- 2.Aiello L P, Avery R L, Arrigg P G.et al Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 19943311480–1487. [DOI] [PubMed] [Google Scholar]
- 3.Caldwell R B, Bartoli M, Behzadian M A.et al Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets 20056511–524. [DOI] [PubMed] [Google Scholar]
- 4.Witmer A N, Vrensen G F, Van Noorden C J.et al Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res 2003221–29. [DOI] [PubMed] [Google Scholar]
- 5.Miller J W, Adamis A P, Aiello L P. Vascular endothelial growth factor in ocular neovascularization and proliferative diabetic retinopathy. Diabetes Metab Rev 19971337–50. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe D, Suzuma K, Matsui S.et al Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med 2005353782–792. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez C, Fonollosa A, Garcia‐Ramirez M.et al Erythropoietin is expressed in the human retina and it is highly elevated in the vitreous fluid of patients with diabetic macular edema. Diabetes Care 2006292028–2033. [DOI] [PubMed] [Google Scholar]
- 8.Maxwell P H, Ratcliffe P J. Oxygen sensors and angiogenesis. Semin Cell Dev Biol 20021329–37. [DOI] [PubMed] [Google Scholar]
- 9.Wenger R H, Kvietikova I, Rolfs A.et al Hypoxia‐inducible factor‐1 alpha is regulated at the post‐mRNA level. Kidney Int 199751560–563. [DOI] [PubMed] [Google Scholar]
- 10.Semenza G L. HIF‐1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol 2000881474–1480. [DOI] [PubMed] [Google Scholar]
- 11.Bracken C P, Whitelaw M L, Peet D J. The hypoxia‐inducible factors: key transcriptional regulators of hypoxic responses. Cell Mol Life Sci 2003601376–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hewitson K S, Schofield C J. The HIF pathway as a therapeutic target. Drug Discov Today 20049704–711. [DOI] [PubMed] [Google Scholar]
- 13.Arjamaa O, Nikinmaa M. Oxygen‐dependent diseases in the retina: role of hypoxia‐inducible factors. Exp Eye Res 200683473–483. [DOI] [PubMed] [Google Scholar]
- 14.Zhu H, Bunn H F. Signal transduction. How do cells sense oxygen? Science 2001292449–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham E T, Jr, Adamis A P, Altaweel M.et al A phase II randomized double‐masked trial of pegaptanib, an anti‐vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology 20051121747–1757. [DOI] [PubMed] [Google Scholar]
- 16.Avery R L, Pearlman J, Pieramici D J.et al Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113: 1695 e1–15, [DOI] [PubMed]
- 17.Chun D W, Heier J S, Topping T M.et al A pilot study of multiple intravitreal injections of ranibizumab in patients with center‐involving clinically significant diabetic macular edema. Ophthalmology 20061131706–1712. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y, Zhang Y, Ojwang B A.et al Long‐term tolerance to retinal ischemia by repetitive hypoxic preconditioning: Role of HIF‐1{alpha} and heme oxygenase‐1. Invest Ophthalmol Vis Sci 2007481735–1743. [DOI] [PubMed] [Google Scholar]
- 19.Grimm C, Wenzel A, Acar N.et al Hypoxic preconditioning and erythropoietin protect retinal neurons from degeneration. Adv Exp Med Biol 2006588119–131. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda Y, Yonemitsu Y, Onimaru M.et al The regulation of vascular endothelial growth factors (VEGF‐A, ‐C, and ‐D) expression in the retinal pigment epithelium. Exp Eye Res 2006831031–1040. [DOI] [PubMed] [Google Scholar]
- 21.Epstein A C, Gleadle J M, McNeill L A.et alC. elegans EGL‐9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 200110743–54. [DOI] [PubMed] [Google Scholar]
- 22.Stiehl D P, Wirthner R, Koditz J.et al Increased prolyl 4‐hydroxylase domain proteins compensate for decreased oxygen levels. Evidence for an autoregulatory oxygen‐sensing system. J Biol Chem 200628123482–23491. [DOI] [PubMed] [Google Scholar]
- 23.Demidenko Z N, Rapisarda A, Garayoa M.et al Accumulation of hypoxia‐inducible factor‐1alpha is limited by transcription‐dependent depletion. Oncogene 2005244829–4838. [DOI] [PubMed] [Google Scholar]
- 24.Metzen E, Stiehl D P, Doege K.et al Regulation of the prolyl hydroxylase domain protein 2 (phd2/egln‐1) gene: identification of a functional hypoxia‐responsive element. Biochem J 2005387711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pescador N, Cuevas Y, Naranjo S.et al Identification of a functional hypoxia‐responsive element that regulates the expression of the egl nine homologue 3 (egln3/phd3) gene. Biochem J 2005390189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paulding W R, Czyzyk‐Krzeska M F. Hypoxia‐induced regulation of mRNA stability. Adv Exp Med Biol 2000475111–121. [DOI] [PubMed] [Google Scholar]
- 27.Stein I, Itin A, Einat P.et al Translation of vascular endothelial growth factor mRNA by internal ribosome entry: implications for translation under hypoxia. Mol Cell Biol 1998183112–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L, Simon M C. Regulation of transcription and translation by hypoxia. Cancer Biol Ther 20043492–497. [DOI] [PubMed] [Google Scholar]
- 29.Fernandes A F, Guo W, Zhang X.et al Proteasome‐dependent regulation of signal transduction in retinal pigment epithelial cells. Exp Eye Res 2006831472–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jelkmann W. Molecular biology of erythropoietin. Intern Med 200443649–659. [DOI] [PubMed] [Google Scholar]
- 31.Wiesener M S, Jurgensen J S, Rosenberger C.et al Widespread hypoxia‐inducible expression of HIF‐2alpha in distinct cell populations of different organs. Faseb J 200317271–273. [DOI] [PubMed] [Google Scholar]
- 32.Li Q F, Wang X R, Yang Y W.et al Hypoxia upregulates hypoxia inducible factor (HIF)‐3alpha expression in lung epithelial cells: characterization and comparison with HIF‐1alpha. Cell Res 200616548–558. [DOI] [PubMed] [Google Scholar]
- 33.Grimm C, Wenzel A, Groszer M.et al HIF‐1‐induced erythropoietin in the hypoxic retina protects against light‐induced retinal degeneration. Nat Med 20028718–724. [DOI] [PubMed] [Google Scholar]
- 34.Rankin E B, Biju M P, Liu Q.et al Hypoxia‐inducible factor‐2 (HIF‐2) regulates hepatic erythropoietin in vivo. J Clin Invest 20071171068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]