Abstract
Background
Optimal management of patients with lung cancer requires accurate cell typing of tumours and staging at the time of diagnosis. Endobronchial ultrasound‐guided lymph node aspiration as a method of diagnosing and staging lung cancer is a relatively new technique.
Aim
To report the use of liquid‐based‐thin‐layer cytology for the processing and reporting of these specimens.
Methods
The specimens obtained from 80 patients were processed using the ThinPrep system, with the remainder of the samples being processed as a cell block.
Results
40 of the 81 procedures yielded malignant cells (30 non‐small cell carcinoma, 8 small‐cell carcinoma and 2 combined small‐cell carcinoma/non‐small‐cell carcinoma). The cell blocks were found to contain sufficient material to allow the immunohistochemical characterisation of tumour cells with a range of antibodies.
Conclusion
The use of liquid‐based‐thin‐layer cytological techniques provides high‐quality specimens for diagnostic purposes. When used in conjunction with cell blocks, sufficient material may be obtained to allow immunohistochemical studies to confirm the tumour cell type. Given the current move towards centralisation of pathology services, this approach gives the pathologist high‐quality specimens without the need for direct onsite support at the time of the procedure.
Optimal management of patients with bronchial carcinoma requires histological or cytological conformation of malignancy, tumour classification and accurate staging to allow treatment planning and the identification of patients who will benefit from potentially curative radical treatment.1 CT scanning is usually carried out as a first staging procedure and can determine the presence of enlarged lymph nodes, but the predictive value of nodal enlargement for the presence of metastatic disease is recognised to be poor.2,3 Although recent developments such as flurodeoxyglucose positron emission tomography, especially when combined with CT, have shown significant improvements in the accuracy of mediastinal staging,4 there remains a requirement to confirm the presence of metastatic malignancy by histological or cytological means in order to plan appropriate treatment.5
Mediastinal lymph node sampling for this purpose has been largely carried out by mediastinoscopy or thoracoscopy,6 but these have the disadvantage of being inpatient procedures requiring a general anaesthetic, and in the case of mediastinoscopy only giving access to nodes in the upper anterior mediastinum. Transbronchial7,8 and transthoracic9 lymph node aspiration or biopsy have previously been used to sample mediastinal nodes, but with variable results. The development of endoluminal ultrasound technology however, has, allowed the development of techniques whereby lymph nodes can be aspirated through either the airway (endobronchial ultrasound fine‐needle aspiration (EBUS–FNA)) or the oesophageal wall (endoscopic ultrasound fine‐needle aspiration (EUS–FNA)).10
We and others11,12,13,14 have previously reported the use of EBUS–FNA in assessing patients with lung cancer. Such studies from other centres have used standard cytological smears prepared from the aspirated material usually, but not in all cases,12 with the assistance of a cytologist present at the procedure. In this paper, we report our experience of processing and reporting EBUS–FNA specimens using liquid‐based thin‐layer technology.
Materials and methods
Patients
During the period between May 2005 and January 2006, 81 EBUS–FNA procedures were carried out on a total of 80 patients (41 men and 39 women, aged 30–86 years) who had been referred to the Respiratory Unit, Royal Infirmary of Edinburgh, Edinburgh, UK. Most patients had a presumed, or proven, diagnosis of bronchial carcinoma with hilar or mediastinal nodal enlargement on CT scan but were not considered to be candidates for surgical resection. To confirm the diagnosis, establish the cell type of the tumour or confirm the stage, EBUS–FNA was carried out as we have described previously.11 A few patients were, however, also referred with mediastinal lymphadenopathy of unknown cause or were thought to have mediastinal recurrence of carcinoma.
The number of lymph node stations sampled ranged from 1–4. In all, two or three needle passes were performed on each node in the manner we have described previously.11 All the samples obtained were placed directly into Cytolyt (Cytyc UK, Crawley, West Sussex, UK) and delivered to the Pathology Department, Royal Infirmary of Edinburgh.
Specimen processing
The specimens were processed using the T2000 ThinPrep System (Cytyc UK, Crawley, West Sussex, UK) and the single preparation was stained with Papanicolaou (PAP) stain. Any remaining of the specimen was centrifuged, to form a pellet, suspended in agar, fixed in neutral buffered formalin and processed as a cell block from which a single H&E stained section was cut. Further sections were cut and used for immunohistochemical staining as required, with a range of antibodies using standard methods and the automated staining machines currently in use in the department.
Reporting
All specimens were reported by one of three consultant pathologists with a specific interest in thoracic pathology (WAHW, HMM, DMS) who report both histological and cytological specimens.
Results
The aspirates obtained were of variable cellularity. Evidence of lymph node sampling was assessed by the presence of lymphoid cells or sheets of histiocytes often with associated pigment consistent with the prominent sinus histiocytosis commonly observed in mediastinal nodes. Although some aspirates were relatively sparsely cellular, none were deemed to show evidence of nodal sampling. In addition, all the aspirates contained evidence of varying amounts of endobronchial‐derived material in the form of respiratory epithelial cells and alveolar macrophages. The cell blocks again were of variable cellularity, but many contained “mini biopsies” with fragments of identifiable nodal tissue and in some cases cartilage.
From the 81 procedures carried out, metastatic malignancy was identified in 40 cases. Non‐small‐cell carcinoma (fig 1) was diagnosed in 30 cases (6 squamous carcinoma, 3 adenocarcinoma and 21 unspecified), small‐cell carcinoma (fig 2) in 8 cases and combined small‐cell carcinoma/non‐small‐cell carcinoma in 2 cases. In two cases of squamous carcinoma, malignancy was diagnosed solely on the basis of the cell block, with no identifiable tumour in the PAP‐stained cytological preparations. In addition, in one of the cases of combined small‐cell carcinoma/non‐small cell carcinoma only the latter was present in the cytological preparation and the small‐cell component was only identified in the cell block.
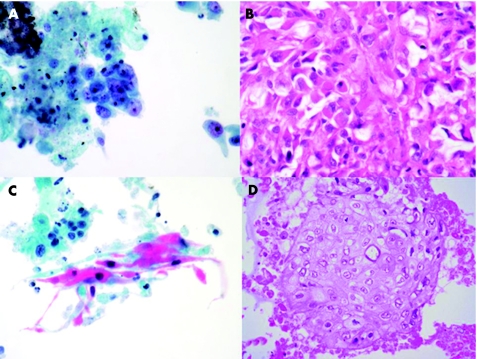
Figure 1 Photomicrographs from endobronchial ultrasound fine‐needle aspiration samples illustrating the appearances of metastatic adenocarcinoma (A, B) and metaststic squamous carcinoma (C, D) in Papanicolaou‐stained ThinPrep preparations and the accompanying H&E stained sections from the cell blocks. (original magnification ×400).
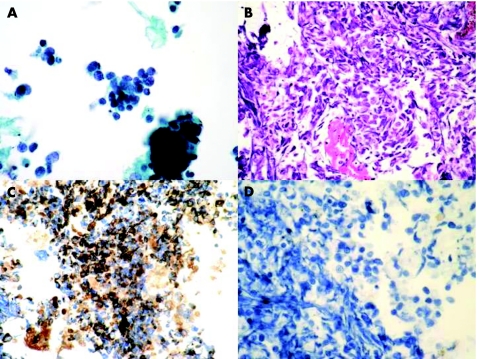
Figure 2 Photomicrographs from endobronchial ultrasound fine‐needle aspiration samples illustrating the appearances of metastatic small‐cell carcinoma in a Papanicolaou‐stained ThipPrep preparation (A) and in a section from a cell block stained with H&E (B). Immunohistochemistry with pan‐cytokeratin shows positive staining of the tumour cells (C) whereas no staining with CD45 was identified (D; original magnification ×400).
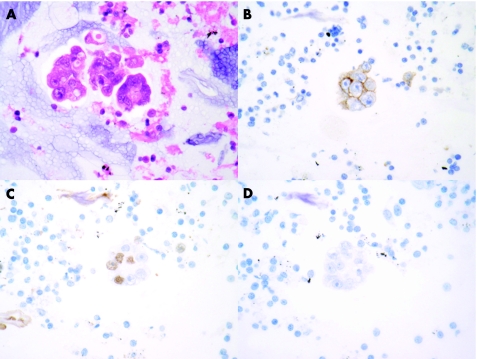
Immunohistochemistry with CD45, pan‐cytokeratin and CD56 was performed in sections obtained from the cell block in the cases of small‐cell carcinoma and this was found to show patterns of staining in the tumour present similar to that seen in more traditional histological specimens (fig 2). Immunohistochemistry with thyroid transcription factor 1 (TTF1) and oestrogen receptor was used to evaluate cases in which the clinical impression was of probable metastatic breast carcinoma. In one case clinically thought to be metastatic breast carcinoma, the cells obtained were shown to be TTF1 positive and oestrogen receptor negative, suggesting a separate lung primary carcinoma. This finding was confirmed by subsequent mediastinoscopy (fig 3).
Figure 3 Photomicrographs of specimens obtained from an endobronchial ultrasound fine‐needle aspiration from a woman with a history of breast cancer and a right hilar/paratracheal mass. Groups of malignant glandular cells consistent with origin from an adenocarcinoma were identified both in the Papanicolaou‐stained ThipPrep preparation and in the H&E‐stained sections from the cell block (A). Immunistochemistry demonstrated that the tumour cells expressed BerEp4 (B) and showed focal nuclear staining with thyroid transcription factor1 (C). No staining with antibodies to oestrogen receptor was identified (D). A subsequent mediastinoscopy confirmed the presence of adenocarcinoma, which was morphologically different from the breast lesion and consistent with origin from a bronchial carcinoma, (original magnification ×400).
The remaining 41 procedures showed no evidence of malignancy. In one patient with unexplained lymphadenopathy, discrete non‐caseating granulomata were identified in the cell block, suggesting the possibility of sarcoidosis. Further material was subsequently submitted for histological and cytological assessment in 11 of these patients; 7 patients underwent mediastinoscopy, 1 had a repeat EBUS–FNA and 3 had further surgical biopsies.
The mediastinoscopies showed two cases of non‐Hodgkin's lymphoma, two metastatic adenocarcinomas and three were negative. Of the latter three, one had a lung biopsy and was diagnosed with Hodgkin's disease and another underwent resection of a primary lung adenocarcinoma, which was found to be node negative. The repeat EBUS–FNA performed in one case demonstrated malignant cells consistent with a non‐small‐cell carcinoma. One patient had a lung biopsy showing a high‐grade sarcoma and two other patients had biopsies of distant metastases (brain and bone) showing non‐small‐cell carcinoma.
Discussion
EBUS–FNA is a relatively new technique that allows aspiration of mediastinal and hilar lymph node groups under direct ultrasound control.10,11,12,13,14 The traditional approach of making spreads from such material may result in large numbers of slides to screen, and assessment can be difficult if the diagnostic material is obscured by blood. The quality of the preparations may be improved if an appropriately trained cytologist or cytology technician is present at the procedure.14 This also has the advantage that the quality of the aspirates can be assessed at the time. Provision of such support is, however, expensive and may be difficult to provide given the current trends to centralise pathology services with increasing sub‐specialisation. We have previously introduced liquid‐based cytology techniques for processing bronchial cytology specimens where similar issues of specimen quality apply. In our experience, the approach we describe provides samples that are relatively straightforward to report. The cells are well visualised, not obscured by blood and air‐drying artefacts are avoided.
Although lymph node FNA provides a reliable way of detecting the presence of malignancy and will often permit cell typing,13 it is often difficult to determine the most likely primary site when there are conflicting clinical possibilities. The use of cell blocks and immunohistochemistry may give some useful information, and in some cases we were able to address the issue by demonstrating TTF1 staining of the tumour cells but in other cases we were unable to make any comment owing to either the sparsity of the material or the absence of staining with any of the tissue‐specific markers. This suggests that case selection for EBUS–FNA is important, and that although it is a valuable tool in staging lung cancer, more complex cases where treatment options are determined by the probable primary site of the tumour rather than simply by the presence or absence of malignancy then lymph node biopsy by mediastinoscopy may be more appropriate.
Take‐home messages
Endobronchial ultrasound fine‐needle aspiration is a useful tool for the diagnosis and staging of lung cancer.
The use of thin‐layer cytological techniques provides good‐quality specimens for diagnostic purposes.
The routine use of cell blocks adds to the diagnostic yield and provides material for immunohistochemical studies.
In our experience to date, we have not identified evidence of lymphoma in three cases where this turned out to be the final diagnosis (two cases of non‐Hodgkin's lymphoma and one case of Hodgkin's lymphoma diagnosed on mediastinoscopy and lung biopsy, respectively). On review, no specific cytological features were seen to suggest the diagnoses. This may suggest that diagnostic material was not obtained in at least the two cases with subsequent positive mediastinoscopies. The potential role of EBUS–FNA cytology in the diagnosis of lymphoma remains controversial and although ancilliary studies such as flow cytometry and molecular studies may play a role, histological assessment and classification remains the “gold standard” for planning treatment schedules. Although we did identify one case where there was evidence of a granulomatous process in the material within the cell block from an aspirated node, the technique should not, in our view, be regarded as a reliable method to assess the aetiology of isolated lympadenopathy when lymphoma and sarcoidosis are the principal clinical differential diagnoses.
As with many cytological samples, a positive result is helpful in patient management but the significance of a negative one is less certain. In our series, most patients with negative aspirates did not undergo mediastinoscopy as they were not considered surgical candidates, and subsequent treatment was guided by the clinical and radiological characteristics of the case. Nevertheless, we would conclude from our experience that the use of liquid‐based thin‐layer cytological techniques for EBUS–FNA samples provides good‐quality specimens for diagnostic purposes and that the use of cell blocks provides additional material for assessment and immunohistochemical staining when this is required.
Abbreviations
EBUS–FNA - endobronchial ultrasound fine‐needle aspiration
PAP - Papanicolaou
TTF1 - thyroid transcription factor1
Footnotes
Competing interests: KMS has received equipment and educational grants for travel from KeyMed (Medical and Industrial Equipment) WAHW, HMM, DMS and MAG have no financial or other interests in the material published.
References
- 1.Scottish Intercollegiate Guidelines Network Management of patients with lung cancer. A national clinical guideline. Edinburgh, 2005. http://www.sign.ac.uk (accessed 10 Feb 2007)
- 2.Kerr K M, Lamb D, Wathan C G.et al Pathological assessment of mediastinal lymph nodes in lung cancer: implications for non‐invasive mediastinal staging. Thorax 199247337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts J R, Blum M G, Arilsden R.et al Prospective comparison of radiologic, thoracoscopic and pathologic staging in patients with early non‐small cell lung cancer. Ann Thorac Surg 1999681154–1158. [DOI] [PubMed] [Google Scholar]
- 4.Shim S S, Lee K S, Kim B T.et al Non‐small cell lung cancer: prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology 20052361011–1019. [DOI] [PubMed] [Google Scholar]
- 5.de Langen A J, Raijmakers P, Riphagen I.et al The size of mediastinal lymph nodes and its relation with metastatic involvement: a meta analysis. Eur J Cardiothorac Surg 20062926–29. [DOI] [PubMed] [Google Scholar]
- 6.Passlick B. Initial surgical staging of lung cancer. Lung Cancer 200342(Suppl 1)S21–S25. [DOI] [PubMed] [Google Scholar]
- 7.Rajamani S, Mehta A C. Transbronchial needle aspiration of central and peripheral nodules. Monaldi Arch Chest Dis 200156436–445. [PubMed] [Google Scholar]
- 8.Hsu L H, Liu C C, Kos J S. Education and experience improve the performance of transbronchial needle aspiration: a learning curve at a cancer centre. Chest 2004125532–540. [DOI] [PubMed] [Google Scholar]
- 9.Protopapasz, Westcott J L. Transthoracic needle biopsy of mediastinal lymph nodes for staging lung and other cancers. Radiology 1996199489–496. [DOI] [PubMed] [Google Scholar]
- 10.Hearth F J. Mediastinal staging—the role of endobronchial and endo‐oesophageal sonographic guided needle aspiration [review]. Lung Cancer 200445(Suppl 2)S63–S67. [DOI] [PubMed] [Google Scholar]
- 11.Rintoul R C, Skwarski K M, Murchison J T.et al Endobronchial and endoscopic ultrasound guided real‐time FNA for mediastinal staging. Eur Respir J 200525416–421. [DOI] [PubMed] [Google Scholar]
- 12.Herth F, Becker H D, Ernst A. Conventional vs endobronchial ultrasound–guided transbronchial needle aspiration. A randomised trial. Chest 2004125322–325. [DOI] [PubMed] [Google Scholar]
- 13.Yasufuku K, Chiyo M, Koh E.et al Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer 200550347–354. [DOI] [PubMed] [Google Scholar]
- 14.Shannon J J, Bude R O, Orens J B.et al Endobronchial ultrasound‐guided needle aspiration of mediastinal adenopathy. Am J Respir Crit Care Med 19961531424–1430. [DOI] [PubMed] [Google Scholar]